Abstract
Over the last 15 years, it has become increasingly apparent that a single class of compounds, the acylated homoserine lactones (AHLs), elicit effects on many levels of biological and ecological organization. Despite the fact that the distribution of AHL production in the prokaryotic phylogenetic tree is restricted to a small set of genera, representatives of these genera are abundant in the environment and are responsible for processes of much interest to humans. As well as driving interactions between clones, AHLs have been shown to mediate interactions between different species of bacteria and between bacteria and higher organisms, either through the phenotypes they regulate or directly through their own chemical behaviour. Understanding the biological activity of AHLs and the ecological consequences of these activities may provide us with an opportunity to manipulate the composition and function of complex biological assemblages. Ultimately, this broadens the biotechnological focus of AHL-based research beyond the attenuation of virulence in humans and plant pathogens.
Keywords: acylated homoserine lactone, microbial ecology, microbial communities
1. The emerging status of acylated homoserine lactones
The structural elucidation of the diffusible metabolite that regulates luminescence in the marine symbiont Vibrio fischeri was published by Eberhard et al. (1981). Over a decade after the discovery of its autoinducer activity, the metabolite was identified as an acylated homoserine lactone (AHL). In the early 1990s, AHLs went from being an obscure, yet curious, regulator of gene expression in marine luminescent bacteria to a regulator of virulence in high profile plant and animal pathogens (Jones et al. 1993). Since then, AHLs have emerged as one of the most fascinating secondary metabolites in biology (Keller & Surette 2006).
AHLs are a discrete group of biologically active metabolites produced by a small number of bacterial taxa. While a comprehensive, unbiased screen of cultivable microbial taxa for AHL production has never been conducted, existing research roughly maps out where, in the prokaryotic phylogenetic tree, AHL production is providing a selective advantage. Despite extensive screening of environmental isolates, an AHL-producing strain outside the α-, β- or γ-classes of the Proteobacteria phylum has never been discovered and the genera known to produce AHLs are listed in table 1. Only 7% of the genera in each class contain AHL-producing isolates (9/133 for α-proteobacteria, 4/56 for β-proteobacteria and 12/180 for γ-proteobacteria). This consistent distribution across the α-, β- and γ-proteobacteria suggests that there is no specific relationship between the three classes, or the environments they inhabit, and the selective advantage of AHL production. More than probably, the ability to produce AHLs precedes the divergence of the three classes, and 93% of the genera that have emerged since the divergence have found no selective advantage in producing AHLs, within the classes as a whole.
Table 1.
Current known AHL-producing genera within the Proteobacteria.
| α | Rhodobacter |
| Rhizobium | |
| Agrobacterium | |
| Ruegaria | |
| Bradyrhizobium | |
| Mesorhizobium | |
| Sinorhizobium | |
| Roseobacter | |
| Brucella | |
| β | Burkholderia |
| Ralstonia | |
| Chromobacterium | |
| Nitrosomonas | |
| γ | Vibrio |
| Pseudomonas | |
| Aeromonas | |
| Yersinia | |
| Erwinia | |
| Serratia | |
| Hafnia | |
| Pantoea | |
| Halomonas | |
| Acidothiobacillus | |
| Edwardsiella | |
| Brucella |
While this cursory phylogenetic survey suggests that only a limited number of taxa have found their usefulness in AHL-mediated gene expression, it does not address the environmental or medical relevance of this extraordinary class of small molecule. The fact of the matter is that while AHL production is restricted to 7% of the genera in three out of five classes of one bacterial phylum, the taxa involved are representatives among the most abundant and active in the environment. With this in mind, the relevance of AHL production can never be questioned. This article details the remarkable range of biological activities of AHLs in the context of biological and ecological complexity.
