Abstract
Severe acute respiratory syndrome (SARS) was the first natural disaster that challenged the Chinese people at the beginning of the twenty-first century. It was caused by a novel animal coronavirus, never recognized or characterized before. This SARS coronavirus (SARS-CoV) exploited opportunities provided by ‘wet markets’ in southern China to adapt to the palm civet and human. Under the positive selection pressure of human host, certain mutated lineages of the virus became readily transmissible between humans and thus caused the epidemic of 2002–2003. This review will provide first-hand information, particularly from Guangdong, China, about the initial epidemiology, the identification of the aetiological agent of the disease, the molecular evolution study of the virus, the finding of SARS-like CoV in horseshoe bats and the mechanistic analysis for the cross-host tropism transition. The substantial scientific contributions made by the Chinese scientists towards understanding the virus and the disease will be emphasized. Along with the description of the scientific discoveries and analyses, the significant impact of these researches upon the public health measurement or regulations will be highlighted. It is aimed to appreciate the concerted and coordinated global response that controlled SARS within a short period of time as well as the research strategy and methodology developed along with this process, which can be applied in response to other public health challenges, particularly the future emerging/re-merging infectious diseases.
Keywords: severe acute respiratory syndrome, SARS coronavirus, epidemiology, evolution, zoonotic disease, genomics
1. Introduction
For the Chinese people, the dawn of the twentieth century was a painful memory of poverty and abasement. Although patriotic revolutionaries and reforms have dramatically changed China since then, the beginning of the twenty-first century was not easy either. Besides other difficulties, a new ‘plague’, originally called ‘atypical pneumonia’ and later designated as severe acute respiratory syndrome (SARS), emerging in the southeastern Guangdong Province of China in November 2002, was one of the most serious natural disasters that unexpectedly challenged the Chinese people.
On 21 February 2003, a urologist (patient A; Ruan et al. 2003), from the HZS-2 Hospital of the capital city Guangzhou, Guangdong Province (Chinese 2004), spent a single night at hotel ‘M’ in Hong Kong. He transmitted an infection to 16 other guests during his stay (Peiris et al. 2004). These, in turn, seeded outbreaks of the disease in Hong Kong, Toronto, Singapore and Vietnam (CDCP 2003). Within weeks, atypical pneumonia had spread to affect more than 8000 people in 25 countries and regions across five continents (World Health Organization (WHO), http://www.who.int/csr/sars/country/table2004_04_21/en/index.html). By the end of the global outbreak (5 July 2003), it had killed 774 people. Although it is a small number in comparison with the fatalities during the previous pandemics of plague and influenza, as a novel infectious disease with unknown aetiological agent, but rapidly spread by air travel at the beginning of the epidemic, the panic thus caused was enormous. With today's globalization of economic activity, this panic eventually caused far more pronounced impact of this epidemic than any previous ones. It was estimated by the Asian Development Bank (November 2003) that the global economic loss was 59 billion USD with 17.9 billion USD of Mainland China (approx. 1.3% of its annual gross domestic product (GDP), a 0.7% decrease in the GDP growth rate) and 12 billion USD of Hong Kong (approx. 7.6% of its annual GDP).
On the other hand, due to the globally concerted and coordinated response contributed by both administrative measures and scientific research, the disease was quickly controlled within couple of months. The clinical syndrome was quickly described (Lee et al. 2003; Poutanen et al. 2003; Tsang et al. 2003) and the viral aetiological agent was identified (Drosten et al. 2003a; Ksiazek et al. 2003; Peiris et al. 2003b). With this knowledge, diagnostic tests were devised (Drosten et al. 2003a; Poon et al. 2003) and the genome of the virus was completely sequenced (Marra et al. 2003; Rota et al. 2003). Meanwhile, epidemiology studies (Lipsitch et al. 2003; Riley et al. 2003) indicated the effectiveness of public health intervention strategy and thus supported the corresponding administrative measures enforced by the government, which eventually proved to be successful. Just one and a half years later, the first phase 1 vaccine trials are underway, and several other vaccine candidates are under evaluation in animal models (Enserink 2004). It was a triumph for global public health and provided a new paradigm for the detection and control of future threats caused by emerging infectious diseases.
This process has recently been thoroughly reviewed, addressing aspects of the clinical presentation (Peiris et al. 2003c; Christian et al. 2004; Rainer 2004), aetiology (Drosten et al. 2003b), virology (Holmes 2003; Davidson & Siddell 2003; Stadler et al. 2003), laboratory diagnosis (Poon et al. 2004), epidemiology (Anderson et al. 2004 and WHO, http://www.who.int/csr/sars/en/whoconsensus.pdf), infection control, clinical management and public health (Poutanen & Low 2004; Poutanen & McGeer 2004; Weinstein 2004). A more detailed review emphasizing the aspects of pathogenesis and their correlation to clinical outcome and discussing the progress that has been made towards antiviral treatment and vaccine development is also available (Peiris et al. 2004). Because I was deeply involved in the molecular epidemiology studies of this disease and had an opportunity to contact many Chinese scientists, physicians and public health workers during the research, I would like to share my knowledge with readers about the real epidemic story and the research efforts made in China, especially, in Guangdong Province, where the disease initiated. I hope that, through reading this review, people of the world may realize how the Chinese scientists together with the public health and medical workers of this largest developing country, considered being ‘defeated’ at the early stage of the combat against SARS (Enserink 2003), found their way to quickly understand and control an emerging zoonotic epidemic in the genomics era.
2. Beginning of SARS: initial epidemiology of the pandemic
Although I was not with the people of Guangdong at the very beginning of the epidemic, I have spent substantial time with them since the beginning of May 2003. The glorious contributions made by the scientists there in the process of combating SARS, particularly in the early stage of the epidemic, are worth recording.
On 2 January 2003, the People's Hospital of Heyuan, a city approximately 200 km northeast of the capital city Guangzhou (not connected), Guangdong Province, reported formally, and for the first time, two patients (one index patient and one secondary infection) diagnosed as ‘infectious atypical pneumonia’ (Peng et al. 2003). Although atypical pneumonia was no surprise to medical experts, this particular case was considered unique owing to its propensity to cause clusters of disease in families and healthcare workers (Zhong & Zeng 2003). The index patient was a restaurant chef working in the city of Shenzhen. He began to have fever and flu-like syndrome on 10 December 2002, but was not hospitalized until 15 December when he returned back to his hometown, Heyuan. He suffered a severe disease course but eventually recovered after further treatment in Guangzhou. He infected seven hospital staff and one patient during his hospitalization in Heyuan (onset dates ranging from 16 to 28 December), and it was the first recognized case of SARS-related hospital infection.
It was reported (He et al. 2003a,b) that medical experts examining this case recognized that previous to this one, a similar case had been encountered in the city of Foshan, directly west of Guangzhou. The index case of Foshan began to have fever higher than 39°C and respiratory symptoms (e.g. cough, headache, shortness of breath) on 16 November 2002 and was hospitalized on the 20th. Owing to the failure of antibiotics treatment and the chest radiograph changes suggestive of pneumonia, he was further treated in the intensive care unit (ICU) and recovered on 8 January 2003. Besides his wife, he further infected three other relatives (onset dates ranging from 7 November to 4 December), but none of his children or hospital staff were infected. Although no biological samples were collected from this patient suitable for virus isolation, retrospective serological studies confirmed this Foshan case as the first index case of the SARS epidemic (unpublished personal communication with R. H. Xu of Centers for Disease Control and Prevention (CDCP), Guangdong Province, 2003; Zhong et al. 2003; Xu et al. 2004a).
From the end of December 2002 to the whole month of January 2003, several cities around the capital city Guangzhou, including itself, in the area of Pearl River Delta of Guangdong Province, continuously suffered from this atypical pneumonia. The information on the index cases is described below (table 1; unpublished result, CDCP Guangdong; He et al. 2003a; Chinese 2004).
Table 1.
SARS case comparison of the early-phase patients versus the Super-Spreader Event index patient of the middle phase from the HZS-2 Hospital. (R, relatives and friends infected; H, hospital infection; T, total number of people infected and D, number of deaths.)
| cases by cities | onset date | no. of secondary infections | no. of tertiary infections | ||||||
|---|---|---|---|---|---|---|---|---|---|
| dd mm yy | R | H | T | Da | R | H | T | D | |
| Foshan index | 16 Nov 2002 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| Heyuan index | 10 Dec 2002 | 0 | 8 | 8 | 0 | 0 | 0 | 0 | 0 |
| Jiangmen index | 21 Dec 2002 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zhongshan index | 26 Dec 2002 | 2 | 6b | 8b | 0 | 1 | 3 | 4 | 0 |
| Zhongshan 2nd | 1 Jan 2003 | 2 | 6b | 8b | 0 | ||||
| Shunde index | 2 Jan 2003 | 1 | 2 | 3 | 1 | 0 | 0 | 0 | 0 |
| Shunde SGQ | 8 Jan 2003 | 1 | 5c | 6c | 1 | ||||
| Guangzhou index | 2 Jan 2003 | 0 | 7 | 7 | 0 | 0 | 0.5d | 0.5d | 0 |
| Shenzhen index | 15 Jan 2003 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Zhaoqing index | 17 Jan 2003 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| Dongguan index | 10 Mar 2003 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| sum (early phase): 11 | 10 | 35 | 45 | 3 | 3 | 3.5 | 6.5 | 0 | |
| SQS (Shunde to Guangzhou) | 18 Jan 2003 | 0 | 7e | 7e | 5e | 5e | |||
| ZZF (Guangzhou) | 22 Jan 2003 | 23 | 55e | 78e | 4 | 5 | 35e | 40e | 1 |
| sum (Hospital HZS-2): 2 | 23 | 62 | 85 | 4 | 5 | 44 | 45 | 1 | |
The hospital infection of the Zhongshan City was caused by both the index case and the second case. Therefore, secondary infections in the hospital are shown as the average contribution of these two patients.
The hospital infection of Shunde City was believed to be contributed by SGQ and another patient. Therefore, the secondary infections in the hospital are shown as the average contribution of these two patients, except for one member of the medical staff who was known to have had only direct contact with SGQ. SGQ also probably infected two people in the HZS-2 Hospital of Guangzhou.
The tertiary infection of the Guangzhou index case involved only one person, but was likely to have two hospital infection origins. Therefore, only the average number (0.5) was used as the contribution made by the secondary cases.
Because SQS and ZZF were together in Hospital HZS-2 for a few days, it is difficult to clearly identify the transmission path. Therefore, estimates were made by the staff of HZS-2 Hospital according to the contact history and the onset dates of the patients for the secondary, tertiary and quaternary infections. The secondary infection by ZZF includes numbers of Hospital HZS-3 and Hospital GZS-8. The same principle was applied for the tertiary and the quaternary infections. It was estimated that the quaternary infections originated from ZZF included one friend and 13 hospital-infected patients.
The index patient is included.
Jiangmen is a city located directly southwest of Foshan. The onset date for the index case was 21 December 2002, but no further infection was recorded. It is interesting to note that all the cases that occurred in January 2003 in Jiangmen apparently had no further infection records, although similar cases did arise one after another.
Zhongshan is a city located at the entrance of Pearl River to the sea and directly south of Guangzhou. The index case of this city was a chef and he began to have fever on 26 December 2002. He and two to three early independent index cases infected at least 24 relatives and hospital staff (secondary and tertiary infections).
Shunde is a city located directly south of Foshan and west of Guangzhou. The index case was a seller of snakes and worked in a wet market selling chickens and exotic animals. His onset date was 2 January 2003. He passed away on January 23. He infected his daughter and two hospital staff members.
