Abstract
The term quorum sensing (QS) is used to describe the communication between bacterial cells, whereby a coordinated population response is controlled by diffusible molecules produced by individuals. QS has not only been described between cells of the same species (intraspecies), but also between species (interspecies) and between bacteria and higher organisms (inter-kingdom). The fact that QS-based communication appears to be widespread among microbes is strange, considering that explaining both cooperation and communication are two of the greatest problems in evolutionary biology. From an evolutionary perspective, intraspecies signalling can be explained using models such as kin selection, but when communication is described between species, it is more difficult to explain. It is probable that in many cases this involves QS molecules being used as ‘cues’ by other species as a guide to future action or as manipulating molecules whereby one species will ‘coerce’ a response from another. In these cases, the usage of QS molecules cannot be described as signalling. This review seeks to integrate the evolutionary literature on animal signalling with the microbiological literature on QS, and asks whether QS within bacteria is true signalling or whether these molecules are also used as cues or for the coercion of other cells.
Keywords: quorum sensing, cooperation, signalling, cue, coercion
1. Cooperation within the microbial world
Understanding altruistic behaviours, those actions that increase another individual's fitness at a cost to your own, is one of the greatest challenges to evolutionary biologists, as natural selection appears to favour selfish and uncooperative individuals (Hamilton 1963, 1964a,b). Despite this, there are many examples in the animal kingdom where this form of cooperation has been successfully demonstrated. However, it is only recently that social behaviour in micro-organisms has been studied with respect to evolutionary theory (Crespi 2001), and so there is a strong potential to develop complementary research in this area from both molecular and adaptive (Darwinian) perspectives (West et al. 2006).
Bacteria exhibit remarkable social behaviours, which some workers have suggested are similar to those performed by insects, vertebrates and humans (table 1). For example, Myxococcus xanthus cells exhibit socially dependent swarming across surfaces (Velicer & Yu 2003), which allows the population to seek out bacterial prey in a manner that has been likened to hunting wolf packs (Dworkin 1996; Crespi 2001). Biofilms are a collection of bacterial cells (both single and mixed species) enclosed in a polysaccharide matrix and have been likened in nature to ant nests or beehives (Crespi 2001; Webb et al. 2003; Parsek & Greenberg 2005). Biofilms, for example those found on the human teeth, can contain up to 500 species of bacteria (Kolenbrander et al. 2002), providing an environment that is ripe for social interactions both within and between species.
Table 1.
Social traits exhibited by bacteria compared with examples from vertebrates and invertebrates.
| cooperative behaviour | group-derived benefits | microbe examples | higher organism comparisons |
|---|---|---|---|
| chemical communication (quorum sensing) | coordinated population behaviour | Vibrio fischeri, Pseudomonas aeruginosa, Staphylococcus aureus, etc. | pheromone production in many social animals |
| biofilm formation | protection from adverse environmental conditions | many species of bacteria | Burrows, nests, hives, cities |
| nitrogen fixation: mutualistic behaviour | nutrients and niche protection in nodules | Rhizobium spp. with legume plants | yucca plant and yucca moth |
| foraging/hunting: nutrient acquisition | enhanced growth and colonization sometimes in specialized niches | siderophore production for iron acquisition in many bacteria | wolves, lions, humans |
| autolysis (suicide) | provides nutrients and DNA for biofilm development | P. aeruginosa | apoptosis in eukaryotic cells |
| motility (swarming) | coordinated motility to a nutrient source | Yersinia spp., Myxococcus xanthus, P. aeruginosa | ants, termites |
| antibiotic resistance | production of extracellular enzymes (e.g. β-lactamase) to break down antimicrobials | Escherichia coli, Klebsiella spp. | group defence, antipredator vigilance |
| immune modulation | modulation of immune response to facilitate survival within the host | P. aeruginosa, Porphyromonas gingivalis, Helicobacter pylori | helminth parasites |
Perhaps the paradigm for bacterial cooperation and social behaviour can be seen in the diverse quorum-sensing (QS) systems found in both Gram-negative and Gram-positive bacteria (Swift et al. 2001). It is generally assumed that QS represents both intra- and interspecies signalling and that QS cooperation is for the benefit of the local group or population as a whole. However, as previously mentioned, cooperative communication can require specific conditions for it to evolve. Therefore, this raises the question as to whether QS in microbes is truly cooperative behaviour (Redfield 2002; Keller & Surette 2006).
This review aims to promote awareness of some of the evolutionary problems provided by QS. A key issue in research on communication is the careful and consistent use of terminology (Maynard Smith & Harper 2003). We (i) provide the standard definitions for terms such as signalling, (ii) discuss the conditions required for signalling to evolve, and be stable to invasion from mutants that exploit the signalling of others to their selfish benefit, and (iii) use specific microbial examples to illustrate whether the molecules produced are primarily used as signals, cues or for coercion.
2. When is a signal not a signal?
When we see cell A produce a substance X that elicits a response in cell B, it is tempting to conclude that the substance produced is a signal, i.e. cell A is trying to tell cell B something. The word ‘signal’ is widely used to define such substances in the context of QS or communication between bacterial cells. However, the broad use of this term can confuse or even obscure the details of the interaction between the cells it attempts to describe. This has been well illustrated by research on communication and signalling in animals, where considerable confusion has arisen through different researchers using the same term to mean different things or different terms to mean the same thing (Maynard Smith & Harper 2003).
This problem can be avoided if the different kinds of interactions that we observe when cell A elicits a response in cell B are differentiated, depending upon their consequences for cells A and B (table 2; Maynard Smith & Harper 2003). Specifically, a signal is defined as ‘any act or structure that alters the behaviour of other organisms, which evolved owing to that effect, and which is effective because the receiver's response has also evolved’. This definition distinguishes a signal from a cue where the production of substance X by cell A has not evolved owing to its effect on cell B. For example, substance X may be a waste product produced by cell A that is detected by cell B. To demonstrate that substance X is a signal and not a cue, it is necessary to show that it evolved owing to the response it elicits. If the production of substance X by cell A forces a costly response from cell B, we differentiate this from signalling and term it as coercion or chemical manipulation.