2. Acylated homoserine lactones and the individual
While AHLs have traditionally been viewed as serving an intercellular signalling function, it is important in the context of ecology to consider that AHLs may play non-signalling roles. The issue here is that signal production (AHL synthesis) represents a resource cost to the individual. This is not necessarily an issue in rich culture medium where many of our experiments are performed, but is likely to come into play in the environment where organisms experience multiple resource limitations. Indeed, because AHL production represents a resource cost, it is unlikely that luxI homologues evolved to coordinate group activities without originally serving some other function selected for at the level of individual cells. Such functions may, or may not, still exist in different AHL-producing bacteria. Two concepts that circulate outside the existing literature are those of waste disposal and iron scavenging. For example, AHL production, which currently consumes S-adenosylmethionine (SAM), may historically have been a means of removing the toxic demethylated product of SAM, S-adenosylhomocysteine (SAH; Barbes et al. 1990). The fact that SAM serves as a substrate for the AHL synthase LuxI, while SAH does not, sheds some doubt on the possibility (Schaefer et al. 1996). Another hypothesis, derived from the observation that AHLs bind iron at high concentrations (T. Charleton 2001, unpublished data), suggests that they may have once played the role of ancient siderophores. A siderophore assay in which a test compound competes with known siderophores revealed that AHLs could not compete with extant siderophores for iron sequestration (M. Manefield 2003, unpublished data). While AHLs are not currently known to play a role in central metabolism in bacteria and luxI mutants are viable under typical culturing conditions, the possibility that they serve or once served a non-signalling function remains. A test of this hypothesis would be to compete luxI mutant cells with wild-type cells or to monitor the evolution of luxI mutants from wild-type cells in a chemostat over several generations.
3. Acylated homoserine lactones and intraspecific intercellular phenomena
AHLs are best known for their ability to stimulate transcription of functional genes through their interaction with homologues of the LuxR protein of V. fischeri (Welch et al. 2000). Owing to the size and polarity of AHLs, most of them can move freely across cellular membranes (those that cannot are transported; Evans et al. 1998). Owing to this, the transcriptionally relevant intracellular concentrations of AHL are dependent on the increases in concentration in the extracellular environment. The nature of changes in the environment reflected by the extracellular accumulation of AHLs is a fascinating subject that requires testing in realistic experimental systems.
The popular hypothesis, referred to as ‘quorum sensing’, suggests that the selective advantage of AHL production lies in linking gene expression with cell density (Fuqua et al. 1994). In quorum sensing, of the two parameters that define cell density (cell number and volume), it is an increase in cell number, rather than a decrease in volume, that is thought to activate transcription. There are numerous elegant scenarios whereby expressing certain genes when a population has increased in size is plausibly beneficial. One example involves virulence factor production in the plant pathogen Erwinia carotovora, and it suggests that producing virulence determinants at low cell densities would alert host immune systems to the infection before the bacterial population was at a density capable of resisting an immune response. This has been tested experimentally through the inoculation of AHL− E. carotovora mutants on AHL-producing plants (Fray et al. 1999). In this experiment, the production of virulence factors at low densities did not interfere with the ability to mount a successful infection, casting some doubt on this ‘stealth invasion’ model.
Other hypotheses explaining the selective advantage of AHL-mediated gene expression involve elements of environmental sensing. For example, increases in extracellular AHL concentration may indicate that a population of cells is confined in a small space, such as the light organ of a squid or the xylem of a plant. Alternatively, given that AHLs lose LuxR binding activity at basic pH, AHLs could function as an extracellular sensor of hydrogen ion concentration. Another plausible hypothesis explaining the selective advantage of this intercellular signalling-dependent gene expression mechanism is that of orchestration. AHLs may act to make sure the expression of certain genes is simultaneous throughout a population, independent of cell density. This may be important in the expression of genes encoding antibiotics and antibiotic resistance. If some cells in a population started producing antibiotics while others were not expressing resistance genes, the result would be deleterious. While one or a number of these hypotheses may be most applicable for different bacteria, the selective advantage of AHLs may, in part, lie outside the regulation of intraspecific intercellular phenomena.