Guangzhou is the capital city of Guangdong Province, and the Pearl River runs through the city. The index case began to have atypical pneumonia syndrome on 2 January 2003. He raised a guinea-pig as pet, but it died before his onset date. He infected primarily seven relatives, hospital patients and staff members. Subsequently, mixed infections occurred in the hospital, ending with approximately more than 15 secondary and tertiary infected cases.
The onset date of the formally reported index case of Shenzhen, the city of the first Chinese special economic zone between Hong Kong Special Administrative Region (SAR) and the mainland, located on the east coast of the Pearl River, was 15 January 2003. It was one month later than the Heyuan case mentioned previously, although the latter was infected in Shenzhen. This index case was a 46-year-old male working for an electronic equipment company and no contact history was observed. He was hospitalized on 20 January and transferred to another hospital on 26 January. He passed away on 1 February. He infected one close contact (onset date: 29 January), but no hospital infection was observed. He attended a meeting in Hong Kong on 14 January.
Zhaoqing is a city located directly northwest of Foshan. The index case was a private seller, who began to have atypical pneumonia syndrome on 17 January 2003, two weeks after her visit to Guangzhou. She infected only her son and a medical doctor.
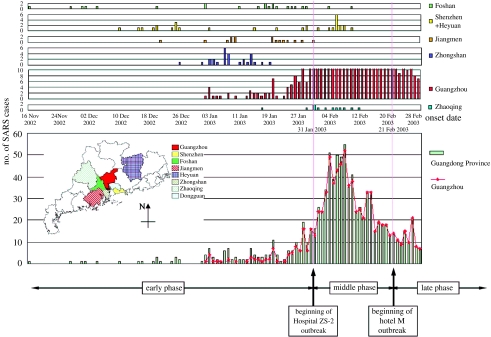
From the first Foshan index case of mid-November 2002 to the middle of January 2003, the atypical pneumonia cases in Guangdong Province seemed to occur independently and their geographical distribution was scattered. During this period of time, it is significant that, even as late as 10 March (the Dongguan case, see below), all of the independent onsets of the epidemic were limited to the regions located directly west of the capital city, Guangzhou (including Guangzhou), down to the southern city Shenzhen, while no cases were reported in the north or east of Guangzhou (figure 1; unpublished result, CDCP Guangdong). This region, known as the Pearl River Delta, has been enjoying rapid economic development since the late 1970s, resulting in more fashionable behaviour involving exotic animals as pets or food in contrast to the other less affluent regions of Guangdong (Chinese 2004).
Figure 1.
The triphasic SARS epidemic in Guangdong Province, China. Shown are the number of daily documented SARS cases reported from individual cities of the Guangdong Province, China, up to February 2003. The early, middle and late phases of the epidemic are defined in the text. The map shows the geographical distribution of cases belonging to the early phase by administrative districts of Guangdong Province. Original epidemiological data were collected and analysed by the Guangdong Center for Disease Control and Prevention. The cases reported from the cities of Heyuan and Shenzhen were combined and treated as Shenzhen cases, because the Heyuan index case was infected in Shenzhen, and after this nosocomial infection no additional infections were reported in Heyuan. The order of the cities is arranged from top to bottom based on the disease onset date of their respective index cases, starting from the earliest to the latest dates of onset. Adapted from Chinese (2004).
Up to the end of January 2003, several hospitals in Guangzhou (mainly HSZ, GZS-8, HZS-2 and HZS-3) had received and treated quite a few severe patients transferred from its surrounding cities in addition to the local patients. Meanwhile, hospital infections did occur a couple of times in Guangzhou, with victims who came into contact with the index patients, such as hospital staff, hospitalized patients and relatives and friends of the index patients. However, it was the super spreader event (SSE) that took place in the HZS-2 Hospital which brought the disease into a real pandemic.
On 18 January 2003, two patients (brothers, SGQ and SQS, both working in a restaurant) from Shunde were hospitalized in the HZS-2 Hospital of Guangzhou. Later, one patient from Guangzhou (ZZF) was received by the same hospital on 24 January. The atypical pneumonia-related hospital outbreak began in this hospital on 31 January. During this SSE, the number of daily reported cases of atypical pneumonia from Guangzhou was significantly higher than 10 for more than a month after that day. In particular, from 5 to 10 February, the number exceeded 50 (figure 1; Chinese 2004).
Because the three patients were received by the same hospital within one week, it was difficult to identify the exact transmission path for individual patients. Later, viral genomic sequencing data (Chinese 2004) indicated that only one kind of viral genotype was observed in the secondary and tertiary infections of the HZS-2 Hospital and other related hospitals (see below). Therefore, the following epidemiological facts are critical for chasing the origin of this viral lineage.
The onset date for SGQ was 8 January 2003 and he was transferred from a Shunde local hospital to the HZS-2 Hospital in Guangzhou on 18 January 2003. He infected his brother (SQS, onset date, 18 January 2003) and several hospital staff in a Shunde hospital (maybe with other atypical pneumonia patients in that hospital). He was confined to the ICU of the HZS-2 Hospital until death on 31 January 2003 and probably infected one ICU staff member and a patient.
The onset date for SQS was 18 January 2003. First, he was treated in a local hospital of Shunde and then transferred to the HZS-2 Hospital on 24 January, where he came into contact with many hospital staff members as well as other patients in the ward specialized for respiratory infectious diseases. He had relatively mild symptoms. He recovered and was released from the hospital on 16 February 2003.
The third patient, ZZF, is a 44-year-old businessman who specialized in the wholesale of fish. His onset date was 22 January 2003. First, he visited a local clinic on 26 January 2003 without notable infectious disease symptoms and was then admitted to the HZS-2 Hospital on 30 January 2003. Besides other atypical pneumonia syndromes, he began to have diarrhoea on 1 February and was transferred to the HZS-3 Hospital. During his less than 48 h stay in the HZS-2 Hospital, he infected more than 30 hospital staff members and patients, and two of them finally died. Later, within an 8-day stay in the HZS-3 Hospital, he infected 21 hospital staff members and one of them died. He was further transferred to Hospital GZS-8 on 8 February 2003. He probably infected three hospital staff members there, while he had an intubation procedure for ventilatory support. Apparently, he did not cause further hospital infections in Hospital GZS-8. He began to recover on 10 February 2003 and was released from the hospital on 21 March 2003.
ZZF infected 23 relatives, visiting friends and workers with close contacts. Two of them finally died. Tertiary and quaternary infections did occur in the HZS-2 Hospital but not in the HZS-3 Hospital, which is clearly due to better isolation control. The infection in the Ward L2 of Hospital GZS-8 seems to have been caused by his hospitalized friends and relatives. Later, sequencing of the viral genome from a tertiary infected patient of this ward (HGZ8L2; Chinese 2004) was the same as those detected in the HZS-2 Hospital, but remarkably different from another isolate (HGZ8L1-A) of Ward L1 of the same hospital (Hospital GZS-8), where none of the patients had contact history with ZZF. Because all the viral sequences of the HZS-2 Hospital isolates were basically the same, it could be inferred that ZZF was the original host of the outbreak virus of the HZS-2 Hospital, although the genotype of SQS's virus is still unknown. Therefore, unless the viral genotype of the brothers of Shunde is the same as ZZF's, the majority of the secondary infections should have been caused by ZZF. This set of epidemiological data for ZZF and SQS was based on two independent surveys made on the HZS-2 Hospital staff about the contact history and the onset dates of the patients (table 1).
Retrospectively, similar diseases in six patients and two death cases occurred between 4 and 14 January 2003 and were recognized in Guangxi Province, located directly west of Guangdong Province. However, the major event that led to the dramatic spreading of the disease was unbelievably in Shanxi Province, thousands of kilometres northwest away from Guangdong Province. A lady doing treasury business, a native of Shanxi, went to Guangdong for trading in late February and there she became sick. Because she was not aware of the atypical pneumonia, she went back home and infected eight family members and five hospital staff. Looking for better treatment, the family went to Beijing on 1 March, but there they further infected the hospital staff, which was actually the first index case in Beijing. Retrospective analysis indicated that this transmission line accounted in total for nearly 20 patients in Shanxi.
Because patient A seeded infection in hotel M of Hong Kong on 21 February 2003, which was considered to be the beginning of the global transmission of the atypical pneumonia, it is important to review his epidemiological records. Doctor A (patient A), a urologist of the HZS-2 Hospital, began to have fever on 16 February, with a leucocyte count above 12×109 l−1. Because both his chest radiography and health condition quickly improved and he did not have apparent contact history with atypical pneumonia patients, he visited Hong Kong on 21 February, where he stayed in hotel M. On the 22nd, he was directly admitted to the ICU of hospital A with respiratory failure. Subsequent development of the epidemic has been extensively described. However, some patients in Hong Kong during that period of time were also found to have contact history in Shenzhen. A patient (CUHKW1) hospitalized in the Prince Wales Hospital, the teaching hospital of Hong Kong Chinese University, did travel to Shenzhen, Guangdong Province, six days before the onset of his symptoms on 15 March 2003 (Tsui et al. 2003). This case was one of the clearly identified sources of infection besides the patient A.
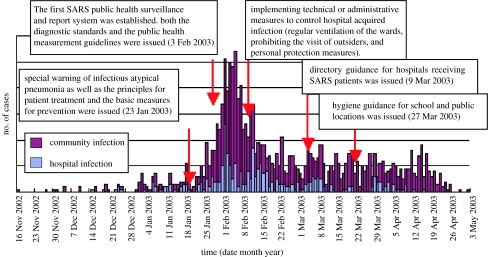
As a respiratory tract infectious disease, the route of transmission is critical for the control of SARS. In the early phase of the epidemic, CDCP of Guangdong was the first to propose what was later confirmed by others (Seto et al. 2003; Wong et al. 2004b), that droplet infection, aerosolization and fomites in short distance were the major routes of transmission of SARS. Based on the pathogenesis and epidemiological studies, a detailed and logical case definition was developed and the incubation period was estimated as 1–12 days (average 4.5 days). It was particularly important at that time to recognize that no infectivity was detected in patients at the stage of incubation or convalescence. These parameters are critical for a public health strategy designed for SARS prevention and control. In fact, the first active public health surveillance and report system for SARS was established and implemented throughout Guangdong Province on 3 February 2003, which was the first daily zero case report system for SARS, as well as the first local SARS surveillance and report system being set up worldwide (figure 2; Fang et al. 2003; He et al. 2003b; Peng et al. 2003; Qin et al. 2003b; Wang et al. 2003b; Zhou et al. 2003; Xu et al. 2004a).
Figure 2.
The time distribution of the onsets of SARS cases as well as the implementation dates for the corresponding public health control measures in Guangdong Province during the 2002–2003 epidemic.
The last ‘independently’ occurring SARS case of the 2002–2003 epidemic in Guangdong was found in Dongguan, a prosperous neighbouring city located directly south of Guangzhou and truly part of the Pearl River Delta region. The index case was a chef and his onset date was 10 March 2003. He went to a local hospital three times and was finally hospitalized in Hospital GZS-8 of Guangdong on 18 March. His disease course was relatively minor and he did not have obvious contact history with SARS patients.
Epidemiologically, this was the end of the independent onset of SARS in this pandemic. Although we observed a similar situation almost 10 months later in the same region, it is significant to realize and characterize the early phase of the epidemic by a series of seemingly independent cases, with scattered but limited geographical distribution and limited transmission/infectivity (Chinese 2004). On the basis of these epidemiological investigations, we divided the course of the epidemic into early, middle and late phases (Chinese 2004). The early phase is defined as the period from the first emergence of SARS to the first documented SSE of the HZS-2 Hospital of Guangzhou at the end of January, as previously described. The middle phase refers to the ensuing events up to the first cluster of SARS cases in a hotel (hotel M) in Hong Kong, while cases following this cluster fall into the late phase.