Table 2.
Types of communication are distinguished depending upon their fitness consequences to the sender and the responder. (Consequences are either beneficial (+) or costly (−).)
| evolved owing to the effect on the sender (cell A) | benefits the receiver to respond (cell B) | |
|---|---|---|
| signal | + | + |
| cue | − | + |
| coercion | + | − |
Does it matter and should we bother? The answer is yes for two reasons. First, it is important for general understanding if there is a consensus on the use of terms. This is a lesson hard learned by biologists working on signalling in higher organisms (Maynard Smith & Harper 2003; see also West et al. 2007). Second, and more importantly, we can make very different predictions about the behaviour of bacterial cells depending on whether they are communicating by a signal, a cue or coercion (table 2). For example, if a molecule is a signal, then we can say several things. (i) It is beneficial to cell B to respond. (ii) The response of cell B benefits cell A. (iii) It might be possible for a signaller to cheat in the amount of signal that they produce either to: (a) free ride on the back of other signallers (avoiding the cost of producing substance X) or (b) manipulate responders (signal can become coercive). (iv) There must be some mechanism that provides a shared interest to cells A and B, otherwise cheaters would invade and make the signalling unstable—in §3c we discuss how kin selection provides a solution to this problem. (v) A signalling system is likely to be more complex than a system involving a cue to remain stable in the face of evolution for individuals to make less substance X or for individuals to respond less.
3. Evolutionary theory for quorum sensing
(a) The problems of communication and cooperation
Two of the greatest problems for evolutionary biology are explaining cooperation and communication (Hamilton 1964b; Maynard Smith & Szathmary 1995; Maynard Smith & Harper 2003). However, they appear to come together in QS, causing a double problem (Brown & Johnstone 2001; Redfield 2002; Keller & Surette 2006). In this section, we consider the conditions under which QS coordinating cooperation can be evolutionarily stable.
The problem of cooperation is why should an individual carry out a behaviour that is costly to perform, but benefits other individuals or the local group (Hamilton 1963, 1964a,b)? Such cooperation is vulnerable to invasion by cheaters who do not cooperate, but gain the benefit from others cooperating. This problem is well known in the fields of economics and human morality, where it is termed the tragedy of the commons (Hardin 1968). The tragedy is that as a group, individuals would do better with cooperation, but this is not stable because each individual gains by selfishly pursuing his or her own short-term interests.
We have recently reviewed this problem in a microbial context elsewhere (West et al. 2006). An obvious case in which it arises is when cells produce extracellular products for nutrient acquisition (Dinges et al. 2000; Greig & Travisano 2004; Griffin et al. 2004), antibiotics (Riley & Wertz 2002), immune modulation molecules (Brown 1999; Tateda et al. 2003; Hooi et al. 2004), antibiotic degradation compounds (e.g. β-lactamases; Ciofu et al. 2000; Dugatkin et al. 2005) and biosurfactants (e.g. rhamnolipids) for motility (Velicer & Yu 2003; Daniels et al. 2004). These products are costly to the individual to produce, but provide a benefit to the individuals in the local group or population. Economic and evolutionary theory refers to such things as public goods (Dionisio & Gordo 2006). Many bacterial products termed ‘virulence factors’ are likely to be public goods—their coordinated production leading to damage to the host. The problem in these cases is that cheaters who do not pay the cost of producing such goods can still gain the benefit from neighbouring cooperators who do (for an experimental demonstration, see Griffin et al. 2004). This makes the cooperative production of public goods unstable, unless a mechanism such as kin selection operates (see §3c; Brown 1999; West & Buckling 2003).
The problem of communication is how can communication be reliable (Maynard Smith & Harper 2003)? Why do individuals convey honest information about themselves, to the benefit of other individuals? Why would they not give a false signal to their selfish advantage? If communication is not reliable, then why should the receiver listen to it? The problem is reviewed for communication in general by Maynard Smith & Harper (2003) and within the specific context of bacteria by Keller & Surette (2006).
(b) The problem of quorum sensing
QS is generally assumed to coordinate cooperative behaviours in bacteria. Specifically, QS appears to provide a means for individual bacteria to assess local cell density and to engage in cooperation once a threshold density has been reached. Many cooperative ventures will not be worthwhile until a sufficient number of cells are present, so one would expect facultative cooperation based on the presence of cues, such as QS molecules that act as a proxy for cell density. The idea is that signalling molecules are released, and that this rate of release is increased further by signal molecules. This leads to positive feedback at high cell densities, and a dramatic increase in cooperative effort (Williams et al. 2000; Swift et al. 2001).
However, this communication may potentially be invaded by cheaters that exploit this system (Brown & Johnstone 2001; Redfield 2002; Keller & Surette 2006). One possibility is a cheater that does not produce QS molecules, and so benefits from monitoring the local cell density without investing effort into the dissemination of this information. An alternate possibility for a cheater would be to overproduce the costly signal, but not respond to it, hence coercing its neighbours into greater production of public goods. The crucial point here is that both signalling and responding to a signal with the production of public goods are costly. Consequently, there must be benefits that outweigh these—otherwise, the system could be invaded by cheaters that did not signal or cooperate.
(c) A kin selection model of quorum sensing
Kin selection theory provides an explanation for cooperation or communication between relatives (Hamilton 1964b). By helping a close relative to reproduce, an individual is still passing on its own genes to the next generation, albeit indirectly. This theory is formalized by Hamilton's rule (Hamilton 1963, 1964a,b), which states that altruistic cooperation is favoured when rb−c>0, where c is the fitness cost to the altruist; b is the fitness cost to the beneficiary; and r is their genetic relatedness. This predicts that individuals should be more likely to cooperate when social partners are more closely related (higher r). For example, high levels of production of public goods are predicted when relatedness is higher among the interacting bacteria (West & Buckling 2003). Relatedness could often be extremely high in bacteria because limited dispersal and clonal reproduction can lead to the individuals interacting over a small area being predominantly clone-mates (West et al. 2006).
