Abstract
We examined the cardioprotective profile of the new A3 adenosine receptor (AR) agonist CP-532,903 in an in vivo mouse model of infarction and an isolated heart model of global ischemia/reperfusion injury. In radioligand binding and cAMP accumulation assays using HEK 293 cells expressing recombinant mouse ARs, CP-532,903 was found to bind with high affinity to mouse A3ARs (ki = 9.0±2.5 nM) and with high selectivity versus mouse A1AR (100-fold) and A2AARs (1,000-fold). In in vivo ischemia/reperfusion experiments, pretreating mice with 30 or 100 µg/kg of CP-532,903 reduced infarct size from 59.2 ± 2.1% of the risk region in vehicle-treated mice to 42.5 ± 2.3% and 39.0 ± 2.9%, respectively. Similarly, treating isolated mouse hearts with CP-532,903 (10, 30, or 100 nM) concentration-dependently improved recovery of contractile function following 20 min of global ischemia and 45 min of reperfusion, including developed pressure and ±dP/dt. In both models of ischemia/reperfusion injury, CP-532,903 provided no benefit in studies using mice with genetic disruption of the A3AR gene (A3KO mice). In isolated heart studies, protection provided by CP-532,903 and ischemic preconditioning induced by 3 brief ischemia/reperfusion cycles were lost in Kir6.2 KO mice lacking expression of the pore-forming subunit of the sarcolemmal ATP-sensitive potassium (KATP) channel. Whole-cell patch clamp recordings provided evidence that the A3AR is functionally coupled to the sarcolemmal KATP channel in murine cardiomyocytes. We conclude that CP-532,903 is a highly selective agonist of the mouse A3AR that protects against ischemia/reperfusion injury by activating sarcolemmal KATP channels.
Introduction
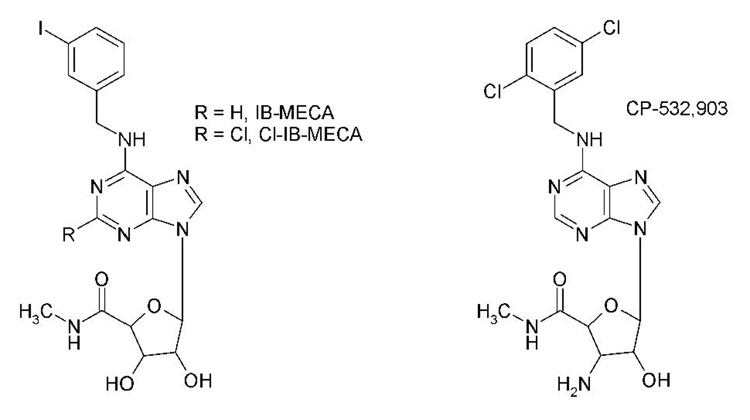
A3 adenosine receptor (AR) agonists have been shown to effectively limit infarct size and reduce contractile dysfunction in several different animal models of ischemia/reperfusion injury (Auchampach et al., 1997b; Tracey et al., 1997; Tracey et al., 1998; Jordan et al., 1999; Thourani et al., 1999; Auchampach et al., 2003; Tracey et al., 2003; Ge et al., 2004; Ge et al., 2006). A3AR agonists are attractive as cardioprotective agents, since they do not alter systemic hemodynamic parameters in non-rodent species and are effective if administered prior to the ischemic event or only during reperfusion (Auchampach et al., 1997b; Tracey et al., 1997; Tracey et al., 1998; Jordan et al., 1999; Thourani et al., 1999; Auchampach et al., 2003; Tracey et al., 2003; Ge et al., 2004; Ge et al., 2006). The most widely available A3AR agonists that have been tested in experimental animal models of ischemia/reperfusion injury include the N6-benzyladenosine-5’-N-methylcarboxamide derivative IB-MECA [N6-(3-iodobenzyl)adenosine-5’-Nmethyluronamide] and its 2-chloro derivative Cl-IB-MECA (Figure 1). While IB-MECA and Cl-IB-MECA are potent A3AR agonists, they exhibit moderate selectivity. In radioligand binding assays, the selectivity of IB-MECA and Cl-IBMECA has been reported to range from 6-2,500-fold versus the A1AR and 4-1,400-fold versus the A2AAR, depending on the species and the assay conditions (Gallo-Rodriguez et al., 1994; Kim et al., 1994; Hill et al., 1997; Takano et al., 2001; Murphree et al., 2002).
Figure 1.
Chemical structures of IB-MECA, Cl-IB-MECA, and CP-532,903.
In the present investigation, we characterized the cardioprotective profile of the A3AR agonist CP-532,903 in an isolated mouse heart model of global ischemia and reperfusion and an in vivo mouse model of infarction. The goal of this work was to examine the cardioprotective effectiveness of CP-532,903 and to confirm whether it mediates cardioprotection via the A3AR. A second goal of this investigation was to determine whether A3AR activation provides ischemic protection by facilitating opening of the sarcolemmal isoform of the ATP sensitive potassium (KATP) channel. This second question was addressed using Kir6.2 gene knock-out (KO) mice lacking the pore-forming subunit of the sarcolemmal KATP channel (Suzuki et al., 2002).
Materials and Methods
Materials
Cell culture reagents, G418, and Lipofectamine were purchased from Invitrogen (Carlsbad, CA). CP-532,903 was provided by Pfizer Inc. (Groton, CT), adenosine deaminase and Liberase Blendzyme I were from Roche Applied Biosciences (Indianapolis, IN), and all other drugs and reagents were purchased from Sigma-Aldrich (St. Louis, MO). Stably transfected human embryonic kidney (HEK 293) cells expressing mouse ARs and N6-(4-amino–3-([125I]iodobenzyl)-adenosine-5’-N-methylcarboxamide ([125I]I-AB-MECA) were prepared, as described previously (Auchampach et al., 1997a; Auchampach et al., 1997b; Kreckler et al., 2006). cAMP and histamine radioimmunoassay kits were obtained from GE Healthcare (Piscataway, NJ) and Immunotech (Marseille, France).
Animals
All experiments were performed with 10- to 14-week old male mice weighing ~24–32 g. Wild-type C57BL/6 mice were purchased from Taconic Farms Inc. (Germantown, NY). A3KO mice were provided by Dr. Marlene Jacobson (Merck Research Laboratories, West Point, PA). Kir6.2 KO mice were provided by Dr. Susumu Seino (Kobe University, Kobe, Japan) and were generated by Dr. Aaron Fisher (University of Pennsylvania), as previously described (Miki et al., 1998). All animals used in the study received humane care in accordance with the guidelines established by the Medical College of Wisconsin, which conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Radioligand Binding Assays
Competition radioligand binding assays were conducted with membranes prepared from HEK 293 cells expressing recombinant mouse A1 or A3ARs using the agonist radioligand [125I]I-AB-MECA (Auchampach et al., 1997a; Auchampach et al., 1997b; Kreckler et al., 2006). Incubations were conducted in 100 µl buffer (10 mM Na-HEPES [pH 7.4], 1 mM EDTA, 5 mM MgCl2, 1 U/ml adenosine deaminase) with ~0.3 nM [125I]I-AB-MECA and competitors at room temperature for three hours, after which bound and free radioligand were separated by filtration over GF/C grade glass fiber filters (Brandel, Gaithersburg, MD). Non-specific binding was defined by the presence of 100 µM adenosine-5’-N-ethylcarboxamide (NECA) in the assays. Ki values for high affinity agonist binding were calculated as previously described (Auchampach et al., 1997a; Auchampach et al., 1997b; Kreckler et al., 2006).
cAMP Assays
HEK 293 cells expressing recombinant mouse ARs were detached using PBS containing 5 mM EDTA and re-suspended at 50,000 cells/tube in DMEM with 25 mM HEPES (pH 7.4), 1 U/ml adenosine deaminase, and 20 µM Ro 20,1724 to inhibit phosphodiesterases. Cells were incubated with agonists for 15 minutes at 37°C with shaking. Reactions were terminated by addition of 0.15 N HCl. [cAMP] in the acid extract was determined by radioimmunoassay. MRS 1754 (1 µM) was included in assays with HEK 293 cells expressing A2A or A3ARs to block endogenous A2BARs expressed in HEK 293 cells.