3. SARS AND SARS-CoV: clinical symptoms and aetiological agent
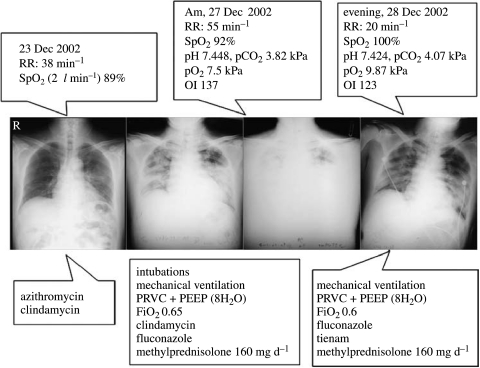
The early recognition of the clinical symptoms of SARS was certainly contributed by the medical staff of Guangdong Province, China (figure 3; Zhong & Zeng 2003). In particular, the first three SARS autopsies were performed in Guangzhou on 11, 12 and 25 February 2003 (Ding et al. 2003a,b). These samples not only greatly helped the characterization of SARS pathogenic changes, but also offered key biological samples for aetiological agent identification and isolation. However, identification of a novel coronavirus as the causative aetiological agent responsible for the epidemic of SARS in Guangdong was fulfilled by the international concerted efforts and announced during 11–12 April 2003. The WHO confirmed this result on 16 April 2003. The formal reports on the clinical symptoms of SARS (Lee et al. 2003; Poutanen et al. 2003; Tsang et al. 2003) and the identification of SARS coronavirus (SARS-CoV; Drosten et al. 2003a; Ksiazek et al. 2003; Peiris et al. 2003b) as the causative viral aetiological agent were published in May 2003.
Figure 3.
Chest radiographs (the middle panel) of a SARS patient obtained with clinical presentations (the upper panel) during treatment (the lower panel) (Zhong & Zeng 2003). Indices reflecting the patient's respiratory function status: oxygenation index (OI), arterial oxygen pressure (PO2), oxygen saturation (SpO2), fraction of inspired oxygen (FiO2), respiratory rate (RR). Indices reflecting mechanical ventilation mode: pressure regulated volume control ventilation (PRVC), positive end-expiratory pressure (PEEP).
As briefly reviewed previously (Peiris et al. 2004), SARS is a lower respiratory tract disease (Lee et al. 2003; Peiris et al. 2003b,c; Poutanen et al. 2003; Tsang et al. 2003). It is typical that the affected individuals have slightly decreased platelet counts, prolonged coagulation profiles and mildly elevated serum hepatic enzymes, besides fever, malaise and lymphopenia. Chest radiography reveals infiltrates with subpleural consolidation or ‘ground glass’ changes compatible with viral pneumonitis.
Although the main clinical symptoms are those of severe respiratory illness, quite a few SARS patients did have watery diarrhoea, and virus could be cultured from the faeces and urine, as well as from the respiratory tract (Peiris et al. 2003b; Chan et al. 2004a,b). Quantitative studies of viral load have provided insights into the pathogenesis of SARS. Viral load is higher in the lower respiratory tract than in the upper airways (Cheng et al. 2004; Drosten et al. 2004). Viral load in the upper respiratory tract (Peiris et al. 2003a) and faeces (Cheng et al. 2004) is low during the first 4 days and peaks at around day 10 of the illness. In marked contrast, viral load in influenza peaks soon after the onset of clinical symptoms (Kaiser et al. 1999). This unusual feature of SARS-CoV infection explains its low transmissibility early in the illness. In addition, RT-PCR has identified the virus in the serum, plasma and peripheral blood leucocytes (Li et al. 2003a; Ng et al. 2003). Thus, the first-generation RT-PCR diagnostic tests on the upper respiratory tract and faecal specimens collected early in the illness had very low sensitivity (Poon et al. 2004), and many laboratories (R. W. Chiu & Y. M. Lo, 2004, personal communication) as well as our experiences have indicated that faecal samples collected during the middle phase of the illness are ideal sources for SARS-CoV isolation and detection, particularly for patients with mild symptoms.
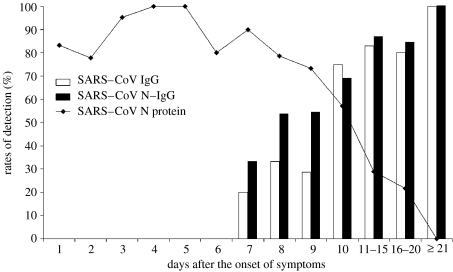
Besides the RT-PCR-based viral RNA detection and ELISA-based antibody detection commonly used for SARS diagnostics (Hui et al. 2003; Che et al. 2004), Xiao-Yan Che (Che et al. 2004) looked at the advantages of using the nucleocapsid protein, or ‘N protein’, of the coronavirus as a diagnostic marker. Using a specially developed immunosorbent assay, they were able to establish N-protein concentrations in the samples. The approach resulted in a test specificity of 99.9%. The technique could be used in medical centres lacking sophisticated equipment and in mass screening to track the origins of SARS (Khamsi 2004). This group of scientists analysed 420 serum samples taken from 317 SARS patients, and found that the protein could be detected as early as day 1 after the onset of symptoms and until day 18 (figure 4).
Figure 4.
The profile of N protein detection in blood and antibody response to SARS-CoV from the onset of symptoms to the convalescent phase (Che et al. 2004).
Individuals with SARS also have a pronounced peripheral T-cell lymphocytopenia: numbers of CD4+ and CD8+ cells are both reduced and more than one-third of individuals have a CD4+T-cell count of less than 200 cells mm−3 (Wong et al. 2003; Li et al. 2004a). Therefore, it suggests the increased susceptibility to secondary infections. The mechanisms underlying the T-cell lymphopenia remain to be elucidated (Peiris et al. 2004).
Approximately 20–30% of individuals with SARS require management in ICU (Peiris et al. 2003c) and the overall fatality rate is approximately 15% (WHO, http://www.who.int/csr/sars/en/whoconsensus.pdf). Some of the death cases were certainly caused by secondary infection and a fatal aspergillosis in a patient with SARS treated with corticosteroids was reported (Wang et al. 2003a). The age dependence of disease severity and mortality is notable. During the outbreak in Hong Kong, the mortality rates of affected individuals who were 0–24, 25–44, 45–64 and above 65 years old were 0, 6, 15 and 52%, respectively (WHO, http://www.who.int/csr/sars/en/WHOconsensus.pdf). None of the 1–12-year-olds infected with SARS-CoV had disease severe enough to require intensive care or mechanical ventilation (Hon et al. 2003; Leung et al. 2004a). This progressive age dependence in mortality is not totally explained by co-morbid factors and the underlying biological basis remains unclear (Peiris et al. 2004).
4. The SARS-CoV genome: from sequencing to molecular epidemiology
After the aetiological agent of SARS was identified as a new coronavirus, not previously endemic in humans (Drosten et al. 2003a; Ksiazek et al. 2003; Peiris et al. 2003b), the genome was completely sequenced at an unprecedented speed from the isolates of Canada, strain Tor2 (Marra et al. 2003), and Vietnam, strain Urbani (Rota et al. 2003). Meanwhile, the complete genome of strain BJ01, an isolate related to the Shanxi index patient hospitalized in Beijing (refer to §2 of this review for detailed description of the case), was sequenced by Beijing Genomics Institute (BGI), Chinese Academy of Sciences (Qin 2003). Immediately following, the genome of strain GD01 (originally designated GZ01), isolated from the first autopsy sample of Guangdong (refer to the first paragraph of §3 of this review for detailed description of the autopsy) was completely sequenced by the Institute of Microbiology and Epidemiology, Chinese Academy of Military Medical Sciences (Qin et al. 2003a), while other isolates of the BJ group, two isolates related to the Shanxi index patient, BJ02 and BJ03, and one isolate (BJ04) from the lung autopsy of one of the early patients of the HZS-2 Hospital SSE outbreak, Guangzhou (the ambulance driver for patients of SGQ/SQS brothers and ZZF of the HZS-2 Hospital; refer to §2 about the patients' information), were sequenced by the BGI (unpublished personal communication, 2003; Bi et al. 2003). Among these ‘first-round’ sequencing efforts, it is also important to emphasize the contributions made by Hong Kong and Singapore scientists. In particular, the Singapore group promptly recognized the significance of employing the limited sequence information with accurate epidemiology information to trace the genotypic variation of the viral transmission paths (Ruan et al. 2003). They also established the practical methodology, which allowed the efficient usage of cell cultured or partially sequenced SARS-CoV genomic sequences.
I went to Guangzhou on 2 May 2003 searching for samples that might be used for testing the potential of newly developed antibodies as diagnostics. A zoologist, Shuyi Zhang, of the Institute of Zoology, Chinese Academy of Sciences also arrived in Guangzhou. Based on his knowledge about zoonotic diseases, he speculated that warm-blooded animals were probably the origins of SARS-CoV and was happy to learn that the Ministry of Agriculture and the Guangdong Province had collaborated in searching for SARS-like coronaviruses from animals. With his expertise in personal interviews/surveys, he contacted as many early patients as possible. Meanwhile, as a member of the special expert envoy sent by the Task Force Group for Science and Technology Research (TFGSTR) under the State Council to Guangdong and Hong Kong, I had a chance to review the detailed epidemiology records made by the CDCP of Guangdong and some related information in Hong Kong, which I summarized previously. It is clear that many of the affected individuals in November and December 2002 had contact with the live-game trade, including restaurants serving exotic animal dishes (Xu et al. 2004a). The lack of serological evidence of previous infection in healthy humans suggested that SARS-CoV had recently emerged in the human population and that animal-to-human interspecies transmission seemed the most probable explanation for its emergence.
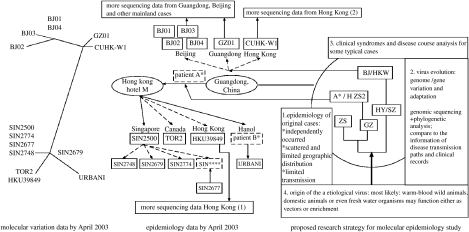
During the visit, I gradually formed the idea for molecular epidemiology research. Although it was then a popular idea to sequence hundreds of SARS-CoV genomes from strains isolated from Beijing, Hong Kong and Taiwan to understand the rule of the viral evolution (figure 5), I realized that it would be difficult, not only due to the lack of proper samples, but also due to the lack of detailed, consistent and accurate biological information useful for the analysis. On the other hand, I learned that the CDCP of Guangdong Province, with the help of CDCP of Guangzhou and local hospitals, collected samples from the very early days of the epidemic and these samples could well be associated with the epidemiological information of the patients. I discussed this idea with the senior epidemiologists in Guangzhou, as well as with some young scientists working in the front lines. All of them showed tremendous enthusiasm to support this effort. Therefore, on the 20 May 2003, I was asked to present this strategy to the CDCP of Guangdong Province with only a single slide (figure 5). However, this might be the most important scientific slide I have ever made in my life. I strongly emphasized that genomic sequencing of approximately 30 representative strains of SARS-CoV isolated from early-phase patients' samples with accurate epidemiological information might eventually not only help the identification of the animal origin of the virus, but also delineate its evolution direction, which, in turn, may support further efforts in controlling the disease. It was a successful effort and the collaboration started right after the meeting.
Figure 5.
Strategic design for molecular epidemiology study of SARS. The unrooted phylogenetic tree for SARS-CoV isolates available by April 2003 (the left panel) and the corresponding SARS transmission epidemiology information (the middle panel) were adapted from Ruan et al. (2003). This slide was presented to the CDCP Guangdong Province on 20 May 2003 for the first time.