Brown & Johnstone (2001) developed a kin selection model of QS. They made the following assumptions.
Signalling is costly to the individual. The fitness of an individual cell is negatively correlated with the amount of signalling by that individual.
The production of public goods, in response to QS, is costly to the individual. The fitness of an individual cell is negatively correlated with the amount of public goods produced by that individual.
The production of public goods provides a benefit to the local group to interacting cells (the group). The fitness of an individual cell is positively correlated with the average amount of public goods produced by the local individuals.
The benefit of producing public goods is greater at higher population densities. The fitness benefit to an individual cell of a certain level of local public goods production is positively correlated with cell density.
Brown & Johnstone (2001) then made predictions for the evolutionarily stable level of signalling (production of signalling molecule) and cooperation (public goods production). A behaviour is described as an evolutionarily stable strategy (ESS) if it cannot be invaded or beaten by a mutant performing any other strategy once it has been adopted by the majority of individuals (Maynard Smith & Price 1973). In particular, they examined the consequences of variation in mean population density and relatedness (r). They found the results as follows.
Result 1. The ESS level of signalling and public goods production both increased with greater population densities. At low densities, there is little to be gained from the cooperative production of public goods.
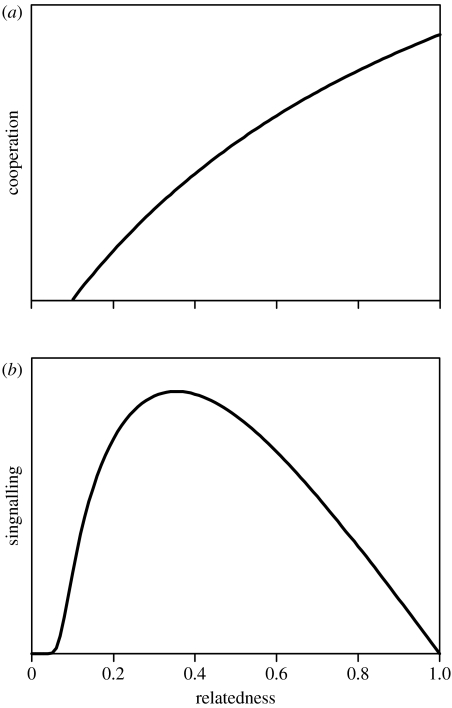
Result 2. The ESS level of production of public goods increased with higher relatedness between the interacting bacteria (figure 1a). This is expected because greater levels of cooperation are favoured with a higher relatedness. However, appreciable levels of cooperation can be predicted even when relatedness is relatively low.
Result 3. The ESS level of signalling showed a domed relationship with relatedness (figure 1b). At high relatedness, there is a shared interest in cooperation and cheap signalling. At low relatedness, there is no selection for cooperation, and hence no selection for signalling to coordinate this. With intermediate relatedness, there can still be selection to cooperatively produce public goods, but it is in the individual's interest to produce less public goods than the other local cells (because r<1). This favours higher levels of signalling in an attempt to manipulate competitors to cooperate more (which in turn leads to the signal being increasingly ignored). This is termed ‘competitive devaluation of signal strength’.
Figure 1.
Brown and Johnstone's theoretical model of quorum signalling. (a) Cooperation effort increases with increasing relatedness, because the inclusive fitness benefits of cooperation are maximal at high relatedness and minimal at low relatedness. (b) Signalling effort is a dome-shaped function of relatedness, because at low relatedness there is little inclusive fitness benefit to be accrued from organizing a cooperative venture, and at high relatedness there is little conflict so that a cheap signal is favoured, whereas at intermediate relatedness cooperation is worthwhile, yet there is also a scope for conflict, hence a costly signal is required to initiate competition.
(d) Other models of quorum sensing
Brown & Johnstone's (2001) model provides a clear and elegant application of kin selection theory to QS. However, as they stress, it makes many simplifications, the relaxing of which may have important consequences. Furthermore, much more has been learnt about QS since, and we should also consider alternative possible explanations for QS.
Brown & Johnstone's (2001) model could be extended to investigate the consequences of several biological complexities. It has been found that signalling molecules can have multiple functions, and this would alter the relative cost and benefit of their production, as well as how this would vary with the social context. For example, they can also function as antibiotics (Stein 2005), potentially as public goods, such as iron-scavenging molecules (Kaufmann et al. 2005; Diggle et al. 2007) and potent immune modulators (Telford et al. 1998; Tateda et al. 2003; Hooi et al. 2004). Signal molecule production and secretion may also be linked to the production of other molecules through excretion in membrane vesicles (Mashburn & Whiteley 2005). Another possibility is that different types of signal need to be considered, with different costs or specificity. It appears that specificity and cost vary across signals, with cheap-to-produce signals being used very generally across species, and more expensive signals being more specific, within species, possibly even within lineage (Keller & Surette 2006).
Kin selection is not the only possible explanation for cooperation (Sachs et al. 2004; West et al. 2006). An alternative explanation for cooperation is that it provides a direct benefit to the individual that performs the behaviour, which outweighs the cost of performing the behaviour (i.e. it is mutually beneficial, not altruistic; West et al. 2006). An example of this would be if the waste product of one species provided a benefit to individuals of a second species (by-product benefit), and hence the second species could be selected to cooperatively help individuals of the first species in order to increase the by-product benefits (Sachs et al. 2004). It would be extremely interesting to see whether communication between species can be evolutionarily stable in such cases. There are several other forms of direct benefit to cooperation that could be examined from a QS and communication perspective—for example, when cooperation is stabilized between non-relatives by policing or punishment of non-cooperators (Frank 2003).