4. Acylated homoserine lactones and interspecific bacteria–bacteria interactions
The discovery that bacteria use extracellular metabolites to regulate gene expression gave rise to the hypothesis that cohabiting bacteria could manipulate gene expression in responsive non-self species. Over the last decade, the possibility that interspecies ‘crosstalk’ may be selected for at some level has grown steadily more plausible. McKenney et al. (1995) demonstrated that cell-free supernatants from Pseudomonas aeruginosa stimulate virulence factor production in its occasional lung cohabitant Burkholderia cepacia. Riedel et al. (2001) strengthened the idea using AHL reporters to demonstrate that AHLs from P. aeruginosa can stimulate gene expression in B. cepacia in mixed biofilms in flow chambers and in a mouse lung. Further evidence for interspecies signalling in the environment comes from the demonstration that naturally coexisting bacterial species on wheat or tomato roots induce AHL-mediated gene expression in non-isogenic species (Pierson et al. 1998; Steidle et al. 2001).
Another compelling example of AHL-mediated interspecies control of gene expression involves Escherichia coli, which harbours the LuxR homologue SdiA, but does not produce AHLs itself. Yao et al. (2006) have recently shown that SdiA does indeed bind AHLs, indicating that E. coli encodes an AHL receptor protein that will only ever encounter the regulatory metabolite produced by cohabitant species. The discovery that Bacillus species produce lactonases that degrade AHLs and that this can be used to modulate AHL-mediated gene expression represents additional evidence that AHLs control phenotypes of importance in interspecies bacterial interactions (Dong et al. 2001).
Recently, Mason et al. (2005) demonstrated that AHL-mediated gene expression can be affected by the presence of non-AHL-producing and non-AHL-degrading species. In elegantly constructed artificial microcolonies, the presence of non-AHL-producing cells appeared to foster the accumulation of AHLs produced by cohabiting cells in the microcolonies. This constitutes an example where non-AHL-producing species regulate gene expression in AHL-producing species.
One final example where AHLs affect cohabiting bacterial populations involves the consumption of the metabolite as an energy source. A Variovorax species has been shown to use the acyl chain of different AHLs as an energy source and can rely on homoserine lactone as a sole source of nitrogen (Leadbetter & Greenberg 2000). Arthrobacter, Ralstonia and Pseudomonas species have also been shown to use AHLs as growth substrates (Huang et al. 2003). These studies show that AHLs play a role in trophic relationships in microbial communities.
Despite the fact that AHLs can and most probably do impact on cohabiting bacterial species, the impact of this on changes in the size of impacted populations, and therefore community composition and function, remains unclear. The design of experiments to distinguish between the impact of interspecies interactions mediated directly through AHL activity and interspecies interactions mediated through AHL-regulated phenotypes is non-trivial.
5. Acylated homoserine lactone-mediated gene expression and interspecific bacteria–eukaryote interactions
Microbial communities in the environment are invariably associated with eukaryotes. As hinted previously, there are many examples where AHL-mediated gene expression plays an important role in interactions between bacteria and higher organisms. The most extensively studied involve the production of virulence factors by plant or animal pathogens, including E. carotovora and P. aeruginosa. These have been reviewed extensively elsewhere (Whitehead et al. 2001).
An interesting phenomenon that has received less attention was discovered by Matz et al. (2004), whereby the ingestion of as few as three Chromobacterium violaceum cells by bacteriovorous nanoflagellate protozoa resulted in the death of the predator. Violacein production in C. violaceum is regulated by AHLs and it was shown that AHL mutants of C. violaceum did not kill the grazing eukaryote. This indicates that predator populations could be adversely affected by an AHL-regulated phenotype. If AHL accumulation only occurs in flocs or biofilms, AHL-mediated production of violacein may act to protect aggregated biomass by limiting proliferation of populations of protozoa that graze on aggregates. In this hypothetical, planktonic cells remain vulnerable to predation, thereby establishing a selection pressure for other bacterial species to integrate into flocs with C. violaceum. The potential impact of AHL-mediated gene expression on the ecology of natural and industrial environments becomes apparent.
Another example, where AHL-mediated gene expression may play a role in eukaryote ecology, involves the red algae Delisea pulchra. This benthic marine plant produces compounds (halogenated furanones) that interfere with AHL-mediated gene expression by triggering proteolytic degradation of the AHL receptor protein (Manefield et al. 2002). It has been proposed that the bioactive secondary metabolite chemistry of D. pulchra has evolved in response to the insistent pressure of AHL-mediated bacterial colonization and phytopathogenic phenotypes (Kjelleberg et al. 1997). If this were so, then AHL-mediated gene expression not only drives ecological interactions with higher organisms but can illicit eukaryotic evolutionary responses as well. The finding that developing gnotobiotic plantlets of Lotus species demonstrate AHL degradation activity lends weight to this assertion (Delalande et al. 2005).