While we were working on this project, scientists of Hong Kong University and the CDCP of Shenzhen, Guangdong Province, China, isolated and characterized a SARS-CoV-like virus with more than 99% nucleotide homology to the human SARS-CoV from specimens collected from animals, particularly Himalayan palm civets (Paguma larvata) and raccoon dogs (Nyctereutes procyonoides) of a live wild-game animal market in Shenzhen (Guan et al. 2003). Meanwhile, many workers who handled animals in these wet markets were proved to have antibodies against SARS-CoV, although they had no history of a SARS-like disease (Guan et al. 2003; Yu et al. 2003).
The discovery of SARS-CoV-like virus from the palm civet and the identification of its genomic sequence greatly facilitated the molecular evolution research. It promptly offered a proper anchor sequence, indicating the possible animal origin of the virus. In addition, its significant sequence variation compared with that of the late-phase SARS-CoV largely derived from isolates linked to the hotel M cluster, on the one hand, and the high sequence similarity to that of GD01, the early-phase human SARS-CoV isolate from Guangzhou, on the other hand, were clear indications of molecular evolution. We were able to determine 29 SARS-CoV genomic sequences obtained from 20 patients from Guangdong Province, with disease onset dates in the early and middle phases of the epidemic and from four patients of the late phase, one from Guangdong, one from Inner Mongolia and the other two from Hong Kong. These sequences supplied a bridge not only for the genotype gaps of different isolates, but also for the time gaps of SARS-CoV evolution and the geographical gaps of the SARS epidemiology.
For the early phase of the epidemic, we identified two major genotypes. Isolates from Guangzhou contained a 29-nucleotide (nt) sequence, which is absent in most of the publicly available SARS-CoV sequences, but these were the same as the isolates from the palm civets. Because this type of sequence was the longest among all the SARS-CoV isolated and the closest to the virus isolated from animals, a representative strain of this kind, GZ02 (GenBank accession no.: AY390556) from the first SARS autopsy sample as that of GD01 (originally designated as GZ01, GenBank accession no.: AY278489) and therefore, epidemiologically the earliest isolate, was used as the reference. Another group of isolates was from Zhongshan with a previously unreported 82-nt deletion in the same region of the genome, Orf8. A sequence with an identical 82-nt deletion was also observed in coronaviruses isolated from farmed civets in Hubei Province, China. It was interesting that a lung biopsy of a patient from the middle phase was found to contain two SARS-CoV genotypes, with the 29-nt and the 82-nt deletions, respectively. Because this patient did have contacts with two patients (SQS and ZZF of HZS-2 Hospital, see §2) from different cities at the time between the early and the middle phases, we may never know whether he was infected with two different kinds of viruses or the virus with 29-nt deletion had further deleted 53 nucleotides. It was also remarkable that another genotype with a 415-nt deletion resulting in the loss of the whole Orf8 region was isolated and confirmed in two Hong Kong patients, with the disease onset from mid-May 2003 (Chiu et al. 2005).
We also identified single nucleotide variations (SNVs), genotype markers characteristic of different lineages (epidemic phases) of SARS-CoV. At the GZ02 reference nucleotide residues 17 564, 21 721, 22 222, 23 823 and 27 827, these genotypes were G : A : C : G : C for the early phase, including the animal SARS-like coronavirus isolates G : A : C : T : C for majority isolates of the middle phase. This genotype was basically representative not only for the SSE of the HZS-2 Hospital, Guangzhou, but also for the isolates from Shanxi (the BJ group) and Shenzhen. It was particularly interesting to observe another distinguished genotype, G : G : C : T : C, in the HZS-2 Hospital represented by only one patient, who was a worker of the hospital and her job was to help the transfer of patients from the outpatient department to the respective wards. Her onset date was 7 February 2003 with flu-like syndromes (chills for 3 days and fever for 1 day), but she was only hospitalized on 9 February 2003. She was considered to be the tertiary infected patient originated from ZZF and she had a chance to contact doctor A (onset date: 21 February 2003) in the outpatient department during her initial flu-like syndrome period of 7–8 February 2003 while she was still working regularly. She had mild disease course and was released from hospital on 24 February 2003. Although this genotype was one step closer to the late-phase genotype, the genotypes connected with the hotel M outbreak, T : G : T : T : T, had three base substitutions representing the extremely stable late-phase viral genome. However, besides the C→T transition in S gene, which caused significant non-synonymous mutation in the S protein, the biological significance of the other two is difficult to explain. Nevertheless, this genotype is even conserved in the late 415-nt deletion variants in Hong Kong, with the exception of the nucleotide 27 827, which falls within the deleted segment.
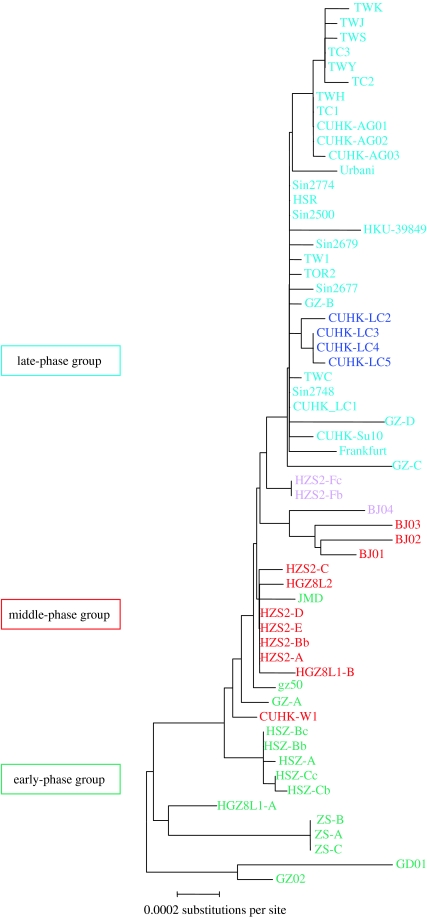
Aided by the accuracy of sequencing and sampling time, the neutral mutation rate for SARS-CoV during this epidemic was noted to be almost constant and estimated to be 8.26×106 nt−1 d−1. This is similar to the values obtained for known RNA viruses and is about one-third that for the human immunodeficiency virus. On the basis of this neutral mutation rate, the most recent common ancestor (MRCA) was estimated to lie in mid-November 2002, which is consistent with the onset date of 16 November 2002 for the earliest index patient from Foshan. This is further evident from the remarkable correlation between the molecular clustering (refer to the GZ02-rooted phylogenetic tree, figure 6) and epidemiological grouping of the genotypes throughout the epidemic (Chinese 2004).
Figure 6.
GZ02-rooted neighbour-joining phylogenetic tree for SARS-CoV genomic sequences of the 2002–2003 epidemic. Colours of strain designations correspond to the epidemiological phases. In general, three colours are used for the three epidemic phases: green for the early, red for the middle and blue for the late. In detail, purple represents strains of the middle phase with a characteristic SNP switch reflecting the genotypic transition of SARS-CoV between the middle and late phases (from the G:A:C:T:C [red] for majority isolates of the middle phase to the T:G:T:T:T [blue] for majority isolates connected with the Hotel M outbreak, via this rare G:G:C:T:C [purple] isolate of the HZS-2 Hospital); dark blue represents the strains of a cluster of very late cases in Hong Kong with significant deletions in the orf8 region of the SARS-CoV genome. The map distance between individual sequences represents the extent of genotypic difference. This figure indicates the strong correlation between the molecular phylogeny of the viral strains and the epidemiological phases of their corresponding transmission paths. Adapted from Chinese (2004).
In contrast to the constant rate of synonymous variations, the non-synonymous mutation rates were variable for the three epidemic phases. Between the coronavirus sequences of the palm civets (SZ3 or SZ16) and each of the human SARS-CoV sequences, the ratios of the rates of non-synonymous to synonymous changes (Ka/Ks) for the S gene sequences were always greater than 1, indicating an overall positive selection pressure. However, pairwise analysis of the Ka/Ks for the genotypes in each epidemic group shows that the average Ka/Ks for the early phase was significantly larger than that for the middle phase, which, in turn, was significantly larger than the ratio for the late phase, which, in fact, was significantly less than 1. These data indicate that the S gene showed the strongest positive selection pressures initially, with subsequent purifying selections and eventual stabilization. For Orf1a, we observed a pattern similar to that for the S gene. However, in contrast, Orf1b (nt coordinate: 13 398–21 485) seems to be undergoing purifying selection during the whole course of the epidemic. Indeed, it is the most conserved genomic region of SARS-CoV.
Tracing the molecular evolution of SARS-CoV in China, we observed that the epidemic started and ended with deletion events, together with a progressive slowing of the non-synonymous mutation rates and a common genotype that predominated during the latter part of the epidemic. Although further work is required for the mechanistic explanation for the selective adaptation and purification processes that led to such genomic evolutionary changes in SARS-CoV, this study has provided valuable clues to aid further investigation of this remarkable evolutionary tale.
5. SARS as a zoonotic disease: from animal-to-human to human-to-human transmission
The identification of SARS-CoV-like virus in the palm civets and raccoon dogs from a live wild-game animal market in Shenzhen (Guan et al. 2003) was the first step towards understanding the zoonotic character of SARS. Further serological studies and/or PCR tests on animals, both wild and domestic, were carried out and positives for SARS-CoV were reported, although in low frequencies (Duan et al. 2003; Liu et al. 2003, 2004; Yi et al. 2004). Meanwhile, a well-controlled animal infectious experiment indicated that the palm civet was sensitive to the infection of human SARS-CoV (Wu et al. 2005). The control experiments performed on 60 individuals of Hydra (water snakes) and 55 individuals of wild rats were all negative with respect to the human SARS-CoV infection. Owing to the unsuccessful domestication of 120 individuals of fruit bats, the SARS-CoV infection test on this animal failed to provide a conclusion. Clinical observations of the infected 10 individuals of palm civets indicated that the in vivo viral amplification and secretion of the infected palm civets were significantly more efficient than any other infection experiments conducted on animal models, such as monkey (Kuiken et al. 2003; Bukreyev et al. 2004; Rowe et al. 2004), ferret (Martina et al. 2003), cat (Martina et al. 2003), mouse (Subbarao et al. 2004), rabbit, pig and guinea-pig (Peiris et al. 2004), etc. This research should be an important contribution for SARS-CoV vaccine and drug development if it can be further supported and continued.
On the other hand, the epidemiology data indicated that by January 2003, among 135 scattered SARS patients of Guangzhou community who did not have clear contact history with SARS patients, 8.14% of them, i.e. 11 individuals, were in the business of dealing with animals. In particular, of the 10 independently occurring index cases of seven to eight cities in the Pearl River Delta region, at least six had direct contact history with animals (particularly wild animals in restaurants), while the possible contact history with animals of the other four could not be excluded. This extraordinary epidemiology fact is a strong indication for the animal origin of SARS (table 1, unpublished data from Guangdong CDCP; He et al. 2003b). Meanwhile, the high percentage of positive tests for anti-SARS-CoV antibodies in serological surveys for people specializing in the retail business of palm civet (72.7%) or wild animals (13.02%) also inferred that the exposure to the animal precursor of SARS-CoV seems to have resulted in asymptomatic infection in most cases of contact (Guan et al. 2003; He et al. 2003b; Yu et al. 2003; Xu et al. 2004a).