Another possibility, suggested by Redfield (2002), is that autoinducer molecules are not released to signal other cells. Redfield argues that autoinducer secretion and response may have a more direct benefit, by allowing individual cells to determine how rapidly secreted molecules move away from the cell. This diffusion sensing could allow cells to regulate secretion of public goods to minimize losses owing to extracellular diffusion and mixing. This is an alternative explanation for QS evolution, and diffusion effects could also be incorporated into kin selection models. It should also be considered that production of these molecules may have initially evolved for one reason (e.g. diffusion sensing), but it is now maintained for another (e.g. QS). The hypotheses need not be alternatives, as it may be the case that diffusion-sensing benefits are crucial for the maintenance of this trait, yet they are still monitored for QS purposes. Probably, both functions will be of importance in understanding when and why these molecules are produced.
4. Defining signalling in bacteria
The phrase ‘quorum sensing’ was first termed by Fuqua et al. (1994) and is generally used to describe the phenomenon whereby the accumulation of ‘signalling’ molecules enables a single cell to sense the number of bacteria (cell density) and therefore the population as a whole can make a coordinated response. To date, several classes of extracellular signal molecule have been described in bacteria, including N-acylhomoserine lactones (AHLs; Swift et al. 2001), 2-alkyl-4(1H)-quinolones (AHQs; Déziel et al. 2004; Diggle et al. 2006), cyclic di-peptides (Holden et al. 1999), autoinducer-2 (AI-2; Bassler et al. 1997) and small modified peptides (Novick 2003). However, as discussed earlier, the fact that a compound produced by cell A elicits a response in cell B does not necessarily mean that there is true signalling between the cells and may represent cell B using the molecule as a ‘cue’ or cell A coercing cell B into a certain action. In this section we discuss examples of QS between single populations and mixed populations of bacteria and suggest whether this can be considered as signalling, a response to a cue or coercion (see also Keller & Surette 2006).
Generally communication in bacteria can be divided into three main areas, which are as follows.
Intraspecies. Communication arising or occurring within a single bacterial species.
Interspecies. Communication arising between two or more distinct species of bacteria.
Inter-kingdom. Communication arising between a bacterial species and a higher organism.
(a) Intraspecies communication
In Gram-negative bacteria, the most intensely studied QS systems rely upon the interaction of AHL signal molecules synthesized by LuxI-type AHL synthases with LuxR-type transcriptional regulator proteins. Together, the LuxR–AHL complex activates the expression of specific target genes (Lazdunski et al. 2004). A simple example of this can be seen in the marine bacterium Vibrio fischeri (Nealson et al. 1970). This organism forms a symbiotic relationship with the squid Euprymna scolopes, where it colonizes the light organ (McFall-Ngai & Ruby 2000). At low cell densities, the bacterial population does not luminesce, but at high densities, there is a coordinated switch on of bioluminescence. This production of light has been shown to be mediated by a diffusible AHL molecule (N-(3-oxohexanoyl)homoserine lactone; 3O-C6-HSL) synthesized by the LuxI protein. At a critical concentration, 3O-C6-HSL binds to LuxR and the complex activates expression of the luxCDABE operon resulting in coordinated bioluminescence production (Fuqua et al. 1994). Under laboratory conditions, it is possible to stimulate early induction of bioluminescence simply by providing the cells with exogenous 3O-C6-HSL. It is not clear why V. fischeri cells have a shared interest that favours signalling and cooperation to produce light. The possibilities are a high relatedness between the cells within a light organ or the avoidance of punishment from the host squid if light is not produced (analogous to why rhizobia fix nitrogen for their host plants; West et al. 2002a; Kiers et al. 2003).
As many species of Gram-negative bacteria have been shown to produce AHL signalling molecules, similar examples can also be seen in other species (Swift et al. 2001; Lazdunski et al. 2004). Some bacteria have been shown to regulate production of virulence determinants in a cell density-dependent manner. For example, Erwinia carotovora subsp. carotovora coordinately produces both exoenzymes, which destroy plant tissue, and the antibiotic carbapenem in response to critical concentrations of 3O-C6-HSL (Jones et al. 1993). Similarly, the opportunistic pathogen, Pseudomonas aeruginosa, regulates an arsenal of extracellular virulence factors using a complex hierarchical QS cascade involving two major AHL molecules, namely (N-(3-oxododecanoyl)-l-homoserinelactone (3O-C12-HSL) and N-butanoylhomoserine lactone (C4-HSL; Venturi 2006). In such cases, it is probable that these are examples where QS molecules can be classified as ‘signals’ between cells as the production by cell A has evolved due to its effects on cell B, which in turn has evolved a response to the signal (Maynard Smith & Harper 2003). We suspect that kin selection is the mechanism to explain the evolutionary stability of such signalling, as discussed in §3. Although the AHL family of QS molecules has been described in a wide variety of Gram-negative bacterial species (Lazdunski et al. 2004), crucially they tend to differ between bacterial species. AHLs consist of a conserved homoserine lactone ring connected via an amide bond to an acyl side chain, which can vary in length from 4 to 18 carbons. In addition, these side chains may or may not be modified with a 3-hydroxy or a 3-oxo group, potentially providing a large variety of potential AHL molecules. Many species of bacteria will only respond to their cognate molecule(s), providing a certain degree of specificity, and therefore AHL signalling is generally of an intraspecies nature. Some bacteria, however, are able to ‘exploit’ AHLs produced by another species and this will be discussed later.
While it is plausible to view AHLs as signals between cells of the same species, the situation is often more complicated as some AHLs have been shown to have multiple functions. For example, 3O-C12-HSL produced by P. aeruginosa has been reported to have immunomodulatory properties (Telford et al. 1998; Tateda et al. 2003). It is unlikely that this involves signalling between the host and the bacteria. More probably, this represents 3O-C12-HSL ‘chemically manipulating’ or ‘coercing’ the host immune response to the benefit of the bacterial population.