6. Acylated homoserine lactones and interspecific bacteria–eukaryote interactions
There are a number of remarkable examples where cohabitant organisms outside the prokaryotic world are respondent to AHLs. The first reported bacteria–eukaryote interaction involving AHLs involved the stimulation of interleukin-8 production in respiratory epithelial cells by a long-chain AHL produced by P. aeruginosa (Dimango et al. 1995). Since then, research on the immunomodulatory effects of AHLs have shown that AHLs play a direct role as a virulence factor in mediating pathogenic relationships (Telford et al. 1998; Smith et al. 2002).
An intriguing non-medical example has recently come to light. In the marine environment, AHLs have been shown to act as a settlement queue for motile zoospores of the green alga Enteromorpha (Joint et al. 2002). While the mechanism by which Enteromorpha detects and responds to AHLs is unknown, the discovery raises the possibility that, in the marine environment, Enteromorpha zoospores use AHLs emanating from bacterial biofilms to select appropriate attachment sites. If this were true, AHLs would have a direct influence on the biogeography of marine algae. The fact that AHL production is an ancient phenotype (possessed by the last common ancestor of the α-, β- and γ-proteobacteria) makes the evolution of such biotic interactions plausible. Indeed, in the rhizosphere environment, Mathesius et al. (2003) provided proteomic evidence for extensive and specific responses to AHLs in the model legume Medicago trunculata.
7. Acylated homoserine lactones and wastewater biotechnology
The biological activity of AHLs discussed previously reveals that they are a versatile group of compounds capable of abiotic and biological interactions relevant over different levels of complexity in ecology. While there is doubt that AHLs play a role in the metabolism of individual bacterial cells (individual level), it is clear that they can mediate interactions between conspecific cells (population level), non-specific bacterial cells and between prokaryotes and eukaryotes (community level). In light of this, it is perhaps not surprising that the addition of AHLs to phenol-degrading activated sludge led to shifts in community composition and phenol degradation rates (Valle et al. 2004).
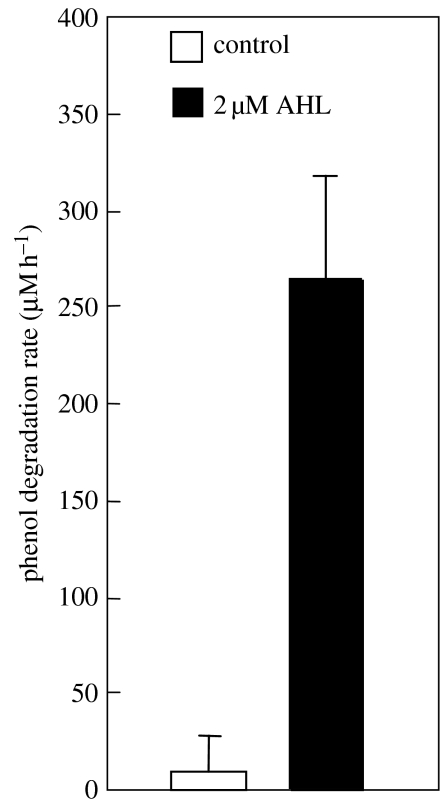
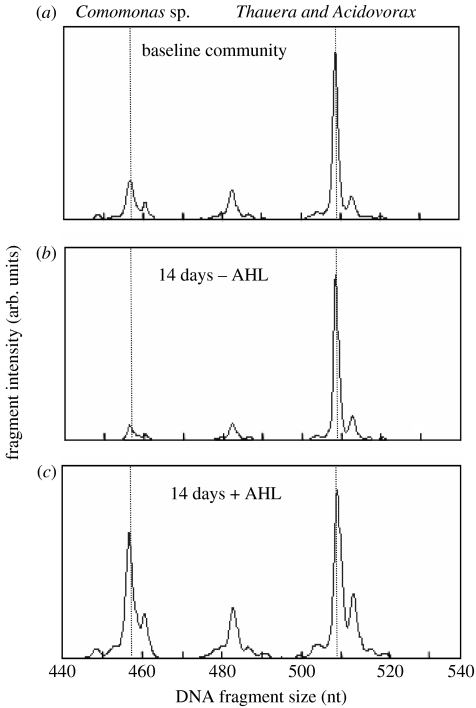
Several known AHL-producing species are commonly found in activated sludge and a screen of cultivable organisms from activated sludge with AHL reporter bioassays showed that they are prevalent, and new genera could be identified that produced AHL-like compounds (Valle et al. 2004). The wastewater system in question operated as part of an industrial coking operation and is critical in order to breakdown highly polluted wastewater streams derived from coke production (approx. 300–500 mg l−1 of organic pollutants with more than 70 major chemical species, phenol being the dominant chemical species). The discovery of these phenotypes indicated that such systems may use AHL inter- and intraspecific signalling as a means to coordinate the complicated breakdown pathways. This assumption was tested during the periods of community shutdown, where wastewater feed to the population was stopped, as occurs during periods of toxicity build-up. Specifically, during toxicity build-up in the coking wastewater streams, the communities are protected by isolating the biological reactors from the wastewater input and storage of the wastewater in holding tanks followed by remedial action, such as dilution. However, during these periods, the communities can rapidly lose the ability to breakdown phenol, the primary pollutant (figure 1), and hence are difficult to restart when the wastewater streams are re-initiated into the