Langendorff-Perfused Mouse Heart Model
Experimental Preparation
Male mice (10 to 12 weeks of age, 23.7 ± 0.4 g body weight) were anesthetized with sodium pentobarbital (100 mg/kg ip). As soon as deep anesthesia was achieved, the hearts were removed and arrested in ice-cold perfusion solution. The hearts were cannulated via the aorta and perfused retrogradely by the Langendorff method at a continuous pressure of 80 mmHg using Krebs-Henseleit buffer containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 0.5 mM EDTA, 25 mM NaHCO3, and 11 mM glucose. The buffer was equilibrated with 95% O2/5% CO2 at 37°C to maintain the pH at 7.4 and filtered through an in-line Sterivex filter unit (0.22 µm; Millipore, Bedford, MA) to remove particulate matter. For each heart, the left ventricle (LV) was drained by inserting a short polyethylene tube through the apex, and a fluid-filled balloon connected to a pressure transducer was inserted into the left ventricle via the mitral valve. The balloon was connected to a pressure transducer (ADInstruments, Colorado Springs, CO) for continuous measurement of left ventricular pressure. The hearts were immersed in perfusion buffer maintained at 37° C, and the balloons were inflated to achieve end-diastolic pressures of 5 to 10 mmHg. Coronary flows were monitored by an in-line flow probe connected to a flow meter (Model T206; Transonics Systems Inc., Ithaca, NY). The left ventricular pressure signals were acquired continuously using a PowerLab data acquisition system (ADInstruments) and processed (Chart software) to yield heart rates and left ventricular dP/dts.
Global Ischemia/Reperfusion Protocol
Hearts were perfused for 20 min to allow for stabilization, and then perfused for an additional 15 min while pacing at 420 beats per min (ventricular pacing with 2 ms square waves at a, voltage of 20% above threshold). Baseline functional measurements were acquired immediately before subjecting the hearts to 20 min of normothermic no-flow ischemia and 45 min of reperfusion achieved by closing and opening an in-line stopcock. To examine the effect of CP-532,903 on functional recovery, hearts were perfused with buffer containing the indicated concentrations of agonists for 10 min before ischemia. In studies of ischemic preconditioning (IPC), the hearts were subjected to 3 cycles of 3-min occlusion/2-min reperfusion prior to the 20-min occlusion.
In vivo Mouse Model of Infarction
Experimental Preparation
Male mice were anesthetized with sodium pentobarbital (100 mg/kg ip) and prepared for surgery, as described previously (Black et al., 2002; Ge et al., 2006). Briefly, the mice were intubated using PE-60 tubing, ventilated (model 845; Hugo Sachs Elektronic, Hugsteten, Germany) at a rate of 100–110 beats/min and a tidal volume of 200–250 µL using room air supplemented with oxygen, and fitted with electrodes to obtain the electrocardiogram (ECG) using the limb lead II configuration. The heart was exposed via a lateral incision at the level of the 4th intercostal space and a ligature (8–0 nylon suture) was placed around the left coronary artery ~1–3 mm from the tip of the left atria. The ligature was used to induce coronary occlusion and reperfusion by gently tightening the snare around a piece of wetted gauze. Approximately 15 min after completing the ischemia/reperfusion protocol, the chest cavity was closed using 6–0 polypropylene suture, and the mice were allowed to recover.
Ischemia/Reperfusion Protocol
After a 30-min stabilization period following surgery, all mice were subjected to 30 min of LAD occlusion and 24 h of reperfusion. Successful performance of occlusion and reperfusion was verified by visual inspection (i.e., change in color of the ischemic myocardium) and by changes in the ECG. CP-532,903 or equivalent vehicle was administered as an i.v. bolus 10 min before the coronary artery occlusion. Heart rate was monitored at baseline and during the 30-min occlusion period from the ECG recording.
Measurement of Ischemic Area and Infarct Size
After 24 h of reperfusion, infarct size was measured by dual staining with phthalo blue dye and triphenyltetrazolium chloride (Black et al., 2002; Ge et al., 2006). To delineate the ischemic area at risk, the LAD was re-occluded in situ while a 5% solution of phthalo blue dye was injected into the aortic root. To delineate infarcted tissue, the left ventricle was sliced into five to six transverse sections and stained for exactly 10 min in a solution of 1% triphenyltetrazolium chloride (TTC) at 37° C. TTC stains viable tissue red leaving infarcted tissue unstained. The slices were weighed and photographed from both sides using a SPOT Insight digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI). The left ventricular, ischemic, and infarcted areas were measured by digital planimetry. Infarct size is presented as a percentage of the ischemic risk region.
Measurement of Systemic Blood Pressure and Plasma Histamine Levels
Parallel studies with separate groups of mice were conducted to examine the effect of CP-532,903 on systemic blood pressure and plasma histamine levels. Blood pressure was measured in pentobarbital-anesthetized mice via a catheter inserted into the left femoral artery, and plasma histamine levels were measured by ELISA (Immuno-Biological Laboratories, Hamburg, Germany) from blood samples obtained by cardiac puncture (Ge et al., 2006).
Electrophysiology
Mouse Cardiomyocyte Isolation
Myocytes from adult mouse hearts were isolated according to methods established by the Alliance for Cellular Signaling (www.signaling-gateway.org; protocol #PP00000015). Briefly, hearts were excised from pentobarbital-anesthetized mice, cannulated via the aorta onto a blunted needle, and perfused for 10 min with warmed (37° C) perfusion buffer (113 mM NaCl, 4.7 mM KCl, 0.6 mM KH2PO4, 0.6 mM Na2HPO4, 1.2 mM MgSO4-7H2O, 0.032 mM phenol red, 12 mM NaHCO3, 10 mM KHCO3, 10 mM HEPES [pH 7.4], 30 mM taurine, and 10 mM 2,3-butanedione monoxime, 5.5 mM glucose) containing 0.25 mg/ml Liberase blendzyme I, 0.14 mg/ml trypsin, and 12.5 µM CaCl2. Following perfusion, the ventricles were dissected free from the hearts and repeatedly passed through a plastic transfer pipette to disaggregate the cells into a single-cell suspension. Subsequently, myocytes were enriched by sedimentation in perfusion buffer containing 5% bovine calf serum while slowly exposing the cells to increasing concentrations of CaCl2 to achieve a final concentration of 1.2 mM. The final cell pellet containing calcium-tolerant myocytes was resuspended in MEM culture media containing Hank’s salts, 2 mM L-glutamine, 5% bovine calf serum, 10 mM 2,3-butanedione monoxime, and 100 U/ml penicillin.