Regulation measures for public health were immediately implemented when the palm civet and other exotic animals were proved to be suspected carriers of SARS-CoV. These included a ban on serving palm civet dishes, cleaning the wild animal market and epidemiologically following the people involved in the wild animal business, such as purchasing and trading. Meanwhile, surveillance control aiming at detecting SARS-CoV or SARS-CoV infection in the palm civets and rats from both local farms and farms of other locations was carried out. A systematic analysis of 103 serum samples from the palm civets was performed by the Animal Hygiene Laboratory of Australia (Tu et al. 2004). They found no positives for samples from palm civet farms of Jiangxi, Hunan, Hubei, Henan and Hebei Provinces, but 40% positives of the farms in Shanwe, Guangdong Province. Particularly significant was the 78% positive results for samples from Guangzhou wild animal market. The wet markets in Guangdong sell live poultry, fishes, reptiles and other mammals, which are commonplace across southeast Asia and southern China to service the cultural demand for freshly killed meat and fish produce. In some regions (e.g. Guangdong province, China), increasing affluence has led to the proliferation of markets housing a range of live ‘wild’ animal species, such as civet cats, linked to the restaurant trade, servicing the demand for these exotic foods. Therefore, it was suggested that the special ecological environment may favour the transmission of SARS-CoV and that the wild animal market of Guangzhou is apparently an important mixture incubator of SARS-CoV (Tu et al. 2004).
Based on the previous studies, we were at a much better position of understanding how to control SARS. I still remember a meeting in Beijing organized by the Ministry of Science and Technology of the State Council, forecasting SARS in the coming winter of 2003 and spring of 2004. I clearly proposed that it would not be surprising to have SARS cases during the winter/spring period. However, there were two possible sources of infection. The viruses from the environment, most probably from wild animals, might have mild symptoms and low infectivity, which, in turn, would be easy to control if the patients could be identified and isolated promptly. On the other hand, if the infection was initiated from the laboratory strains, mostly isolated from the late-phase strains well adapted to human hosts and apparently the most pathogenic and contagious, it would be dangerous and likely to cause human-to-human infection and severe symptoms with bad prognostics, including possible death.
Unfortunately, both cases did occur in the coming winter of 2003 and spring of 2004. Because laboratory infection was basically a problem of administration and management, I will emphasize the community infection that occurred in Guangzhou, Guangdong Province, China, between 16 December and 30 January 2004 (Song et al. 2005). The ban on the sale of wildlife in wet markets in Guangdong imposed during the later period of the SARS outbreak was lifted in September 2003, and the four new cases of SARS that occurred during the 2003–2004 period were all independently infected. None of these patients had contact with each other nor with other previously documented SARS cases. However, all of them had clear direct or indirect contact history with wild animals; in particular, three of them had independent contact history related to the TDL Restaurant where palm civet dishes were provided. In addition, unlike most SARS patients during the 2002/2003 epidemic, these four new patients clinically presented very mild symptoms and none of them had their close contacts infected.
Meanwhile, the sample collection activity in Guangzhou wet market indicated that the SARS-CoV-like virus loading from animals of the market was higher than the previous surveys (Peiris et al. 2004; Song et al. 2005). It seems that the wet market in Guangdong is a special ecological environment for a variety of animals, wild and domestic, to be gathered and to have contact with each other, which may favour the SARS-CoV to explore different hosts and evolve to eventually cause cross-host transmission.
With improved sequencing technology, genomic sequences were determined for SARS-CoVs from two of the four human patients and two palm civets of Guangzhou food market and one sample from the palm civet cage at the restaurant where the second patient worked as a waitress. All of these sequences retained the 29-nt segment marker in orf8a. In addition, the genomes of the SARS-CoV from human patients were almost identical to those of the SARS-CoV-like viruses from the palm civet (0.11% difference). Thus, structurally, there was little difference to distinguish the genomic sequences of SARS-CoV and SARS-CoV-like viruses and, functionally, concerning the animal contact history of the four patients. It is probable that the same virus can infect both palm civet and human.
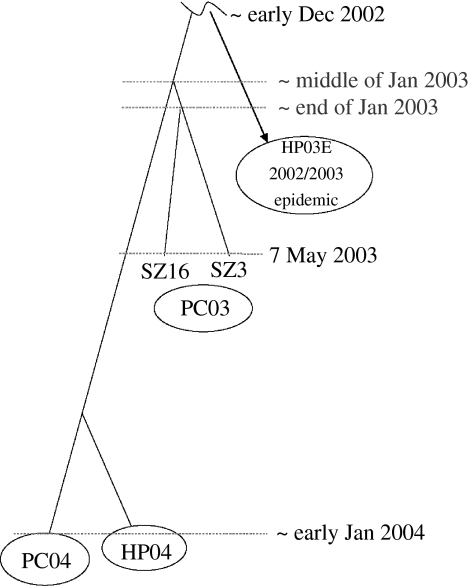
With the newly accessed genomic variation data and the sampling time, the neutral mutation rate and the date for the MRCAs of SARS-CoV were re-estimated in both palm civet and human. The estimate turned out to be approximately 8.00×10−6 nt−1 d−1, which is almost the same as that estimated in our previous work based on 10 samples from the human patients of 2002–2003. This relatively long-term evolutionary analysis once again strongly suggested that SARS-CoV evolves at a relatively constant neutral rate in both human and palm civet. Furthermore, the date estimates of MRCAs for palm civet and human of different transmission lineages were obtained and a rooted phylogenetic tree covering 2 years' epidemic was constructed (figure 7; Song et al. 2005). It clearly indicates that the viral transmission from animal to human occurred independently in these two instances. Because a higher viral load in the palm civet of Guangzhou wet market was suggested during the 2003/2004 outbreak based on the study illustrated previously, SARS-CoV of 2003–2004 might have evolved to be more virulent in, or better adapted to palm civet. This further demonstrated that SARS is a zoonotic disease from still unknown origin that has been evolving not only in human, but also in palm civet hosts.
Figure 7.
A rooted phylogenetic tree for SARS-CoV based on MRCA estimations. The virus isolates are from the palm civets of the 2002–2003 epidemic (PC03) and the 2003–2004 outbreak (PC04), as well as from human patients of the early phase of the 2002–2003 epidemic (HP03E) and the 2003–2004 outbreak (HP04). The branch length is proportional to the time-interval. Adapted from Song et al. (2005).
The direct public health impact of this epidemiological linkage and phylogenetic analysis is the indication of frequent cross-species transmission of SARS-CoV from animal carriers, such as palm civet, to human, although the animal precursor virus is probably not well adapted to efficient human-to-human transmission, but results mostly in mild- or non-symptomatic infections. However, only occasionally has it succeeded in adapting to human-to-human transmission, which clearly occurred in late 2002, and once the virus had adapted to human-to-human transmission in the later part of the outbreak, asymptomatic infection seemed to be rare (Leung et al. 2004b). This time, the findings led to the reintroduction of the ban on wild-game animal markets and there have been no further naturally acquired human cases since. The effectiveness of this public health measure not only changed the food habits of Cantonese, but also clearly reduced the detection rate of anti-SARS-CoV antibody from sera samples of people working in the business of wild animals. It was 25.07% for May 2003, 13.03% for January 2004, but significantly dropped to 5.04% in July of the same year (data from Guangzhou CDCP). Meanwhile, no new cases were detected during the period of winter 2004 to spring 2005. Although this kind of cross-species transition may happen again in the future, given the present understanding and awareness about SARS, we expect that such re-emergence is unlikely to lead to a global outbreak on the scale of 2003.
6. SARS-like CoV from horseshoe bats: the initial success in searching for natural reservoirs
As I have mentioned previously, the caged palm civets from live animal markets in China may have served as an amplification host for interspecies transmission of SARS-CoV in the genesis of the SARS epidemic. However, the search for natural reservoirs of SARS-CoV has never stopped since SARS became an epidemic.
A young zoologist, Shuyi Zhang, of the Institute of Zoology, Chinese Academy of Sciences went to Guangzhou from Beijing on the same day as I made the trip. After only a couple of days of close working relationship, we became very good friends and he started to share his thoughts with me about the possibilities for a natural reservoir for SARS-CoV. ‘Must be warm blood animals, and bats are very likely’, he told me repeatedly. His rationale was simple. Bats, the only flying mammals, account for 20% of the 4800 mammalian species recorded in the world. They are an important reservoir of emerging zoonotic viruses, including Hendra and Nipah, which have recently emerged in Australia and east Asia, respectively (Murray et al. 1995; Chua et al. 2000; Wang & Eaton 2001). Because the fruit-mediated contacts of domestic pigs with fruit bats causing the emerging of Nipah virus infection in human made a vivid similarity with the fruit-eating palm civets as the carrier of SARS-CoV, Shuyi Zhang's interests in bat as the natural reservoir mainly focused on fruit bats (Rousettus, Cynopterus, etc.) for a while. In fact, seropositive fruit bats (Rousettus leschenaulti) were occasionally identified at fairly low frequencies (approx. 1–2%; Li et al. 2005b).
This search was supported by both the Chinese government and the Sixth Framework Programme ‘EPISARS’ from the European Commission. Shuyi Zhang collaborated with many laboratories for this tedious search, but eventually succeeded via an unexpected sampling mistake, i.e. one young scientist collected some horseshoe bats (Rhinolophus) as ‘fruit bats’. After the so-called SARS-like CoV genomic RNA fragments were identified in these bats, sampling species were extended and further efforts in this Rhinolophus genus belonging to the family Rhinolophidae were very successful. In mainland China, among six genera of bat species surveyed (Rousettus, Cynopterus, Myotis, Rhinolophus, Nyctalus and Miniopterus), only three communal, cave-dwelling species from the genus Rhinolophus demonstrated a high SARS-CoV antibody prevalence: 13 out of 46 (28%) in R. pearsoni from Guangxi; 2 out of 6 (33%) in R. pussilus from Guangxi; and 5 out of 7 (71%) in R. macrotis from Hubei (Li et al. 2005b). Meanwhile, this kind of SARS-like CoV was found in Hong Kong from 23 (39%) out of 59 anal swabs of wild Chinese horseshoe bats (R. sinicus) by using RT-PCR (Lau et al. 2005). This high seroprevalence and wide distribution of seropositive and/or RT-PCR positive bats is expected for a wildlife reservoir host of a pathogen (Hudson et al. 2002).
Sequencing and analysis of SARS-like CoV genomes from samples of different bat species collected at different locations of China, i.e. R. sinicus (HKU3-1 isolated from Hong Kong SAR; Lau et al. 2005), R. pearsoni (Rp3 isolated from Guangxi; Li et al. 2005b), as well as R. ferrumequinum (Rf1) and R. macrotis (Rm1) both isolated from Hubei (Ren et al. 2006), showed that this virus is closely related to SARS-CoV from humans and civets and phylogenetic analysis showed that the SARS-like CoV isolated from the horseshoe bats formed a distinct cluster with SARS-CoV as group 2b coronavirus, distantly related to known group 2 coronavirus (Lau et al. 2005; Li et al. 2005b; Ren et al. 2006).
Most differences between the bat SARS-like CoV and SARS-CoV genomes were observed in the spike genes (S), ORF3 and ORF8, which are the regions where most variations were also observed between human and civet SARS-CoV genomes. A detailed analysis of amino acid sequences of S proteins between four SARS-like CoVs and the SARS CoVs in human and civet indicated that the putative S2 domain of all these viruses show high homology to each other (92–96%), and all amino acid residues critical for SARS virus fusion to cell membrane located in the S2 domain are highly conserved, suggesting that they possess the same fusion mechanism during infection to their host cells (Tripet et al. 2004; Xu et al. 2004b,c; Bartlam et al. 2005; Sainz et al. 2005). However, the putative S1 domain of SARS-like CoVs has very low homology to that of SARS CoVs, especially in the N-terminal and the receptor-binding domain (RBD, 318–510 amino acid of Tor2 S1 domain; Wong et al. 2004a). One insertion of six amino acid and three deletion sites are present in S1 domain of SARS-like CoVs, among which two deletion sites (5 and 13 amino acid, respectively) are present in RBD, particularly in the receptor-binding motif (RBM, 424–494 amino acid of Tor2 S1 domain; Li et al. 2005a).