biological reactors. If, however, the isolated community was supplemented with 2 μM of AHL on a daily basis, the ability to breakdown phenol was maintained when tested with a phenol breakdown challenge (figure 1). Under this scenario, AHL addition caused a population shift from the dominant degrader (a Thauera species) to a community that had a more even balance between the Thauera degrader and Comomonas-like organisms (figure 2). There is currently no understanding of the mechanism by which the modulation of population changes occurs or how phenol degradation is maintained; however, unpublished data suggests that the production of surfactants could be linked to the observations.
Figure 1.
The loss of phenol-degrading activity for wastewater treatment samples derived from coking operations, which have been isolated from the standard wastewater input. Standard degradation rates are approximately 250 μM h−1 for healthy sludge (dashed line). During 14 days of cessation of input wastewater feed, the community lost the ability to breakdown a phenol challenge. However, after supplementing cultures with 2 μM AHLs for the 14-day period, the community retains its ability to breakdown phenol challenges.
Figure 2.
Community effects of daily repeated AHL addition during loss of phenol degradation capacity in wastewater communities. The plots represent terminal restriction fragment length polymorphism analyses of bacterial community structure, including (a) baseline community and AHL treatment (b) without or (c) with 2 μM AHL. The 2 μM AHL supplement consisted of a cocktail of equimolar concentrations of N-3-oxohexanoyl-l-homoserine lactone and N-hexanoyl-l-homoserine lactone.
In terms of these important biotechnological applications, AHLs provide an avenue for academics and industrialists to explore their use in maintaining community activity during deleterious changes in operational efficiency, a costly event for industrialists if the biological treatment system they employ fails to work, or is compromised by toxicity issues. This is especially pertinent in the coking industry, where the manufacturing process is required to run on a continuous basis and, therefore, maintaining biological treatment is a main priority during operations. Much work still needs to be performed, such as understanding the mechanisms in switching phenol-degrading dominance during AHL amendment, the stability of AHLs within the system and the ability of isolates to produce them in situ. These investigations require new monitor strains to be produced, which are viable in these toxic ecosystems, hence we can monitor production at the single cell level and methodological developments to address stability in situ and the different classes of AHLs that are bioactive in situ.
In general, the application of microbial communities to wastewater treatment has had a profound influence on human civilization. Despite this biotechnological marvel, our understanding of what influences community dynamics and hence sludge function is still rudimentary, as we have demonstrated within the coking system. Activated sludge represents an experimentally tractable and biotechnologically relevant environment in which to improve our understanding of the role of AHLs in ecology and provide actual solutions to ‘real world’ problems faced by industrialists. Future solutions could involve the direct addition of synthesized AHLs to communities experiencing poor degradation capabilities, or the promotion of naturally occurring strains within the community to aid community stability. Either way, we believe that such solutions will have a place in engineering stability into complex industrial degradation communities, with the ultimate aims of significant cost savings for industrialists and protection of the environment as a whole.