Solutions
Myocytes were placed in an external bath solution containing 5 mM KCl, 132 mM N-methyl-D-glucamine, 1 mM CaCl2, 2 mM MgCl2, 5 mM 4-aminopyridine, and 10 mM HEPES with pH adjusted to 7.4 with HCl. Pinacidil (KATP channel opener), glibenclamide, (KATP channel antagonist), 8-cyclopentyl-1,3-dipropylxanthine (CPX; A1AR antagonist), ZM 241385 (A2AAR antagonist), and CP-532,903 were all prepared as stock solutions in dimethyl sulfoxide (DMSO) and diluted to the desired concentrations before use. The final concentration of DMSO was <0.05%. The pipette solution contained 60 mM K-glutamate, 50 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 0.01 K2-ATP, 11 mM EGTA, and10 mM HEPES with pH adjusted to 7.4 with KOH. For the experiments investigating coupling between the A3AR and the KATP channel, 0.2 mM K2-ATP and 0.5 mM Na-GTP were included in the pipette solution.
Recording Procedure and Data Analysis
ATP-sensitive potassium current, IKATP, was recorded using the whole-cell configuration of the patch clamp technique. Pipettes were pulled from borosilicate glass capillary tubes (Garner Glass, Claremont, CA) using a horizontal two-stage puller (Sachs-Flaming P-97; Sutter Instruments, Novato, CA) and heat polished (microforge MF-830, Narishige, Tokyo, Japan). In standard solutions, the pipette resistance ranged from 3–5 MΩ. Current was measured using a patch clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA) interfaced to a computer via a digitizer (Digidata 1200A, Axon Instruments). Data acquisition and analysis were conducted using the pClamp software package version 9.0 (Axon Instruments). Additional analyses were performed on Origin version 7 (OriginLab, Northampton, MA).
Whole-cell IKATP was monitored during 100-ms test pulses from −120 to +60 mV from a holding potential of −40 mV. Current-voltage relationships were obtained from current amplitude measured at the end of the test pulse and plotted against membrane potential
Mitochondrial Isolation and Functional Assays
Cardiac mitochondria were isolated from adult mouse hearts using the MITOISO1 kit (Sigma-Aldrich). Respiration of isolated mitochondria (1 mg/ml) was monitored at 30° C with an oxygen electrode (Hansatech Instruments Ltd., Norfolk, United Kingdom) in respiration buffer containing 130 mM KCl, 5 mM K2HPO4, 20 mM MOPS (pH = 7.2), 2.5 mM EGTA, 0.001 mM Na4P2O7, 0.1% bovine serum albumin. State 2 respiration was stimulated with a combination of pyruvate and malate (5 M each) as substrates. ADP-stimulated state 3 respiration was measured in the presence of 250 µM ADP, and state 4 respiration was measured after added ADP was consumed. The respiratory control ratio (RCR) was calculated as a ratio of the state 3 rate divided by the state 4 rate.
ATP synthesis rates by isolated mitochondria was assessed using a chemiluminescence-based method utilizing firefly luciferase and luciferin (ATP Determination Kit from Invitrogen, Carlsbad, CA), as described previously (Ljubkovic et al., 2007). The reaction solution contained respiration buffer, 0.2 µM diadenosine pentaphosphate, 5 mM pyruvate, 5 mM malate, 2 mg/L mitochondria, 0.1 g/L luciferin, and 1.25 mg/L luciferase. The reaction was initiated by the addition of 30 µM ADP (made ATP-free by hexokinase treatment). Blanks were obtained through measurements in the absence of substrates. Chemiluminescence was monitored in a Modulus Luminometer (Turner Biosystems, Sunnyvale, CA) at room temperature for 120 seconds. The rate of mitochondrial ATP production was calculated from standard curves generated with defined ATP concentrations.