In addition, all of the ORF8s of bat SARS-like CoV are similar to that of SARS-CoV isolated from the palm civets (Song et al. 2005) and some ‘early-phase’ patients with a 29 bp fragment, which was supposed to be deleted in most of the human SARS-CoVs (Chinese 2004). This genomic variation feature further suggests that the SARS-like CoV has a common ancestor with SARS-CoV.
Antibody against recombinant bat SARS-like CoV nucleocapsid protein (N) was detected in 84% of Chinese horseshoe bats by using an enzyme immunoassay. Neutralizing antibody to human SARS-CoV was also detected in bats with lower viral loads. However, except for one human serum sample from patients with recent infections by human coronavirus OC43, none of the other 37 serum samples from patients with recent infections by human coronavirus OC43, 229E, NL63 and coronavirus HKU-1 was tested to be positive in the recombinant bat SARS-like CoV N protein-based EIA (Lau et al. 2004). Therefore, the cross-reactivity of antibodies against the coronavirus antigens so far studied was mainly retained in their corresponding groups.
The genetic diversity existing among zoonotic viruses in bats increases the possibility of variants crossing the species barrier and causing outbreaks of disease in human populations, such as the SARS epidemic this time and the henipaviruses infection in Malaysia (Abubakar et al. 2004; Harcourt et al. 2005; Reynes et al. 2005). It is therefore essential to enhance human knowledge and understanding of reservoir host distribution, animal–animal and human–animal interactions, particularly within the wet-market system, and virus genetic diversity of bat-borne viruses to prevent future outbreaks.
7. SARS-CoV spike protein: essential residues for species-specific receptor binding
Studies using pseudotyped lentiviruses carrying the spike (S), membrane and envelope surface glycoproteins of SARS-CoV separately and in combination demonstrated that the spike protein is both necessary and sufficient for virus attachment on susceptible cells (Han et al. 2004; Hofmann et al. 2004; Simmons et al. 2004; Yang et al. 2004). The receptor for SARS-CoV was identified as that of the metallopeptidase, angiotensin-converting enzyme 2 (ACE2; Li et al. 2003b; Wang et al. 2004). The soluble ACE2 ectodomain blocks SARS-CoV infection (Hofmann et al. 2004), and amino acids 318–510 of the S protein are predicted to constitute the ACE2 receptor binding site (RBS; Babcock et al. 2004; Wong et al. 2004a).
The S protein is responsible for binding to the ACE2 receptor and thus is the fastest evolving protein of SARS-CoV over the epidemic from animal to human. Although mutations are dispersed over the whole protein, the majority of the mutations is located in the S1 domain (31 out of 48 total SNVs); particularly, in the RBS, 11 SNVs corresponding to 10 amino acid residues (codon 479 is a two-substitution codon involving two non-synonymous changes, N or K/R). Molecular mechanistic studies were directly followed attempting to elucidate the critical amino acid residues responsible for host-specific ACE2 receptor binding and cell entry (Qu et al. 2005; Li et al. 2005c).
Based on the correlation between non-synonymous variations and the SARS-CoV progression and tropism, six putative key amino acid residues within the S protein RBD were identified (344, 360, 472, 479, 480 and 487). Although the computer simulated three-dimensional structure of the S protein (Protein Data Bank code 1T7G) predicted to have these six amino acids exposed at the surface of the S1 domain (Bernini et al. 2004), combinatorial substitution of two key residues (N479K/T487S) in the S protein RBD of the middle-phase human SARS-CoV strain, BJ01, greatly interferes with the human ACE2-mediated pseudotyped virus entry to only 0.1% of the original. On the other hand, any single substitutions or double substitutions with/of the other four residues had no obvious effects. This loss-of-function observation was substantiated with a gain-of-function experiment by substituting amino acid residues K479 and S487 in the typical palm civet SARS-CoV S protein RBD to N479 and T487, individually or in combination. The results showed that compared with the wild-type palm civet strain, SZ3, both the two single amino acid substitutions and the double amino acid substitution greatly enhanced the human ACE2-mediated pseudotyped virus entry of the palm civet SARS-CoV infection, although less efficiently than the case mediated by the BJ01 S protein.
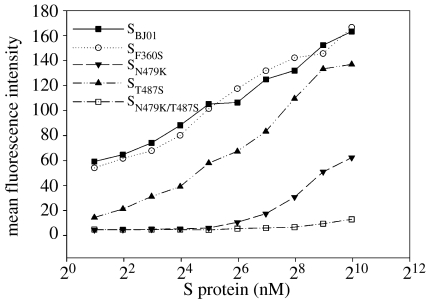
The greatly reduced infectivity mediated with the N479K/T487S substituted human SARS-CoV S protein was correlated with its decreased binding affinity to the human ACE2 (figure 8). The binding affinity of S protein at 100 nM was human SBJ01≈SF360S>ST487S>SN479K≫SN479K/T487S (palm civet mutant). The binding kinetics of SF360S was nearly identical to that of SBJ01. ST487S had a significantly lower affinity than SBJ01 in the low concentration range, but appeared to be almost similar to the level of SBJ01 at a high S protein concentration (0.5 μM). The affinity of SN479K remained much lower than that of ST487S at all concentrations tested and had no detectable binding when the S protein concentration was below 60 nM. SN479K/T487S showed undetectable binding ability even when the S protein concentration reached 1 μM.
Figure 8.
The binding affinity curves of selected mutants of human SARS-CoV S proteins (BJ01) to human ACE2-expressed Hela cells (Hela F5). These mutated S fragments (13-510) were constructed based on the S gene of strain BJ01. They were fused with human Fc. Hela cells expressing human ACE2 were incubated with 100 nM S fragments and the binding affinity of S proteins was detected through FACS analysis using FITC-conjugated anti-human IgG-Fc antibody. The mean fluorescence intensity (MFI) in the y-axis was determined by a series of FACS analysis in which Hela F5 cells were incubated with serially diluted S fragments from 1 μM to 2 nM. Adapted from Qu et al. (2005).
It is particularly interesting to consider these biochemical data with the molecular epidemiological data. The SK(R)497/S487 protein naturally exists in all isolates from the palm civet in 2003 and most isolates of palm civet in 2004. Considering that these double amino acid substituted viruses had extremely low viral entry ability and receptor binding affinity specifically towards human ACE2-expressing cells, it is not surprising that workers who handled or had close contact with palm civet during the 2002–2003 period showed no SARS-like syndrome, although they may have positive antiserum against SARS-CoV. These results strongly suggest that the coexistence of K/R479 and S487 functions as the entry barrier for animal-to-human transmission of the SARS-CoV.
The maintenance of this entry barrier requires the conservation of both K479 and S487. Once one substitution at position 479 or 487 occurred in animal SARS-CoV, the virus could greatly increase its affinity towards the human receptor ACE2 and even cross the species barrier to cause animal-to-human transmission. All the viral isolates from human patients of the 2003–2004 period had a genome very close to four isolates from the palm civet of the same time, i.e. they all had the SN479/S487 protein.
Unlike N479, T487 did not exist in any other natural SARS-CoV isolates except those from human patients of 2002–2003 and, in fact, all the virus isolates of that epidemic, from the earliest strains that have ever been detected, had the same SN479/T487 protein, which is the same as that of the middle-phase strain, BJ01. With the high human ACE2 binding affinity of BJ01 as the reference, the S487T amino acid switch may reflect the virus adaptation to human host and the presence of the SN479/T487 protein might be essential for causing the pandemic, i.e. the successful transition from animal-to-human transmission to human-to-human transmission.
The above analysis supports the claim that it is not difficult to overcome the entry barrier between animal and human and that palm civet may serve as a favourable incubator. The existence of SN479/S487 in the 2003–2004 SARS-CoV isolates both from human patients and some palm civets reveals a dangerous signal. It manifests that some isolates in the palm civet have already evolved to acquire the ability to cross the entry barrier between palm civets and humans. In this direction, it is significant to emphasize that the inhibition of the pseudotyped virus infection caused by the N479K/T487S double substitution of human SARS-CoV S protein was human-specific, as the decrease in infectivity could be observed only in human ACE2-expressing cells but not in cells expressing mouse ACE2. Although one early study has showed that the mouse ACE2 is a relatively poor receptor (Li et al. 2004b), this study showed that the high level and sustained expression of mouse ACE2 was capable of supporting SARS-CoV pseudotyped virus entry. These results are in accordance with the finding from the animal model study that mice can be efficiently infected by human SARS-CoV (Subbarao et al. 2004; Wentworth et al. 2004). Therefore, the potential intermediate host position of rodents for SARS-CoV should be carefully studied as a reasonable extrapolation of these experimental findings.
I certainly believe that the identification of these two critical residues was not the end of understanding the molecular mechanism of SARS-CoV evolution in the direction of cross-host transmission and adaptation. Instead, it is more probably the beginning of the overall molecular mechanistic studies. For instance, the evolutionary relationship between the SARS-like CoV, in the wild-animal reservoir, and the SARS-CoV, causing epidemic in human, is yet to be elucidated and the molecular mechanism of SARS pathogenesis is still largely unclear. However, by reviewing the progress we have made within less than 4 years, we may appreciate the great impact of human genomic research upon today's medical research. Honestly speaking, without the continuous efforts that Chinese scientists have made in human genome research since 1994, it would have been impossible for us to achieve the present success that people have witnessed. The experiences and lessons we may learn from this history are not just experimental design and technology, but also include the wisdom of strategic planning and the capability of team organization. It is my sincere hope that these lessons and experiences will become useful guidance for our future research, particularly in combating the emergence/re-emergence of infectious diseases, which eventually may help our people to construct and develop a safer, healthier and more harmonious society.
Acknowledgments
I do very much appreciate the close collaborative work among nearly 100 individuals of the following 23 institutions: (1) Guangdong Center for Disease Control and Prevention, (2) Guangzhou Center for Disease Control and Prevention, (3) Guangdong Provincial Veterinary Station of Epidemic Prevention and Supervision, (4) College of Veterinary Medicine, South China Agriculture University, (5) Changchun University of Agriculture and Animal Sciences, (6) State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agriculture Sciences, (7) Institute of Virology, Chinese Center for Disease Control and Prevention, (8) Department of Infectious Diseases and Immunology, Nanfang Hospital, (9) The Second Affiliated Hospital of Sun Yat-Sen University, (10) Guangzhou Institute of Respiratory Disease, (11) Department of Chemical Pathology and Department of Microbiology, The Chinese University of Hong Kong, (12) Wuhan Institute of Virology, Chinese Academy of Sciences, (13) Institute of Zoology, Chinese Academy of Sciences, (14) Department of Cell Biology and Genetics, College of Life Sciences, Peking University, (15) School of Pharmacy, Shanghai Jiaotong University, (16) State Key Laboratory for Medical Genomics, Ruijin Hospital Affiliated to Shanghai Second Medical University, (17) Bioinformation Center/Institute of Plant Physiology and Ecology/Health Science Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, (18) Shanghai Center for Bioinformation Technology, (19) School of Life Sciences, Fudan University, (20) Department of Ecology and Evolution, University of Chicago, USA, (21) Biomolecular Structure Research Center and Department of Molecular Biology, University of Siena, Italy, (22) Center for Disease Control and Prevention, USA, and (23) Pasteur Institute, France.
I also appreciate the support from the Ministry of Science and Technology and the Ministry of Agriculture of the Central Government of China, the Governments of Guangdong Province and Shanghai Municipality. This work was supported by the State High Technology Development Program (863, grant no. 2003AA208407), the State Key Program for Basic Research (973, grant no. 2003CB514101), and the European Commission grant EPISARS (no. 511063).