The world of microbial communication is not limited to Gram-negative bacteria. Gram-positive bacteria also produce QS molecules, but tend to use post-translationally modified autoinducing peptides (AIPs). For example, Staphylococcus aureus uses AIPs to regulate the production of exotoxins in response to a critical concentration of peptide (Novick 2003).
Explaining cooperative signalling at the intraspecies level requires some kind of mechanism. The production of a costly signal for the common good makes this type of communication exploitable by cheaters who do not contribute to signal production, but reap the benefits of QS-mediated behaviour; for example, acquisition of nutrients provided by QS-dependent exoenzyme production. In fact, recent work has shown that many P. aeruginosa clinical isolates are QS defective and make very few virulence factors when grown in the laboratory (Denervaud et al. 2004; Schaber et al. 2004; Lee et al. 2005), suggesting that it may be beneficial not to signal under certain environmental conditions, or that cheaters can invade in long-term infections (West et al. 2006). As local populations of cells are likely to be closely related, then one way that cooperation can be maintained is via kin selection, which requires a sufficiently high relatedness between the cooperating individuals (West et al. 2006). Limited dispersal (population viscosity) would tend to keep relatives together (Hamilton 1964a,b). In this case, indiscriminate altruism may be favoured because neighbours will tend to be relatives (West et al. 2002b). This type of mechanism is likely to be of huge importance in micro-organisms, where asexual reproduction means that single cells colonize and grow in a local area. In this case, the individuals interacting over a small area will be clonal, which can be very conducive to the evolution of cooperation.
(b) Interspecies communication: bacterial ‘crosstalk’
A third class of QS signal molecule was described to be produced by the marine bacterium Vibrio harveyi. Bioluminescence in this organism is cooperatively regulated by AHLs and a molecule termed AI-2, which is a furanosyl borate diester produced by the enzyme LuxS (Chen et al. 2002). The identification of the luxS gene required for the production of AI-2 (Surette et al. 1999) sparked an exponential increase into AI-2 signalling research, the reason being that the luxS gene can be found in a wide variety of bacterial genera (Winzer et al. 2002a,b, 2003).
Importantly, representatives of both Gram-negative and Gram-positive bacteria carry this particular gene and, consequently, AI-2 production has been demonstrated in many species of bacteria. This has led to the hypothesis that AI-2 is employed as interspecies communication or ‘bacterial Esperanto’ (Winans 2002). This idea is difficult to explain from an evolutionary point of view, as cooperation between species is even harder to explain than within species. The major difference is that kin selection, as discussed in §3, will not be important across species. There are mechanisms by which cooperation can be favoured between species, such as by-product benefit (Sachs et al. 2004; Foster & Wenseleers 2006) or to avoid punishment (West et al. 2002a; Kiers et al. 2003), but these are expected to be relatively rare (West et al. 2006).
It must therefore be questioned whether AI-2 can be defined as a true signal. For this to be the case, AI-2 must be (i) diffused from the cell, (ii) taken up by a neighbouring cell, (iii) elicit a response from that cell because the receiver's response has evolved, and (iv) benefit both the producer and the receiver. Clearly, points (i) and (ii) are met with respect to AI-2, but there are major doubts about points (iii) and (iv). Despite AI-2 being produced by many genera, there is very little evidence linking it with direct activation of any specific genes. Studies in many different bacteria have shown that luxS mutants differ phenotypically from wild-type strains; however, this can often be explained owing to a defect in a metabolic pathway. It is now well known that LuxS plays an important role in bacterial metabolism, contributing to the recycling of S-adenosyl-l-methionine, of which AI-2 is a metabolic by-product (Winzer et al. 2002b). To date, only bioluminescence in V. harveyi (Surette et al. 1999) and an ABC transporter in Salmonella typhimurium (termed Lsr; Taga et al. 2001) have been shown to be regulated by AI-2. In these species, we can speculate that AI-2 may be used as a cooperative signal in an intraspecies context. Theoretically, these species could also use AI-2 from other organisms to regulate these respective traits. In this case, however, it is inaccurate to use the term interspecies signalling as the receiver's response has not evolved in parallel with the producing bacterial species. In this scenario, we can say that both V. harveyi and S. typhimurium use the metabolic by-product AI-2 as an environmental cue to regulate gene expression. Interspecies signalling has also been suggested between avirulent oropharyngeal flora (OF; AI-2-positive) and P. aeruginosa (luxS and AI-2-negative) within the cystic fibrosis (CF) lung (Duan et al. 2003). Co-incubation of P. aeruginosa with OF bacteria resulted in an increase in virulence gene expression, which was attributed, at least in part, to AI-2. The mechanism for this is unknown as P. aeruginosa does not make AI-2, but we suggest that this is not an example of interspecies signalling. It is more probable that P. aeruginosa is able to use AI-2 as a cue, perhaps to assess its surroundings, or it may be that OF bacteria ‘coerce’ or manipulate P. aeruginosa into increased virulence, which may provide them with more nutrients.
Interspecies signalling between bacterial species using AHL molecules has also been suggested. P. aeruginosa and Burkholderia cepacia often occur together in the lungs of people with CF, where they are associated with high morbidity and mortality (Govan & Deretic 1996; Eberl & Tummler 2004). B. cepacia has been shown to upregulate production of virulence determinants in response to AHLs produced by P. aeruginosa, although this does not appear to happen the other way round. This type of behaviour has also been termed ‘bacterial crosstalk’, which is suggestive of a cooperative venture between two or more species. In this case, it suggests that B. cepacia uses P. aeruginosa AHLs as a cue to alter its behaviour rather than there being signalling between the two bacteria. P. aeruginosa pays the cost of producing the AHLs, possibly for within species signalling, but appears to gain no benefit from B. cepacia in return.