Data Analysis
All data are reported as means ± S.E.M. In vivo blood pressure measurements, plasma histamine concentration, and left ventricular recovery of developed pressure were analyzed by two-way repeated measures ANOVA (time and treatment) to determine whether there was a main effect of time, a main effect of treatment or a time-treatment interaction. If global tests showed a main effect or interaction, post-hoc analysis was performed using unpaired or paired analyses, as appropriate. Infarct size, risk region size, and left ventricular functional recoveries at 45 min of reperfusion in the isolated heart studies were compared using oneway ANOVA followed by Student’s t test with the Bonferroni correction or an unpaired t test, as appropriate.
Results
Radioligand Binding and cAMP Assays
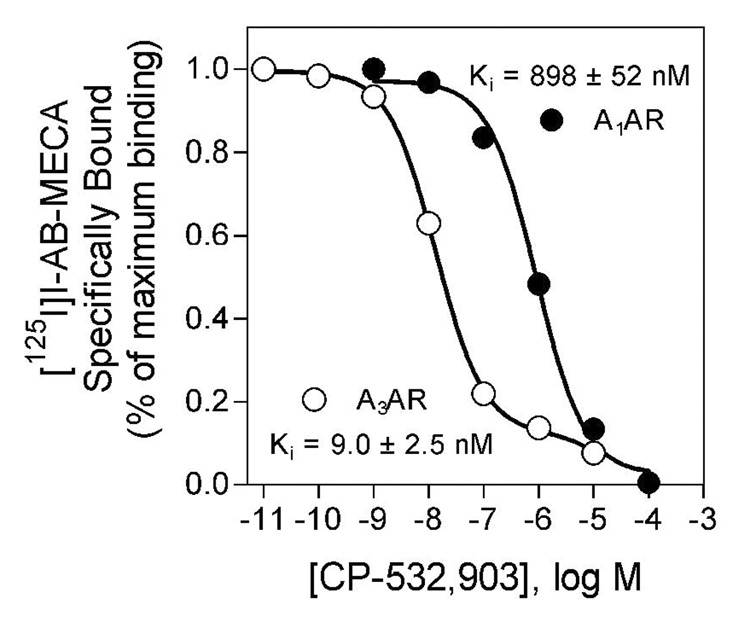
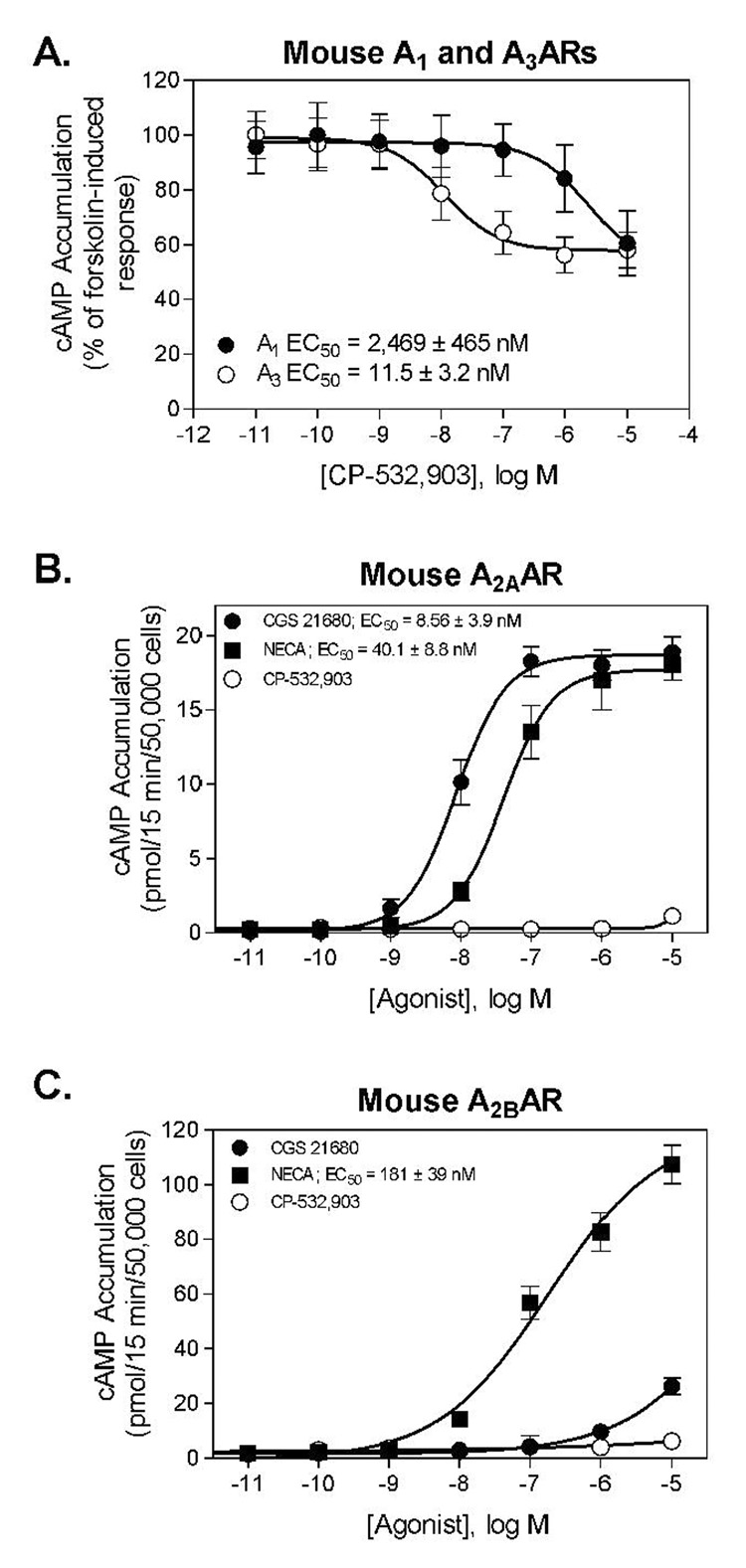
In vitro radioligand binding and cAMP assays using HEK 293 cell lines overexpressing mouse ARs demonstrated that CP-532,903 is a highly selective agonist of the mouse A3AR. In competition binding assays, CP-532,903 bound with ~100-fold higher affinity to the mouse A3AR compared to the mouse A1AR (Figure 2). In HEK 293 cells overexpressing the mouse A3AR, CP-532,903 inhibited forskolin-stimulated cAMP production with greater than 200-fold higher potency compared to assays conducted with HEK 293 cells overexpressing the mouse A1AR (Figure 3). CP-532,903 did not stimulate cAMP production in cells transfected with mouse A2A or A2BARs at concentrations as high as 10 µM, demonstrating that it has little agonist activity for mouse A2ARs (Figure 3).
Figure 2.
Inhibition of [125I]I-AB-MECA binding to HEK 293 cell membranes expressing mouse A1 or A3ARs by CP-532,903. The specific binding data were fitted to one (A1AR) or two (A3AR) binding sites, and dissociation constants for the high affinity sites were calculated, as described previously (ref). Values are the mean ± S.E.M. of triplicate determinations. Protein, 50 µg/incubation; [125I]I-AB-MECA, ~0.3 nM.
Figure 3.
A. Inhibition of forskolin-induced cAMP accumulation in HEK 293 cells expressing mouse A1 or A3ARs by CP-532,903. B and C. Stimulation of cAMP accumulation by CP-532,903, CGS 21680 (A2AAR agonist), and the nonselective AR agonist NECA in HEK 293 cells expressing mouse A2A (B) or A2BARs (C). EC50 values were calculated using the following formula: E = EMIN + (EMAX − EMIN)/(1 +10^((LogEC50 − C or C − EC50)*HillSlope)) where E is the rate of cAMP production observed at the concentration of agonist C and EMAX and EMIN are the maximal and minimum rates of cAMP production, respectively.
Isolated Mouse Heart Studies
Hemodynamic Studies
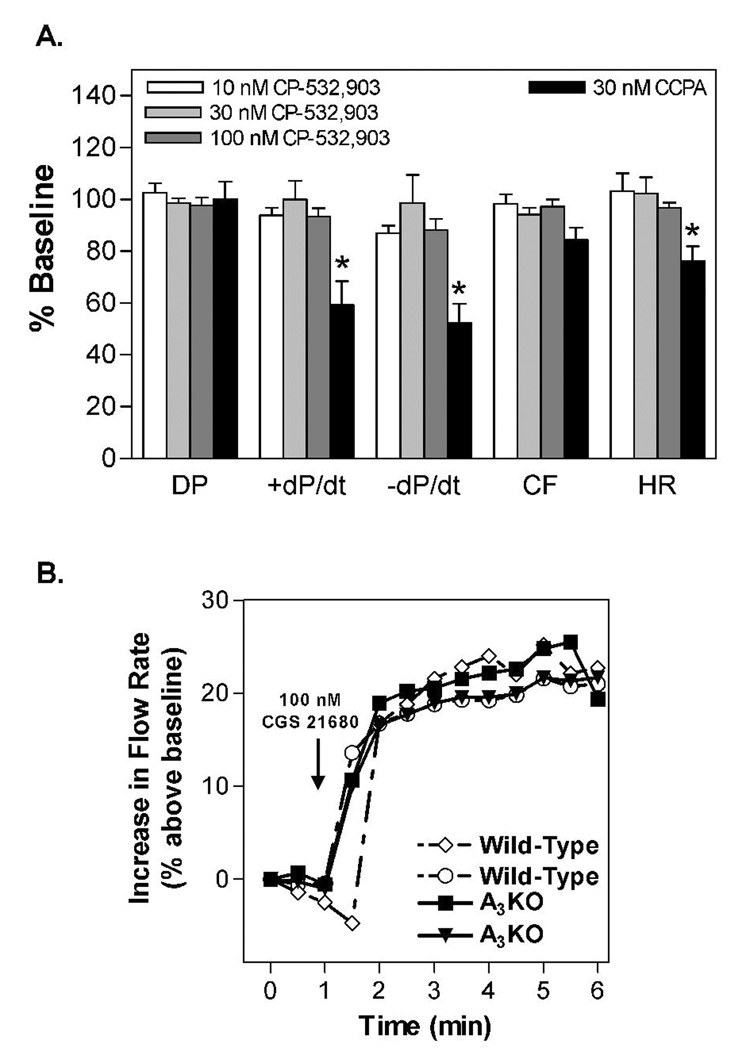
Preliminary studies were conducted with the Langendorff-perfused mouse heart model to examine the effect of CP-532,903 on baseline hemodynamic parameters. Mouse hearts (n = 4) were allowed to stabilize for 20 min (no pacing) and then increasing concentrations of CP-532,903 were administered into the perfusion buffer while heart rate, left ventricular pressure, and coronary flow were recorded for 5 min. The effect of the A1AR agonist 2-chloro-N6-cyclopentyladenosine (CCPA; 30 nM) was also tested. As shown in Figure 4, CP-532,903 produced no changes in any of the hemodynamic parameters measured, whereas CCPA markedly decreased heart rate, +dP/dt, and −dP/dt. CCPA also tended to decrease coronary flow, but not significantly. These results demonstrate that administration of CP-532,903 at concentrations as high as 100 nM did not activate A1 or A2AARs in this model system.