I would like to thank Dr N. S. Zhong and Dr Z. Chen for offering their advisory guidance to the writing and Dr R. H. Xu for proof-reading the manuscript. I would like to acknowledge the close collaboration and essential technical support offered by Dr J. Xu, Dr X. Y. Che, Dr H. K. Deng, Dr C. C. Tu, Mr Y. Y. He and Mr Y. G. Miao. I should also acknowledge that a certain portion of this paper was written based on the reviews by J. S. M. Peiris, Y. Guan and K. Y. Yuen, 2004 and a draft by Y. X. Li (2005 personal communication), now published as Hao et al. 2006.
Footnotes
One contribution of 14 to a Theme Issue ‘Biological science in China’.
References
- Abubakar S, Chang L.Y, Ali A.R, Sharifah S.H, Yusoff K, Zamrod Z. Isolation and molecular identification of Nipah virus from pigs. Emerg. Infect. Dis. 2004;10:2228–2230. doi: 10.3201/eid1012.040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M, Fraser C, Ghani A.C, Donnelly C.A, Riley S, Ferguson N.M, Leung G.M, Lam T.H, Hedley A.J. Epidemiology, transmission dynamics and control of SARS: the 2002–2003 epidemic. Phil. Trans. R. Soc. B. 2004;359:1091–1105. doi: 10.1098/rstb.2004.1490. doi:10.1098/rstb.2004.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock G.J, Esshaki D.J, Thomas W.D, Jr, Ambrosino D.M. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J. Virol. 2004;78:4552–4560. doi: 10.1128/JVI.78.9.4552-4560.2004. doi:10.1128/JVI.78.9.4552-4560.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlam M, Yang H, Rao Z. Structural insights into SARS coronavirus proteins. Curr. Opin. Struct. Biol. 2005;15:664–672. doi: 10.1016/j.sbi.2005.10.004. doi:10.1016/j.sbi.2005.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernini A, et al. Prediction of quaternary assembly of SARS coronavirus peplomer. Biochem. Biophys. Res. Commun. 2004;325:1210–1214. doi: 10.1016/j.bbrc.2004.10.156. doi:10.1016/j.bbrc.2004.10.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S, et al. Complete genome sequences of the SARS-CoV: the BJ Group (Isolates BJ01–BJ04) Genomics Proteomics Bioinformatics. 2003;1:180–192. doi: 10.1016/S1672-0229(03)01023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A, Lamirande E.W, Buchholz U.J, Vogel L.N, Elkins W.R, St Claire M, Murphy B.R, Subbarao K, Collins P.L. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. doi: 10.1016/S0140-6736(04)16501-X. doi:10.1016/S0140-6736(04)16501-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDCP. Update: outbreak of severe acute respiratory syndrome-worldwide. MMWR Morb. Mortal. Wkly Rep. 2003;52:269–272. [PubMed] [Google Scholar]
- Chan K.H, et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg. Infect. Dis. 2004a;10:294–299. doi: 10.3201/eid1002.030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K, et al. Laboratory diagnosis of SARS. Emerg. Infect. Dis. 2004b;10:825–831. doi: 10.3201/eid1005.030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X.Y, et al. Nucleocapsid protein as early diagnostic marker for SARS. Emerg. Infect. Dis. 2004;10:1947–1949. doi: 10.3201/eid1011.040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.K, Wong D.A, Tong L.K, Ip S.M, Lo A.C, Lau C.S, Yeung E.Y, Lim W.W. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. doi:10.1016/S0140-6736(04)16255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese S.M.E.C. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. doi:10.1126/science.1092002 [DOI] [PubMed] [Google Scholar]
- Chiu R.W, Chim S.S, Tong Y.K, Fung K.S, Chan P.K, Zhao G.P, Lo Y.M. Tracing SARS-coronavirus variant with large genomic deletion. Emerg. Infect. Dis. 2005;11:168–170. doi: 10.3201/eid1101.040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M.D, Poutanen S.M, Loutfy M.R, Muller M.P, Low D.E. Severe acute respiratory syndrome. Clin. Infect. Dis. 2004;38:1420–1427. doi: 10.1086/420743. doi:10.1086/420743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. doi:10.1126/science.288.5470.1432 [DOI] [PubMed] [Google Scholar]
- Davidson A, Siddell S. Potential for antiviral treatment of severe acute respiratory syndrome. Curr. Opin. Infect. Dis. 2003;16:565–571. doi: 10.1097/00001432-200312000-00009. doi:10.1097/00001432-200312000-00009 [DOI] [PubMed] [Google Scholar]
- Ding Y, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003a;200:282–289. doi: 10.1002/path.1440. doi:10.1002/path.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.Q, et al. Study on etiology and pathology of severe acute respiratory syndrome. Zhonghua Bing Li Xue Za Zhi. 2003b;32:195–200. [PubMed] [Google Scholar]
- Drosten C, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003a;348:1967–1976. doi: 10.1056/NEJMoa030747. doi:10.1056/NEJMoa030747 [DOI] [PubMed] [Google Scholar]
- Drosten C, Preiser W, Gunther S, Schmitz H, Doerr H.W. Severe acute respiratory syndrome: identification of the etiological agent. Trends Mol. Med. 2003b;9:325–327. doi: 10.1016/S1471-4914(03)00133-3. doi:10.1016/S1471-4914(03)00133-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C, et al. Evaluation of advanced reverse transcription-PCR assays and an alternative PCR target region for detection of severe acute respiratory syndrome-associated coronavirus. J. Clin. Microbiol. 2004;42:2043–2047. doi: 10.1128/JCM.42.5.2043-2047.2004. doi:10.1128/JCM.42.5.2043-2047.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J.-H, Wu J, Lin L.-F, Pei F.-Q, Yi J.-R, Lu W.-C, Cai S.-W. Preliminary report on SARS coronavirus detection from vector rat and cockroach by RT-PCR. Chin. J. Vector Biol. Control. 2003;14:332–334. [Google Scholar]
- Enserink M. SARS in China. China's missed chance. Science. 2003;301:294–296. doi: 10.1126/science.301.5631.294. doi:10.1126/science.301.5631.294 [DOI] [PubMed] [Google Scholar]
- Enserink M. One year after outbreak. SARS virus yields some secrets. Science. 2004;304:1097. doi: 10.1126/science.304.5674.1097. doi:10.1126/science.304.5674.1097 [DOI] [PubMed] [Google Scholar]
- Fang Y, Xu Y, Dai J.-Y, Peng G.-W, Yu D.-W. The establishment of an epidemic reporting system for the infectious atypical pneumonia of Guangdong Province. South China J. Prev. Med. 2003;29:57–58. [Google Scholar]
- Guan Y, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. doi:10.1126/science.1087139 [DOI] [PubMed] [Google Scholar]
- Han D.P, Kim H.G, Kim Y.B, Poon L.L, Cho M.W. Development of a safe neutralization assay for SARS-CoV and characterization of S-glycoprotein. Virology. 2004;326:140–149. doi: 10.1016/j.virol.2004.05.017. doi:10.1016/j.virol.2004.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao P, Chen M, Zhang G, He W, Li Y. Bioinformatics research on the SARS coronavirus in China. Curr. Pharm. Des. 2006;12:4565–4572. doi: 10.2174/138161206779010404. [DOI] [PubMed] [Google Scholar]
- Harcourt B.H, et al. Genetic characterization of Nipah virus, Bangladesh. Emerg. Infect. Dis. 2005;11:1594–1597. doi: 10.3201/eid1110.050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.F, Peng G.W, Zheng H.Z, Luo H.M, Liang W.J, Li L.H, Guo R.N, Deng Z.H. An epidemiological study on the index cases of severe acute respiratory syndrome occurring in different cities among Guangdong province. Zhonghua Liu Xing Bing Xue Za Zhi. 2003a;24:347–349. [PubMed] [Google Scholar]
- He J.F, et al. Severe acute respiratory syndrome in Guangdong Province of China: epidemiology and control measures. Zhonghua Yu Fang Yi Xue Za Zhi. 2003b;37:227–232. [PubMed] [Google Scholar]
- Hofmann H, Geier M, Marzi A, Krumbiegel M, Peipp M, Fey G.H, Gramberg T, Pohlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 2004;319:1216–1221. doi: 10.1016/j.bbrc.2004.05.114. doi:10.1016/j.bbrc.2004.05.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K.V. SARS coronavirus: a new challenge for prevention and therapy. J. Clin. Invest. 2003;111:1605–1609. doi: 10.1172/JCI18819. doi:10.1172/JCI200318819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon K.L, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. doi:10.1016/S0140-6736(03)13364-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson P.J, Rizzoli A, Bryan G, editors. The ecology of wildlife diseases. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- Hui D.S, Wong P.C, Wang C. SARS: clinical features and diagnosis. Respirology. 2003;8(Suppl):S20–S24. doi: 10.1046/j.1440-1843.2003.00520.x. doi:10.1046/j.1440-1843.2003.00520.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser L, Briones M.S, Hayden F.G. Performance of virus isolation and Directigen Flu A to detect influenza A virus in experimental human infection. J. Clin. Virol. 1999;14:191–197. doi: 10.1016/s1386-6532(99)00058-x. doi:10.1016/S1386-6532(99)00058-X [DOI] [PubMed] [Google Scholar]
- Khamsi R. Simple SARS detection. Nature. 2004;432:163. doi:10.1038/432163a [Google Scholar]
- Ksiazek T.G, et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. doi:10.1056/NEJMoa030781 [DOI] [PubMed] [Google Scholar]
- Kuiken T, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. doi:10.1016/S0140-6736(03)13967-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K, et al. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in SARS patients by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 2004;42:2884–2889. doi: 10.1128/JCM.42.7.2884-2889.2004. doi:10.1128/JCM.42.7.2884-2889.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl Acad. Sci. USA. 2005;102:14 040–14 045. doi: 10.1073/pnas.0506735102. doi:10.1073/pnas.0506735102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. doi:10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- Leung C.W, et al. Severe acute respiratory syndrome among children. Pediatrics. 2004a;113:e535–e543. doi: 10.1542/peds.113.6.e535. doi:10.1542/peds.113.6.e535 [DOI] [PubMed] [Google Scholar]
- Leung G.M, et al. SARS-CoV antibody prevalence in all Hong Kong patient contacts. Emerg. Infect. Dis. 2004b;10:1653–1656. doi: 10.3201/eid1009.040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wo J, Shao J, Zhu H, Wu N, Li M, Yao H, Hu M, Dennin R.H. SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J. Clin. Virol. 2003a;28:239–244. doi: 10.1016/S1386-6532(03)00195-1. doi:10.1016/S1386-6532(03)00195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003b;426:450–454. doi: 10.1038/nature02145. doi:10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 2004a;189:648–651. doi: 10.1086/381535. doi:10.1086/381535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Greenhough T.C, Moore M.J, Vasilieva N, Somasundaran M, Sullivan J.L, Farzan M, Hyeryun C. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J. Virol. 2004b;78:11429–11433. doi: 10.1128/JVI.78.20.11429-11433.2004. doi:10.1128/JVI.78.20.11429-11433.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Li W, Farzan M, Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005a;309:1864–1868. doi: 10.1126/science.1116480. doi:10.1126/science.1116480 [DOI] [PubMed] [Google Scholar]
- Li W, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005b;310:676–679. doi: 10.1126/science.1118391. doi:10.1126/science.1118391 [DOI] [PubMed] [Google Scholar]
- Li W, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005c;24:1634–1643. doi: 10.1038/sj.emboj.7600640. doi:10.1038/sj.emboj.7600640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. doi:10.1126/science.1086616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.-H, Huang R, Lin H.-Z, Zhang P.-H, Liu X.-M. Investigation and analysis of coronaviruses carried by rodents. Chin. J. Epidemiol. 2003;24:1140. [Google Scholar]