(c) Communication across the prokaryote/eukaryote division
Several recent reports have demonstrated that bacterial QS molecules (specifically AHLs) can affect gene expression in eukaryotes, as many eukaryotic hormones structurally resemble AHLs. This has been generally termed inter-kingdom signalling or global sensing (Shiner et al. 2005). AHL molecules have been experimentally demonstrated to affect a number of animal cell types, including murine and human primary cells (Telford et al. 1998), breast cancer cells (Li et al. 2004), bone marrow macrophages (Tateda et al. 2003) and primary porcine arterial smooth muscle cells (Lawrence et al. 1999). In addition, plant behaviour has also been shown to be modified by AHLs. The zoospores of the seaweed Enteromorpha have been shown to settle preferentially to AHL-producing biofilms of the marine bacterium Vibrio anguillarum (Joint et al. 2002). Furthermore, higher organisms have mechanisms that appear to downregulate QS in micro-organisms. For example, the marine red alga Delisea pulchra produces a halogenated furanone that disrupts QS in several species of bacteria, including the swarming motility of Serratia liquefaciens (Givskov et al. 1996). This furanone has also been shown to disrupt P. aeruginosa biofilms (Hentzer et al. 2002). These AHL ‘mimics’ attract interest as possible alternatives to antibiotic therapy. Whether these examples demonstrate signalling using small molecules between prokaryotes and eukaroytes is open to debate. In general, studies performed to date appear to show that either (i) the signalling bacterium manipulates or coerces its host into a certain action rather than there being a truly evolved signalling system between the two or (ii) as in the example of the zoospore settlement, the eukaryote uses bacterial AHLs as an environmental cue as a guide to future action.
5. Conclusions
Our aim has been to integrate the evolutionary literature on animal signalling with the microbiological literature on QS. We suspect that many cases of QS within species will represent signalling. This is because the natural history of many micro-organisms means that the interactions will be between relatives, in which case signalling and cooperation can be favoured by kin selection. In contrast, we suspect that most cases of QS between bacterial species or across kingdoms will represent cues or coercion. Although signalling and cooperation can be favoured between species, this requires very special conditions that are likely to be only rarely met. However, perhaps the most important point is that this is speculation—the major task for the future is experimental studies that determine the costs and benefits of communication to both the sender and the responder.
Acknowledgments
We would like to thank Klaus Winzer and Steve Atkinson for their useful comments and suggestions. We would also like to gratefully acknowledge the Royal Society as S.P.D., S.A.W., A.G. and A.S.G. are all supported by Royal Society University Research Fellowships.
Footnotes
One contribution of 12 to a Theme Issue ‘Bacterial conversations: talking, listening and eavesdropping’.
References
- Bassler B.L, Greenberg E.P, Stevens A.M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 1997;179:4043–4045. doi: 10.1128/jb.179.12.4043-4045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.P. Cooperation and conflict in host-manipulating parasites. Proc. R. Soc. B. 1999;266:1899–1904. doi:10.1098/rspb.1999.0864 [Google Scholar]
- Brown S.P, Johnstone R.A. Cooperation in the dark: signalling and collective action in quorum-sensing bacteria. Proc. R. Soc. B. 2001;268:961–965. doi: 10.1098/rspb.2001.1609. doi:10.1098/rspb.2001.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler B.L, Hughson F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. doi:10.1038/415545a [DOI] [PubMed] [Google Scholar]
- Ciofu O, Beveridge T.J, Kadurugamuwa J, Walther-Rasmussen J, Hoiby N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000;45:9–13. doi: 10.1093/jac/45.1.9. doi:10.1093/jac/45.1.9 [DOI] [PubMed] [Google Scholar]
- Crespi B.J. The evolution of social behavior in microorganisms. Trends Ecol. Evol. 2001;16:178–183. doi: 10.1016/s0169-5347(01)02115-2. doi:10.1016/S0169-5347(01)02115-2 [DOI] [PubMed] [Google Scholar]
- Daniels R, Vanderleyden J, Michiels J. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev. 2004;28:261–289. doi: 10.1016/j.femsre.2003.09.004. doi:10.1016/j.femsre.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Denervaud V, TuQuoc P, Blanc D, Favre-Bonte S, Krishnapillai V, Reimmann C, Haas D, van Delden C. Characterization of cell-to-cell signaling-deficient Pseudomonas aeruginosa strains colonizing intubated patients. J. Clin. Microbiol. 2004;42:554–562. doi: 10.1128/JCM.42.2.554-562.2004. doi:10.1128/JCM.42.2.554-562.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déziel E, Lépine F, Milot S, He J.X, Mindrinos M.N, Tompkins R.G, Rahme L.G. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl Acad. Sci. USA. 2004;101:1339–1344. doi: 10.1073/pnas.0307694100. doi:10.1073/pnas.0307694100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle S.P, Cornelis P, Williams P, Camara M. 4-Quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int. J. Med. Microbiol. 2006;296:83–91. doi: 10.1016/j.ijmm.2006.01.038. doi:10.1016/j.ijmm.2006.01.038 [DOI] [PubMed] [Google Scholar]
- Diggle S.P, et al. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multi-functional roles in quorum sensing and iron entrapment. Chem. Biol. 2007;14:87–96. doi: 10.1016/j.chembiol.2006.11.014. doi:10.1016/j.chembiol.2006.11.014 [DOI] [PubMed] [Google Scholar]
- Dinges M.M, Orwin P.M, Schlievert P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio F, Gordo I. The tragedy of the commons, the public goods dilemma, and the meaning of rivalry and excludability in evolutionary biology. Evol. Ecol. Res. 2006;8:321–332. [Google Scholar]