Figure 4.
A. Effect of increasing concentations of CP-532,903 and 30 nM CCPA (A1AR agonist) on hemodynamic parameters of unpaced isolated wild-type mouse hearts. DP, developed presssure; ± dP/dt, maximal rate of contraction/relaxation; CF, coronary flow; HR, heart rate. *p < 0.05 vs. baseline; n=4. B. Effect of the A2AAR agonist CGS 21680 (100 nM) on coronary flow in wild-type and A3KO mice.
Since it has been suggested that some A3AR agonists with limited selectivity (i.e., IB-MECA) may achieve cardioprotection by activating the A2AAR rather than the A3AR (Yang et al., 2003), in an additional series of experiments we examined whether the A2AAR system is functional in A3KO mice by measuring coronary flow responses to the A2AAR agonist CGS 21680. As shown in Figure 4, administration of CGS 21680 (100 nM) increased coronary flow in hearts from A3KO mice to a similar extent as compared to wild-type hearts.
Ischemia/Reperfusion Studies
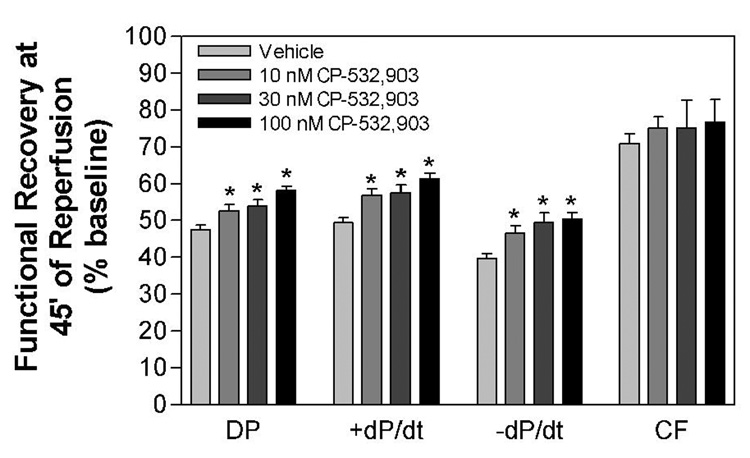
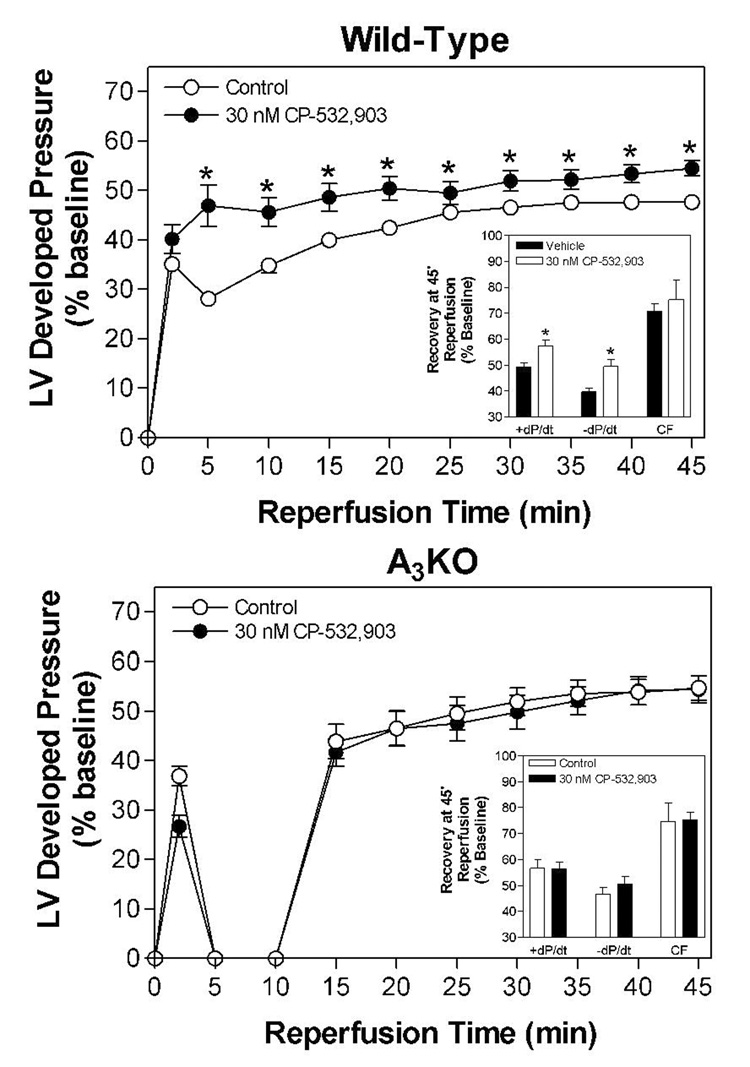
Administration of CP-532,903 concentration-dependently improved functional recovery of hearts subjected to 20 min of global ischemia and 45 min of reperfusion (Figure 5). At a concentration of 100 nM, developed pressure, +dP/dt, and −dP/dt were improved from 47.5 ± 1.1 to 58.2 ± 1.2 %, from 49.4 ± 1.3 to 61.3 ± 1.5 %, and from 39.7 ± 1.3 to 50.5 ± 1.7 % of baseline at 45 min of reperfusion, respectively. Treatment with 30 nM CP-532,903 provided no benefit in studies using hearts obtained from A3AR KO mice (Figure 6). Noticably, recovery of function during the first 10 min of reperfusion was impaired in A3KO compared to wild-type mice, suggesting that endogenous adenosine acting through native A3ARs may provide some degree of protection during ischemia/reperfusion injury.
Figure 5.
Effect of increasing concentrations of CP-532,903 on recovery of left ventricular developed pressure (DP), ±dP/dt, and coronary flow (CF) of isolated mouse hearts from wild-type mice subjected to 20 min of global ischemia and 45 min of reperfusion. *p<0.05 vs. the vehicle-treated group; n = 14–15.
Figure 6.
Effect of 30 nM CP-532,903 on recovery of left ventricular developed pressure (DP) of isolated mouse hearts from wild-type (upper panel) and A3KO mice (lower panel) following 20 min of global ischemia. Insets show recovery of left ventricular ±dP/dt and coronary flow at 45 min of reperfusion. Left ventricular developed pressure at baseline of hearts obtained from wild-type and A3KO mice was 114 ± 3 and 111 ±3 mmHg, respectively. *p < 0.05 vs. the vehicle-treated group; n = 7–14.