- Liu Z.-H, Cheng S.-J, Guo Z.-M, Qu L.-Z, Wei S.-L, Gu W.-W, Huang R. Investigation of source of SARS virus in laboratory animals and zoo animals. ACTA Laboratorium Animals Scientia Sinica. 2004;12:50–52. [Google Scholar]
- Marra M.A, et al. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. doi:10.1126/science.1085953 [DOI] [PubMed] [Google Scholar]
- Martina B.E, Haagmans B.L, Kuiken T, Fouchier R.A, Rimmelzwaan G.F, Van Amerongen G, Peiris J.S, Lim W, Osterhaus A.D. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. doi:10.1038/425915a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. doi:10.1126/science.7701348 [DOI] [PubMed] [Google Scholar]
- Ng E.K, et al. Serial analysis of the plasma concentration of SARS coronavirus RNA in pediatric patients with severe acute respiratory syndrome. Clin. Chem. 2003;49:2085–2088. doi: 10.1373/clinchem.2003.024588. doi:10.1373/clinchem.2003.024588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003a;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. doi:10.1016/S0140-6736(03)13412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003b;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. doi:10.1016/S0140-6736(03)13077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S, Yuen K.Y, Osterhaus A.D, Stohr K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003c;349:2431–2441. doi: 10.1056/NEJMra032498. doi:10.1056/NEJMra032498 [DOI] [PubMed] [Google Scholar]
- Peiris J.S, Guan Y, Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. doi:10.1038/nm1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G.W, et al. Epidemiological study on severe acute respiratory syndrome in Guangdong province. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:350–352. [PubMed] [Google Scholar]
- Poon L.L, Wong O.K, Chan K.H, Luk W, Yuen K.Y, Peiris J.S, Guan Y. Rapid diagnosis of a coronavirus associated with severe acute respiratory syndrome (SARS) Clin. Chem. 2003;49:953–955. doi: 10.1373/49.6.953. doi:10.1373/49.6.953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L.L, Guan Y, Nicholls J.M, Yuen K.Y, Peiris J.S. The aetiology, origins, and diagnosis of severe acute respiratory syndrome. Lancet Infect. Dis. 2004;4:663–671. doi: 10.1016/S1473-3099(04)01172-7. doi:10.1016/S1473-3099(04)01172-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen S.M, Low D.E. Severe acute respiratory syndrome: an update. Curr. Opin. Infect. Dis. 2004;17:287–294. doi: 10.1097/01.qco.0000136924.45049.7e. doi:10.1097/01.qco.0000136924.45049.7e [DOI] [PubMed] [Google Scholar]
- Poutanen S.M, McGeer A.J. Transmission and Control of SARS. Curr. Infect. Dis. Rep. 2004;6:220–227. doi: 10.1007/s11908-004-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen S.M, et al. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. doi:10.1056/NEJMoa030634 [DOI] [PubMed] [Google Scholar]
- Qin E.D.E.A. A complete sequence and comparative analysis of a SARS-associated virus (isolate BJ01) Chin. Sci. Bull. 2003;48:941–948. doi: 10.1007/BF03184203. doi:10.1360/03wc0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin E, et al. A genome sequence of novel SARS-CoV isolates: the genotype, GD-Ins29, leads to a hypothesis of viral transmission in South China. Genomics Proteomics Bioinformatics. 2003a;1:101–107. doi: 10.1016/S1672-0229(03)01014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P.-Z, Zhou R.-H, Liu W.-S, Cai W.-F, Li Z.-R, Liu Y.-F, Wang M. Establishment and evaluation on emergency surveillance and notification system of SARS in Guangzhou. Chin. J. Public Health. 2003b;19:T3–T4. [Google Scholar]
- Qu X.X, et al. Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J. Biol. Chem. 2005;280:29 588–29 595. doi: 10.1074/jbc.M500662200. doi:10.1074/jbc.M500662200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer T.H. Severe acute respiratory syndrome: clinical features, diagnosis, and management. Curr. Opin. Pulm. Med. 2004;10:159–165. doi: 10.1097/00063198-200405000-00003. doi:10.1097/00063198-200405000-00003 [DOI] [PubMed] [Google Scholar]
- Ren W, et al. Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis. J. Gen. Virol. 2006;87:3355–3359. doi: 10.1099/vir.0.82220-0. doi:10.1099/vir.0.82220-0 [DOI] [PubMed] [Google Scholar]
- Reynes J.M, et al. Nipah virus in Lyle's flying foxes, Cambodia. Emerg. Infect. Dis. 2005;11:1042–1047. doi: 10.3201/eid1107.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley S, et al. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–1966. doi: 10.1126/science.1086478. doi:10.1126/science.1086478 [DOI] [PubMed] [Google Scholar]
- Rota P.A, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. doi:10.1126/science.1085952 [DOI] [PubMed] [Google Scholar]
- Rowe T, et al. Macaque model for severe acute respiratory syndrome. J. Virol. 2004;78:11 401–11 404. doi: 10.1128/JVI.78.20.11401-11404.2004. doi:10.1128/JVI.78.20.11401-11404.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y.J, et al. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet. 2003;361:1779–1785. doi: 10.1016/S0140-6736(03)13414-9. doi:10.1016/S0140-6736(03)13414-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz B, Jr, Rausch J.M, Gallaher W.R, Garry R.F, Wimley W.C. Identification and characterization of the putative fusion peptide of the severe acute respiratory syndrome-associated coronavirus spike protein. J. Virol. 2005;79:7195–7206. doi: 10.1128/JVI.79.11.7195-7206.2005. doi:10.1128/JVI.79.11.7195-7206.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto W.H, Tsang D, Yung R.W, Ching T.Y, Ng T.K, Ho M, Ho L.M, Peiris J.S. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519–1520. doi: 10.1016/S0140-6736(03)13168-6. doi:10.1016/S0140-6736(03)13168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Reeves J.D, Rennekamp A.J, Amberg S.M, Piefer A.J, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl Acad. Sci. USA. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. doi:10.1073/pnas.0306446101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.D, et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl Acad. Sci. USA. 2005;102:2430–2435. doi: 10.1073/pnas.0409608102. doi:10.1073/pnas.0409608102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler K, Masignani V, Eickmann M, Becker S, Abrignani S, Klenk H.D, Rappuoli R. SARS—beginning to understand a new virus. Nat. Rev. Microbiol. 2003;1:209–218. doi: 10.1038/nrmicro775. doi:10.1038/nrmicro775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, et al. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. doi:10.1128/JVI.78.7.3572-3577.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet B, Howard M.W, Jobling M, Holmes R.K, Holmes K.V, Hodges R.S. Structural characterization of the SARS-coronavirus spike S fusion protein core. J. Biol. Chem. 2004;279:20 836–20 849. doi: 10.1074/jbc.M400759200. doi:10.1074/jbc.M400759200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. doi:10.1056/NEJMoa030666 [DOI] [PubMed] [Google Scholar]
- Tsui S.K, Chim S.S, Lo Y.M. Coronavirus genomic-sequence variations and the epidemiology of the severe acute respiratory syndrome. N. Engl. J. Med. 2003;349:187–188. doi: 10.1056/NEJM200307103490216. doi:10.1056/NEJM200307103490216 [DOI] [PubMed] [Google Scholar]
- Tu C, et al. Antibodies to SARS coronavirus in civets. Emerg. Infect. Dis. 2004;10:2244–2248. doi: 10.3201/eid1012.040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.F, Eaton B.T. Emerging paramyxoviruses. Infect. Dis. Rev. 2001;3:52. [Google Scholar]
- Wang H, Ding Y, Li X, Yang L, Zhang W, Kang W. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N. Engl. J. Med. 2003a;349:507–508. doi: 10.1056/NEJM200307313490519. doi:10.1056/NEJM200307313490519 [DOI] [PubMed] [Google Scholar]
- Wang M, et al. Study on the epidemiology and measures for control on severe acute respiratory syndrome in Guangzhou city. Zhonghua Liu Xing Bing Xue Za Zhi. 2003b;24:353–357. [PubMed] [Google Scholar]
- Wang P, et al. Expression cloning of functional receptor used by SARS coronavirus. Biochem. Biophys. Res. Commun. 2004;315:439–444. doi: 10.1016/j.bbrc.2004.01.076. doi:10.1016/j.bbrc.2004.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R.A. Planning for epidemics: the lessons of SARS. N. Engl. J. Med. 2004;350:2332–2334. doi: 10.1056/NEJMp048082. doi:10.1056/NEJMp048082 [DOI] [PubMed] [Google Scholar]
- Wentworth D.E, Gillim-Ross L, Espina N, Bernard K.A. Mice susceptible to SARS coronavirus. Emerg. Infect. Dis. 2004;10:1293–1296. doi: 10.3201/eid1007.031119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.S, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Br. Med. J. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. doi:10.1136/bmj.326.7403.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.K, Li W, Moore M.J, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004a;279:3197–3201. doi: 10.1074/jbc.C300520200. doi:10.1074/jbc.C300520200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.W, et al. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg. Infect. Dis. 2004b;10:269–276. doi: 10.3201/eid1002.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, et al. Civets are equally susceptible to experimental infection by two different severe acute respiratory syndrome coronavirus isolates. J. Virol. 2005;79:2620–2625. doi: 10.1128/JVI.79.4.2620-2625.2005. doi:10.1128/JVI.79.4.2620-2625.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.H, et al. Epidemiologic clues to SARS origin in China. Emerg. Infect. Dis. 2004a;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lou Z, Liu Y, Pang H, Tien P, Gao G.F, Rao Z. Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J. Biol. Chem. 2004b;279:49 414–49 419. doi: 10.1074/jbc.M408782200. doi:10.1074/jbc.M408782200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, et al. Characterization of the heptad repeat regions, HR1 and HR2, and design of a fusion core structure model of the spike protein from severe acute respiratory syndrome (SARS) coronavirus. Biochemistry. 2004c;43:14 064–14 071. doi: 10.1021/bi049101q. doi:10.1021/bi049101q [DOI] [PubMed] [Google Scholar]
- Yang Z.Y, Huang Y, Ganesh L, Leung K, Kong W.P, Schwartz O, Subbarao K, Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. doi:10.1128/JVI.78.11.5642-5650.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.-R, Lin L.-F, Duan J.-H, Wu J, Pei F.-Q, Lu W.-C, Cai S.-W, Zheng W.-Y, Ying W.-X. Analysis on murine-like animals carrying SARS coronavirus in the hygienic units related to SARS. Clin. J. Vector Biol. Control. 2004;15:7–9. [Google Scholar]
- Yu D, Li H, Xu R.H, He J.F, Al E. Prevalence of IgG antibody to SARS-associated coronavirus in animal traders-Guangdong Province, China. MMWR Morb. Mortal. Wkly Rep. 2003;52:986–987. [PubMed] [Google Scholar]
- Zhong N.S, Zeng G.Q. Our strategies for fighting severe acute respiratory syndrome (SARS) Am. J. Respir. Crit. Care Med. 2003;168:7–9. doi: 10.1164/rccm.200305-707OE. doi:10.1164/rccm.200305-707OE [DOI] [PubMed] [Google Scholar]
- Zhong N.S, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. doi:10.1016/S0140-6736(03)14630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D.-H, Liu Y.-F, Qin P.-Z, Wang M, Du L. Field study and epidemic report on the infectious atypical pneumonia of Guangzhou City. Chin. J. Epidemiol. 2003;24:360–361. [Google Scholar]