8. Conclusions
We have highlighted only a subset of examples, which indicates the number and breadth of biological activities that AHLs display. From these few examples, it is apparent that this group of small molecules is among the most fascinating and influential non-essential metabolites in biology. In short, while AHLs have no apparent role in central metabolism, AHLs are somehow involved in the biology of individuals, populations and communities (including eukaryotes), and possibly at the ‘event horizon’ of evolution. This is true in the sense of major transitions of evolution (cellular to multicellular biology) and in the sense of controlling current skirmishes between pathogens and hosts (Persson et al. 2005). The vast global research effort that has been directed towards AHLs over the last 15 years reflects both the fascination and the opportunity to apply our understanding (both in terms of stimulating and inhibiting AHL interactions) in industrial and medical contexts for the benefit of society.
Footnotes
One contribution of 12 to a Theme Issue ‘Bacterial conversations: talking, listening and eavesdropping’.
References
- Barbes C, Sanchez J, Yebra M.J, Robert-Gero M, Hardisson C. Effects of sinefungin and S-adenosylhomocysteine on DNA and protein methyltransferases from Streptomyces and other bacteria. FEMS Microbiol. Lett. 1990;57:239–243. doi:10.1111/j.1574-6968.1990.tb04237.x [PubMed] [Google Scholar]
- Delalande L, et al. N-Hexanoyl-l-homoserine lactone, a mediator of bacterial quorum-sensing regulation, exhibits plant-dependent stability and may be inactivated by germinating Lotus corniculatus seedlings. FEMS Microbiol. Ecol. 2005;52:13–20. doi: 10.1016/j.femsec.2004.10.005. doi:10.1016/j.femsec.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Dimango E, Zar H.J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Invest. 1995;5:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.H, Wang L.H, Xu J.L, Zhang H.B, Zhang X.F, Zhang L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411:813–817. doi: 10.1038/35081101. doi:10.1038/35081101 [DOI] [PubMed] [Google Scholar]
- Eberhard A, Burlingame A.L, Eberhard C, Kenyon G.L, Nealson K.H, Oppenheimer N.J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. doi:10.1021/bi00512a013 [DOI] [PubMed] [Google Scholar]
- Evans K, Passador L, Srikumar R, Tsang E, Nezezon J, Poole K. Influence of the MexAB–OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1998;180:5443–5447. doi: 10.1128/jb.180.20.5443-5447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray R.G, Throup J.P, Wallace A, Daykin M, Williams P, Stewart G.S.A.B, Grierson D. Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nat. Biotechnol. 1999;17:1017–1020. doi: 10.1038/13717. doi:10.1038/13717 [DOI] [PubMed] [Google Scholar]
- Fuqua W.C, Winans S.C, Greenberg E.P. Quorum sensing in bacteria: the LuxR–LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.J, Han J.I, Zhang L.H, Leadbetter J.R. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2003;69:5941–5949. doi: 10.1128/AEM.69.10.5941-5949.2003. doi:10.1128/AEM.69.10.5941-5949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint I, Tait K, Callow M.E, Callow J.A, Milton D, Williams P, Cámara M. Cell-to-cell communication across the prokaryote–eukaryote boundary. Science. 2002;298:1207. doi: 10.1126/science.1077075. doi:10.1126/science.1077075 [DOI] [PubMed] [Google Scholar]
- Jones S, et al. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L, Surette M.G. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. doi:10.1038/nrmicro1383 [DOI] [PubMed] [Google Scholar]
- Kjelleberg S, Steinberg P, Givskov M, Manefield M, de Nys R. Do marine natural products interfere with prokaryotic AHL regulatory systems? Aquat. Microb. Ecol. 1997;13:85–93. [Google Scholar]