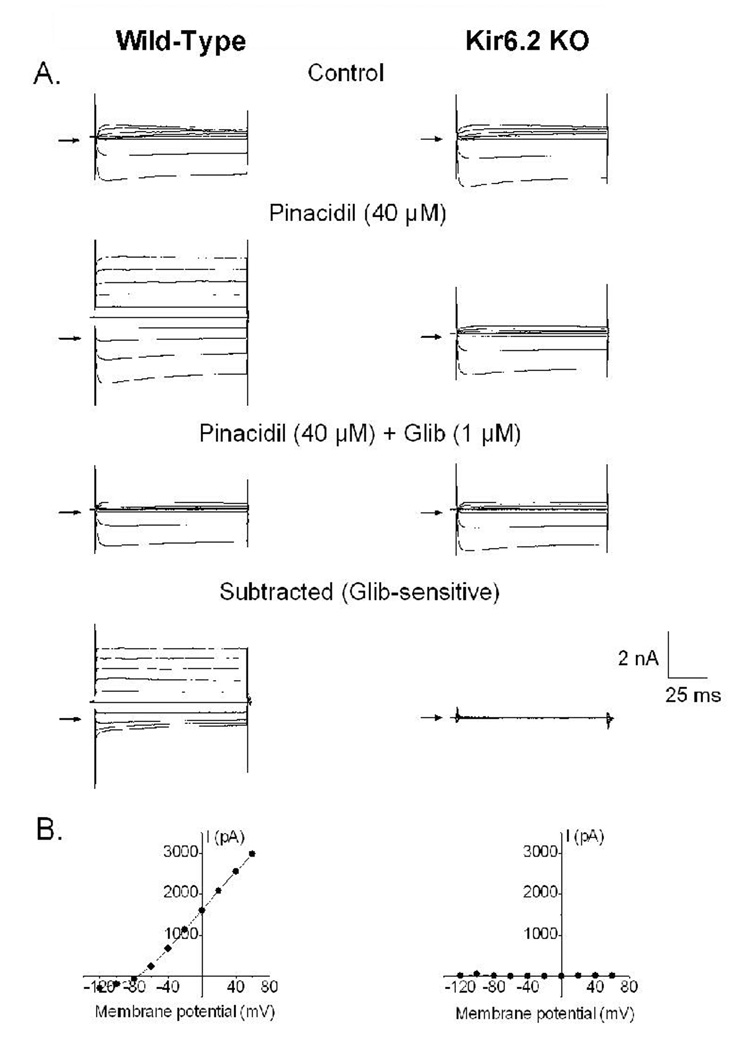
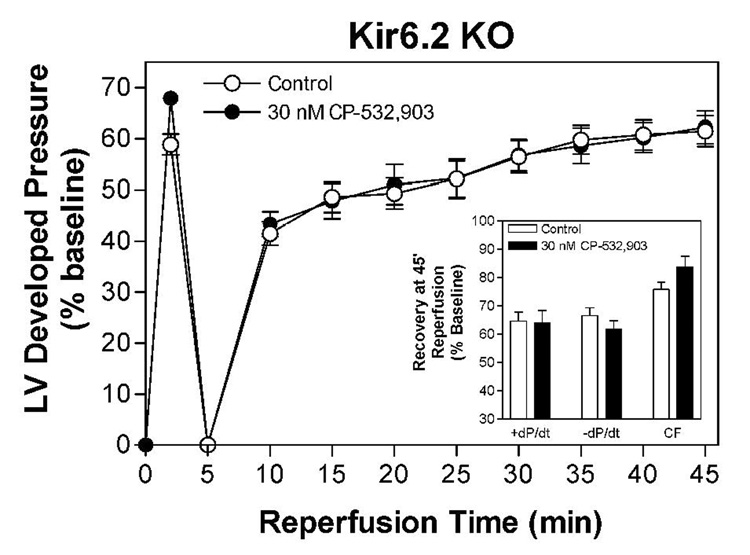
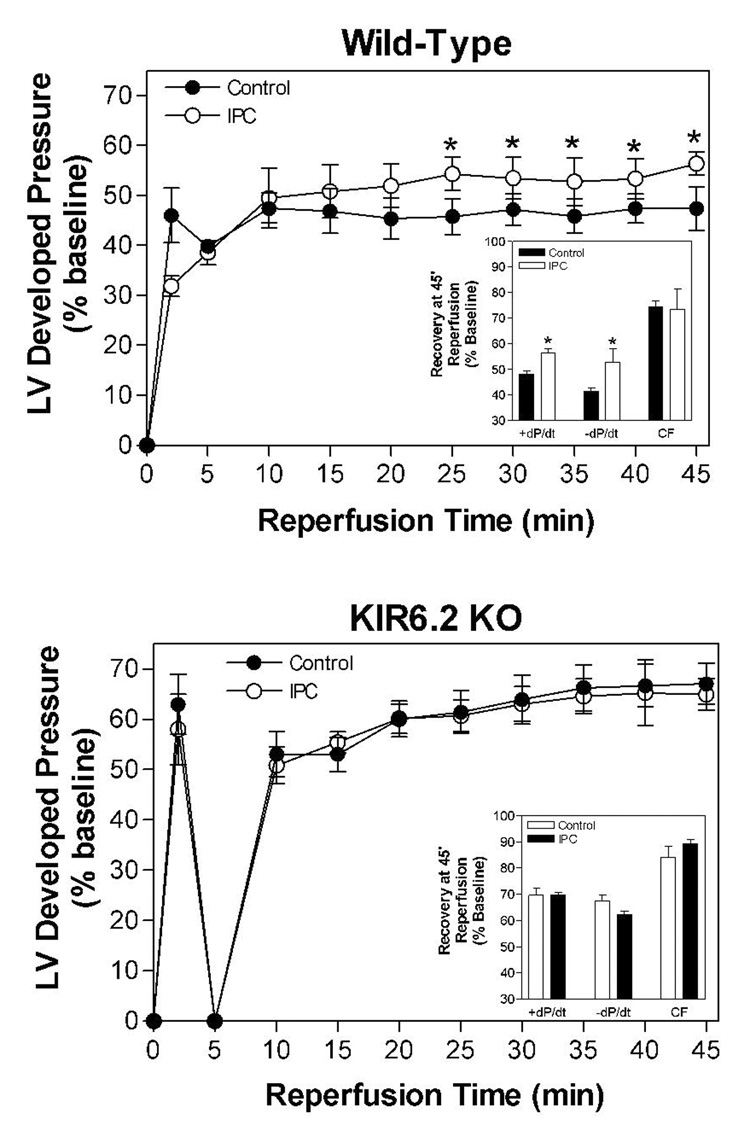
We subsequently examined whether treatment with CP-532,903 provides protection against ischemia/reperfusion injury in hearts obtained from Kir6.2 KO mice. Kir6.2 KO mice lack functional IKATP in cardiac myocytes due to genetic disruption of KCNJ11 encoding the Kir6.2 pore-forming subunit of the sarcolemmal KATP channel (Figure 7; Suzuki et al., 2002). For purposes of comparison, we also examined whether protection provided by IPC is functional in Kir6.2 KO hearts. As shown in Figure 8, recovery of left ventricular developed pressure, +dP/dt, and −dP/dt was not improved by treatment with 30 nM CP-532,903 in Kir 6.2 KO hearts subjected to 20 min of global ischemia and 45 min of reperfusion. Post-ischemic functional recovery was also not improved by IPC induced by three 3-min occlusion/2-min reperfusion cycles, whereas the same IPC protocol improved all measures of contractile function of wild-type hearts from ~45% to nearly 60% of pre-ischemic levels (Figure 9). Collectively, these results demonstrate that protection provided by activation of the A3AR as well as by IPC in isolated hearts is mediated by a mechanism involving the sarcolemmal KATP channel.
Figure 7.