- Duan K.M, Dammel C, Stein J, Rabin H, Surette M.G. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 2003;50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. doi:10.1046/j.1365-2958.2003.03803.x [DOI] [PubMed] [Google Scholar]
- Dugatkin L.A, Perlin M, Lucas J.S, Atlas R. Group-beneficial traits, frequency-dependent selection and genotypic diversity: an antibiotic resistance paradigm. Proc. R. Soc. B. 2005;272:79–83. doi: 10.1098/rspb.2004.2916. doi:10.1098/rspb.2004.2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl L, Tummler B. Pseudomonas aeruginosa and Burkholderia cepacia in cystic fibrosis: genome evolution, interactions and adaptation. Int. J. Med. Microbiol. 2004;294:123–131. doi: 10.1016/j.ijmm.2004.06.022. doi:10.1016/j.ijmm.2004.06.022 [DOI] [PubMed] [Google Scholar]
- Foster K.R, Wenseleers T. A general model for the evolution of mutualisms. J. Evol. Biol. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. doi:10.1111/j.1420-9101.2005.01073.x [DOI] [PubMed] [Google Scholar]
- Frank S.A. Perspective: repression of competition and the evolution of cooperation. Evolution. 2003;57:693–705. doi: 10.1111/j.0014-3820.2003.tb00283.x. doi:10.1554/0014-3820(2003)057[0693:PROCAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fuqua W.C, Winans S.C, Greenberg E.P. Quorum sensing in bacteria—the LuxR–LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givskov M, DeNys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P.D, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J.R.W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig D, Travisano M. The Prisoner's Dilemma and polymorphism in yeast SUC genes. Proc. R. Soc. B. 2004;271:S25–S26. doi: 10.1098/rsbl.2003.0083. doi:10.1098/rsbl.2003.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A.S, West S.A, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. doi:10.1038/nature02744 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Evolution of altruistic behavior. Am. Nat. 1963;97:354–356. doi:10.1086/497114 [Google Scholar]
- Hamilton W.D. Genetical evolution of social behaviour I. J. Theor. Biol. 1964a;7:1–16. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Genetical evolution of social behaviour II. J. Theor. Biol. 1964b;7:17–52. doi: 10.1016/0022-5193(64)90039-6. doi:10.1016/0022-5193(64)90039-6 [DOI] [PubMed] [Google Scholar]
- Hardin G. Tragedy of commons. Science. 1968;162:1243–1248. doi:10.1126/science.162.3859.1243 [PubMed] [Google Scholar]
- Hentzer M, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- Holden M.T.G, et al. Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol. Microbiol. 1999;33:1254–1266. doi: 10.1046/j.1365-2958.1999.01577.x. doi:10.1046/j.1365-2958.1999.01577.x [DOI] [PubMed] [Google Scholar]
- Hooi D.S.W, Bycroft B.W, Chhabra S.R, Williams P, Pritchard D.I. Differential immune modulatory activity of Pseudomonas aeruginosa quorum-sensing signal molecules. Infect. Immun. 2004;72:6463–6470. doi: 10.1128/IAI.72.11.6463-6470.2004. doi:10.1128/IAI.72.11.6463-6470.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint I, Tait K, Callow M.E, Callow J.A, Milton D, Williams P, Camara M. Cell-to-cell communication across the prokaryote–eukaryote boundary. Science. 2002;298:1207–1207. doi: 10.1126/science.1077075. doi:10.1126/science.1077075 [DOI] [PubMed] [Google Scholar]
- Jones S, et al. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G.F, et al. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl Acad. Sci. USA. 2005;102:309–314. doi: 10.1073/pnas.0408639102. doi:10.1073/pnas.0408639102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L, Surette M.G. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 2006;4:249–258. doi: 10.1038/nrmicro1383. doi:10.1038/nrmicro1383 [DOI] [PubMed] [Google Scholar]
- Kiers E.T, Rousseau R.A, West S.A, Denison R.F. Host sanctions and the legume-rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. doi:10.1038/nature01931 [DOI] [PubMed] [Google Scholar]
- Kolenbrander P.E, Andersen R.N, Blehert D.S, Egland P.G, Foster J.S, Palmer R.J. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. doi:10.1128/MMBR.66.3.486-505.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R.N, Dunn W.R, Bycroft B, Camara M, Chhabra S.R, Williams P, Wilson V.G. The Pseudomonas aeruginosa quorum-sensing signal molecule, N-(3-oxododecanoyl)-l-homoserine lactone, inhibits porcine arterial smooth muscle contraction. Br. J. Pharmacol. 1999;128:845–848. doi: 10.1038/sj.bjp.0702870. doi:10.1038/sj.bjp.0702870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski A.M, Ventre I, Sturgis J.N. Regulatory circuits and communication in gram-negative bacteria. Nat. Rev. Microbiol. 2004;2:581–592. doi: 10.1038/nrmicro924. doi:10.1038/nrmicro924 [DOI] [PubMed] [Google Scholar]
- Lee B, Haagensen J.A.J, Ciofu O, Andersen J.B, Hoiby N, Molin S. Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J. Clin. Microbiol. 2005;43:5247–5255. doi: 10.1128/JCM.43.10.5247-5255.2005. doi:10.1128/JCM.43.10.5247-5255.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hooi D, Chhabra S.R, Pritchard D, Shaw P.E. Bacterial N-acylhomoserine lactone-induced apoptosis in breast carcinoma cells correlated with down-modulation of STAT3. Oncogene. 2004;23:4894–4902. doi: 10.1038/sj.onc.1207612. doi:10.1038/sj.onc.1207612 [DOI] [PubMed] [Google Scholar]
- Mashburn L.M, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. doi:10.1038/nature03925 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J, Harper D. Oxford University Press; New York, NY: 2003. Animal signals. [Google Scholar]
- Maynard Smith J, Price G.R. Logic of animal conflict. Nature. 1973;246:15–18. doi:10.1038/246015a0 [Google Scholar]
- Maynard Smith J, Szathmary E. W. H. Freeman; Oxford, UK: 1995. The major transitions in evolution. [Google Scholar]