- Leadbetter J.R, Greenberg E.P. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 2000;182:6921–6926. doi: 10.1128/jb.182.24.6921-6926.2000. doi:10.1128/JB.182.24.6921-6926.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manefield M, Rasmussen T.B, Henzter M, Andersen J.B, Steinberg P, Kjelleberg S, Givskov M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology. 2002;148:1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- Mason V.P, Markx G.H, Thompson I.P, Andrews J.S, Manefield M. Colonial architecture in mixed species assemblages affects AHL mediated gene expression. FEMS Microbiol. Lett. 2005;244:121–127. doi: 10.1016/j.femsle.2005.01.031. doi:10.1016/j.femsle.2005.01.031 [DOI] [PubMed] [Google Scholar]
- Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe B.G, Bauer W.D. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc. Natl Acad. Sci. USA. 2003;100:1444–1449. doi: 10.1073/pnas.262672599. doi:10.1073/pnas.262672599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz C, Deines P, Boenigk J, Arndt H, Eberl L, Kjelleberg S, Jurgens K. Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol. 2004;70:1593–1599. doi: 10.1128/AEM.70.3.1593-1599.2004. doi:10.1128/AEM.70.3.1593-1599.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney D, Brown K.E, Allison D.G. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: evidence of interspecies communication. J. Bacteriol. 1995;177:6989–6992. doi: 10.1128/jb.177.23.6989-6992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson T, Givskov M, Nielsen J. Quorum sensing inhibition: targeting chemical communication in Gram-negative bacteria. Curr. Med. Chem. 2005;12:3103–3115. doi: 10.2174/092986705774933425. doi:10.2174/092986705774933425 [DOI] [PubMed] [Google Scholar]
- Pierson E.A, Wood D.W, Cannon J.A, Blachere F.M, Pierson L.S. Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol. Plant Microbe Interact. 1998;11:1078–1084. [Google Scholar]
- Riedel K, et al. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology. 2001;147:3249–3262. doi: 10.1099/00221287-147-12-3249. [DOI] [PubMed] [Google Scholar]
- Schaefer A.L, Val D.L, Hanzelka B.L, Cronan J.E, Greenberg E.P. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl Acad. Sci. USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. doi:10.1073/pnas.93.18.9505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.S, Kelly R, Iglewski B.H, Phipps R.P. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 2002;169:2636–2642. doi: 10.4049/jimmunol.169.5.2636. [DOI] [PubMed] [Google Scholar]
- Steidle A, et al. Visualization of N-acylhomoserine lactone, mediated cell–cell communication between bacteria colonizing the tomato rhizosphere. Appl. Environ. Microbiol. 2001;67:5761–5770. doi: 10.1128/AEM.67.12.5761-5770.2001. doi:10.1128/AEM.67.12.5761-5770.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford G, Wheeler D, Williams P, Tomkins P.T, Appleby P, Sewell H, Stewart G.S.A.B, Bycroft B.W, Pritchard D.I. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 1998;66:36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A, Bailey M.J, Whiteley A.S, Manefield M. N-Acyl-l-homoserine lactones (AHLs) affect microbial community composition and function in activated sludge. Environ. Microbiol. 2004;6:424–433. doi: 10.1111/j.1462-2920.2004.00581.x. doi:10.1111/j.1462-2920.2004.00581.x [DOI] [PubMed] [Google Scholar]
- Welch M, Todd D.E, Whitehead N.A, McGowan S.J, Bycroft B.W, Salmond G.P. N-Acyl homoserine lactone binding to the CarR receptor determines quorum-sensing specificity in Erwinia. EMBO J. 2000;19:631–641. doi: 10.1093/emboj/19.4.631. doi:10.1093/emboj/19.4.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead N.A, Barnard A.M.L, Slater H, Simpson N.J.L, Salmond G.P.C. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. doi:10.1111/j.1574-6976.2001.tb00583.x [DOI] [PubMed] [Google Scholar]
- Yao Y, Martinez-Yamout M.A, Dickerson T.J, Brogan A.P, Wright P.E, Dyson H.J. Structure of the Escherichia coli quorum sensing protein SdiA: activation of the folding switch by acyl homoserine lactones. J. Mol. Biol. 2006;355:262–273. doi: 10.1016/j.jmb.2005.10.041. doi:10.1016/j.jmb.2005.10.041 [DOI] [PubMed] [Google Scholar]