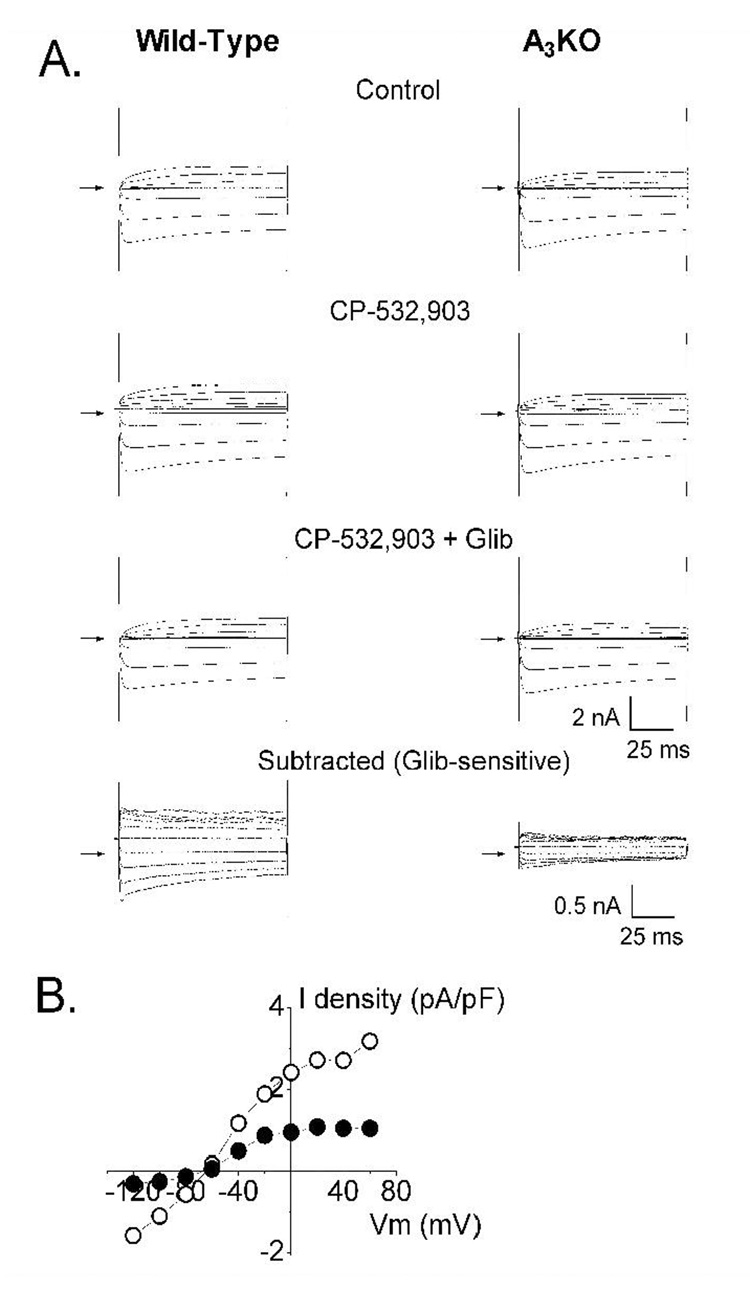
Absence of IKATP in cardiomyocytes obtained from Kir6.2 KO mice. Panel A shows whole-cell current traces recorded from ventricular myocytes isolated from wild-type and Kir6.2 KO mice under control conditions, in the presence of the KATP channel opener pinacidil (40 µM), and in the presence of both pinacidil (40 µM) and the KATP channel blocker glibenclamide (1 µM). The glibenclamide-sensitive current was obtained by digitally subtracting the current obtained in the presence of both pinacidil and glibenclamide fom that obtained with pinacidil alone. Panel B depicts the corresponding current-voltage relationship obtained from wild-type and Kir6.2 KO cardiomyocytes.
Figure 8.
Effect of 30 nM CP-532,903 on recovery of left ventricular developed pressure (DP) of isolated mouse hearts from Kir6.2 KO mice following 20 min of global ischemia. Inset shows recovery of left ventricular ±dP/dt and coronary flow at 45 min of reperfusion. Left ventricular developed pressure at baseline was 98 ± 4 mmHg. n = 7–10.
Figure 9.
Effect of ischemic preconditioning (IPC) induced by 3 cycles of 3-min occlusion/2-min reperfusion on recovery of left ventricular developed pressure (DP) of isolated mouse hearts from wild-type (upper panel) and Kir6.2 KO mice (lower panel) following 20 min of global ischemia. Insets show recovery of left ventricular ±dP/dt and coronary flow at 45 min of reperfusion. Left ventricular developed pressure at baseline of hearts obtained from wild-type and Kir6.2 KO mice was 120 ± 4 and 91 ± 5 mmHg, respectively. *p < 0.05 vs. the vehicle-treated group; n = 9–15.
In Vivo Infarction Studies
Ischemia/Reperfusion Studies
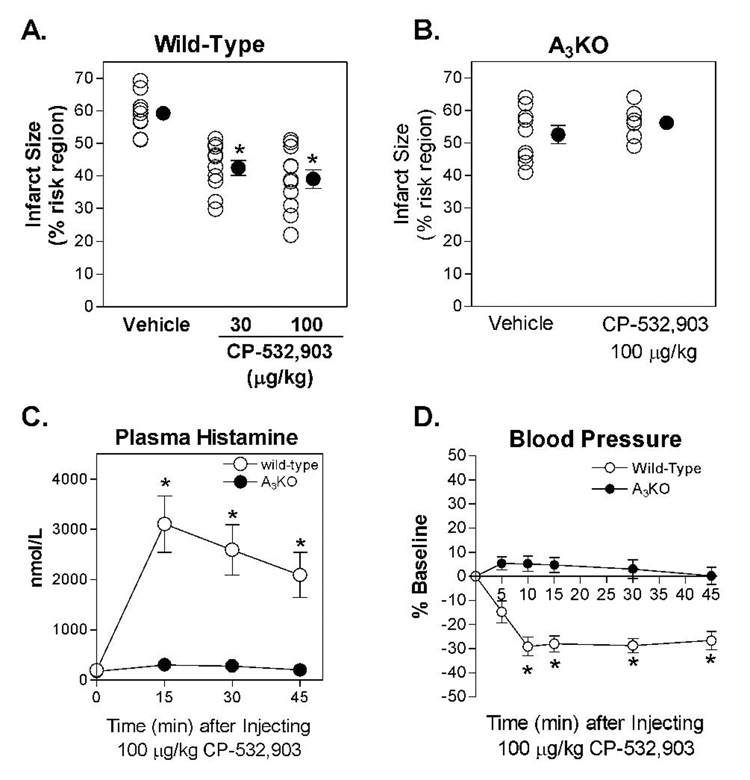
In in vivo studies, infarct size was 59.2 ± 2.1 % of the risk region in vehicle-treated mice subjected to 30 min of LAD occlusion and 24 h of reperfusion. Administration of 30 or 100 µg/kg of CP-532,903 significantly reduced infarct size to 42.5 ± 2.3 % and 39.0 ± 2.9 %, respectively (Figure 10). At a dose of 100 µg/kg, administration of CP-532,903 did not reduce infarct size in studies using A3AR KO mice (Figure 10). The size of the risk region, which ranged from 37.4 ± 2.9 to 40.6 ± 2.4 % of the left ventricle, was not significantly different among the five experimental groups.
Figure 10.
Panel A and B. Myocardial infarct size expressed as a percentage of the risk region in wild-type and A3KO mice treated with 30 or 100 µg/kg of CP-532,903. *p < 0.05 vs. the vehicle-treated group. C and D. Effect of CP-532,903 (100 µg/kg) on plasma histamine levels and mean arterial blood pressure in wildtype and A3KO mice. Baseline mean arterial blood pressure was 90 ± 3 and 86 ± 4 mmHg for wild-type and A3KO mice, respectively. *p < 0.05 vs. baseline; N = 6–10.