- McFall-Ngai M.J, Ruby E.G. Developmental biology in marine invertebrate symbioses. Curr. Opin. Microbiol. 2000;3:603–607. doi: 10.1016/s1369-5274(00)00147-8. doi:10.1016/S1369-5274(00)00147-8 [DOI] [PubMed] [Google Scholar]
- Nealson K.H, Platt T, Hastings J.W. Cellular control of synthesis and activity of bacterial luminescent system. J. Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R.P. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. doi:10.1046/j.1365-2958.2003.03526.x [DOI] [PubMed] [Google Scholar]
- Parsek M.R, Greenberg E.P. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. doi:10.1016/j.tim.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Redfield R.J. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002;10:365–370. doi: 10.1016/s0966-842x(02)02400-9. doi:10.1016/S0966-842X(02)02400-9 [DOI] [PubMed] [Google Scholar]
- Riley M.A, Wertz J.E. Bacteriocin diversity: ecological and evolutionary perspectives. Biochimie. 2002;84:357–364. doi: 10.1016/s0300-9084(02)01421-9. doi:10.1016/S0300-9084(02)01421-9 [DOI] [PubMed] [Google Scholar]
- Sachs J.L, Mueller U.G, Wilcox T.P, Bull J.J. The evolution of cooperation. Q. Rev. Biol. 2004;79:135–160. doi: 10.1086/383541. doi:10.1086/383541 [DOI] [PubMed] [Google Scholar]
- Schaber J.A, Carty N.L, McDonald N.A, Graham E.D, Cheluvappa R, Griswold J.A, Hamood A.N. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 2004;53:841–853. doi: 10.1099/jmm.0.45617-0. doi:10.1099/jmm.0.45617-0 [DOI] [PubMed] [Google Scholar]
- Shiner E.K, Rumbaugh K.P, Williams S.C. Interkingdom signaling: deciphering the language of acyl homoserine lactones. FEMS Microbiol. Rev. 2005;29:935–947. doi: 10.1016/j.femsre.2005.03.001. doi:10.1016/j.femsre.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. doi:10.1111/j.1365-2958.2005.04587.x [DOI] [PubMed] [Google Scholar]
- Surette M.G, Miller M.B, Bassler B.L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl Acad. Sci. USA. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. doi:10.1073/pnas.96.4.1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Downie J.A, Whitehead N.A, Barnard A.M.L, Salmond G.P.C, Williams P. Advances in microbial physiology. vol. 45. Academic Press; London: 2001. Quorum sensing as a population-density-dependent determinant of bacterial physiology. pp. 199–270. [DOI] [PubMed] [Google Scholar]
- Taga M.E, Semmelhack J.L, Bassler B.L. The LuxS-dependent autoinducer Al-2 controls the expression of an ABC transporter that functions in Al-2 uptake in Salmonella typhimurium. Mol. Microbiol. 2001;42:777–793. doi: 10.1046/j.1365-2958.2001.02669.x. doi:10.1046/j.1365-2958.2001.02669.x [DOI] [PubMed] [Google Scholar]
- Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere J.C, Standiford T.J, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 2003;71:5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. doi:10.1128/IAI.71.10.5785-5793.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford G, Wheeler D, Williams P, Tomkins P.T, Appleby P, Sewell H, Stewart G, Bycroft B.W, Pritchard D.I. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3- oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 1998;66:36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer G.J, Yu Y.T.N. Evolution of novel cooperative swarming in the bacterium Myxococcus xanthus. Nature. 2003;425:75–78. doi: 10.1038/nature01908. doi:10.1038/nature01908 [DOI] [PubMed] [Google Scholar]
- Venturi V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 2006;30:274–291. doi: 10.1111/j.1574-6976.2005.00012.x. doi:10.1111/j.1574-6976.2005.00012.x [DOI] [PubMed] [Google Scholar]
- Webb J.S, Givskov M, Kjelleberg S. Bacterial biofilms: prokaryotic adventures in multicellularity. Curr. Opin. Microbiol. 2003;6:578–585. doi: 10.1016/j.mib.2003.10.014. doi:10.1016/j.mib.2003.10.014 [DOI] [PubMed] [Google Scholar]
- West S.A, Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proc. R. Soc. B. 2003;270:37–44. doi: 10.1098/rspb.2002.2209. doi:10.1098/rspb.2002.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.A, Kiers E.T, Simms E.L, Denison R.F. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc. R. Soc. B. 2002a;269:685–694. doi: 10.1098/rspb.2001.1878. doi:10.1098/rspb.2001.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.A, Pen I, Griffin A.S. Conflict and cooperation—cooperation and competition between relatives. Science. 2002b;296:72–75. doi: 10.1126/science.1065507. doi:10.1126/science.1065507 [DOI] [PubMed] [Google Scholar]
- West, S. A., Griffin, A. S., Gardner, A. & Diggle, S. P. 2006 Social evolution theory for microorganisms. Nat. Rev. Microbiol 4, 597–607. [DOI] [PubMed]
- West S.A, Griffin A.S, Gardner A. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 2007 doi: 10.1111/j.1420-9101.2006.01258.x. doi:10.1111/j.1420-9101.2006.01258.x [DOI] [PubMed] [Google Scholar]
- Williams P, et al. Quorum sensing and the population-dependent control of virulence. Phil. Trans. R. Soc. B. 2000;355:667–680. doi: 10.1098/rstb.2000.0607. doi:10.1098/rstb.2000.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S.C. Bacterial Esperanto. Nat. Struct. Biol. 2002;9:83–84. doi: 10.1038/nsb0202-83. doi:10.1038/nsb0202-83 [DOI] [PubMed] [Google Scholar]
- Winzer K, et al. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology. 2002a;148:909–922. doi: 10.1099/00221287-148-4-909. [DOI] [PubMed] [Google Scholar]
- Winzer K, Hardie K.R, Williams P. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch! Curr. Opin. Microbiol. 2002b;5:216–222. doi: 10.1016/s1369-5274(02)00304-1. doi:10.1016/S1369-5274(02)00304-1 [DOI] [PubMed] [Google Scholar]
- Winzer K, Hardie K.R, Williams P. Advances in applied microbiology. vol. 53. Academic Press; London: 2003. LuxS and autoinducer-2: their contribution to quorum sensing and metabolism in bacteria. pp. 291–396. [DOI] [PubMed] [Google Scholar]