Hemodynamic and plasma histamine
In parallel studies, we examined the effect of 100 µg/kg of CP-532,903 on systemic hemodynamic parameters and plasma histamine levels. Although A3AR agonists do not alter hemodynamic variables in non-rodent species including humans, rabbits, and dogs (Auchampach et al., 1997b; Auchampach et al., 2003; van Troostenburg et al., 2004), activation of the A3AR evokes the release of mediators from mast cells in rodents, which indirectly causes hypotension (Hannon et al., 1995; Van Schaik et al., 1996; Ge et al., 2006). As shown in Figure 10, administration of 100 µg/kg of CP-532,903 produced a 30% decrease in mean arterial blood pressure that persisted for at least 45 min without changing heart rate (data not shown). Concomitantly, administration of CP-532,903 significantly increased plasma histamine levels, from 198 ± 22 nmol/L at baseline to 3,104 ± 559 nmol/L 15 min after administration of the drug (Figure 10). In A3KO mice, administration of CP-532,903 had no effect on blood pressure or plasma histamine levels.
Electrophysiology Studies
Our results obtained with Kir6.2 KO mice suggest that the A3AR is functionally coupled to the sarcolemmal KATP channel. To test this hypothesis, whole-cell recording were obtained from myocytes isolated from wild-type mice in the presence of CPX (500 nM) and ZM 241385 (500 nM), antagonists of mouse A1 and A2AARs, respectively (Kreckler et al., 2006). Basal whole-cell current was initially monitored for 20 min to allow for the diffusional exchange of ATP between the pipette solution and the intracellular milieu. Myocytes that exhibited spontaneous activation of outward current during this time period were discarded. Extracellular application of CP-532,903 elicited an outward current that was blocked by glibenclamide (Figure 11). This current was identified as IKATP. In order to confirm that the IKATP elicited by CP-532,903 was indeed due to activation of the A3AR, the experiments were repeated with myocytes isolated from A3KO mice. As shown in Figure 11, the ability of CP-532,903 to elicit opening of the KATP channel was markedly attenuated. These results demonstrate a functional coupling between the A3AR and the KATP channel.
Figure 11.
Evidence for coupling between the A3 receptor and the KATP channel. Panel A shows whole-cell current traces recorded from ventricular myocytes isolated from wild-type and A3AR KO mice in the absence of CP-532,903 (control), in the presence of CP-532,903 (1 µM), and in the presence of both CP-532,903 and glibenclamide (Glib, 1 µM). The glibenclamide-sensitive current was obtained by digitally subtracting the current obtained in CP-532,903 + glibenclamide from that in CP-532,903 alone. CPX (500 nM) and ZM241385 (500 nM) were included in the extracellular solution throughout the recordings. The arrows indicate zerocurrent levels. Panel B depicts the corresponding current-voltage relationship of the glibenclamide-sensitive current elicited by CP-532,903 in wild-type (○) and A3AR KO (●) myocytes. Current is depicted as current density (pA/pF) to normalize for differences in cell size.
Mitochondrial Function Studies
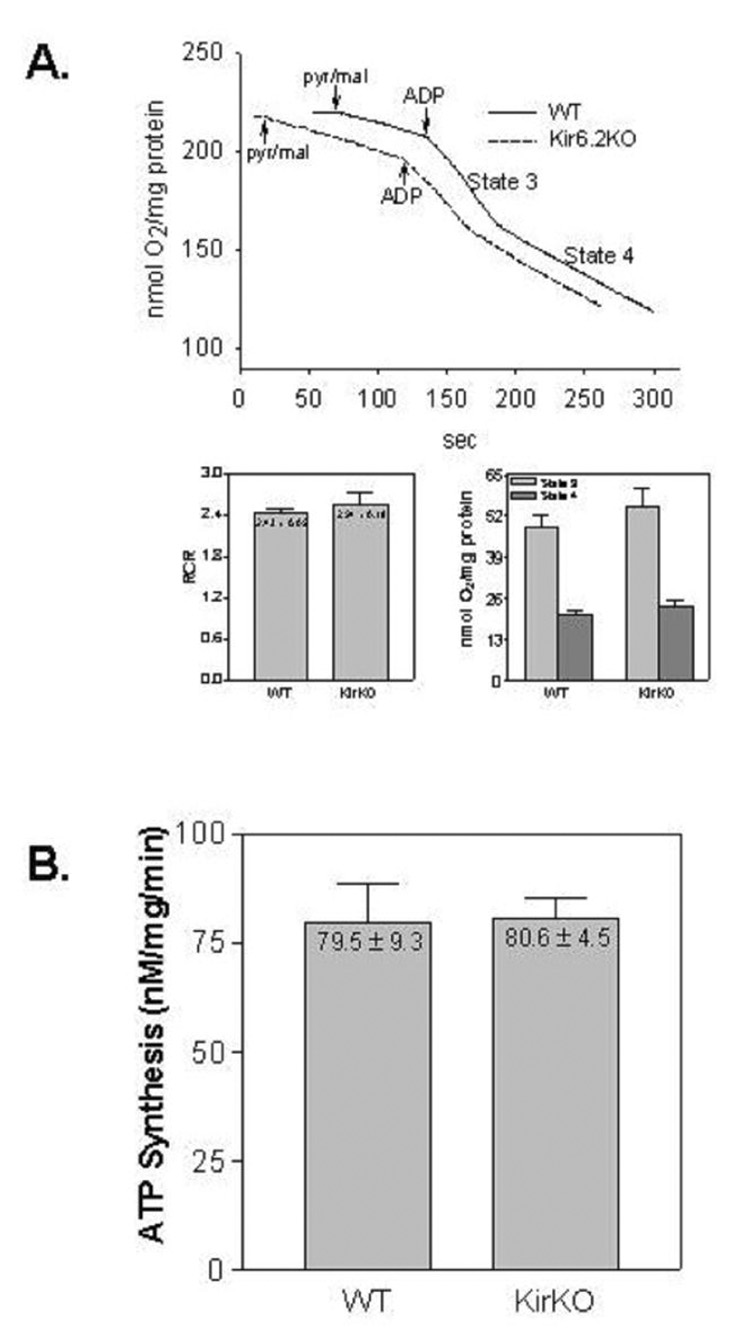
Since it has been hypothesized that mitochondria may express a related isoform of the KATP channel that has yet to be molecularly identified (Hanley and Daut, 2005), we examined respiration and ATP synthesis capacity of mitochondria isolated from Kir6.2 KO mice to determine whether deletion of KCNJ11 impacts normal function of cardiac mitochondria. Compared to wild-type mice, we observed no differences in state 2, 3, or 4 respiration, the respiratory control ratio, or ATP synthesis rates of cardiac mitochondria isolated from Kir6.2 KO mice (Figure 12).
Figure 12.
Functional assessment of isolated cardiac mitochondria obtained from wild-type and Kir6.2 KO mice. Panel A. Representative oxytherm traces comparing oxygen consumption by cardiac mitochondria from wild-type (solid) and Kir6.2 KO (dotted) mice. The rate of state 3 (after addition of ADP) was divided by the rate of state 4 (after ADP depletion) to calculate the respiratory control ratio (RCR). Panel B. ATP synthesis rates of mitochondria isolated from wild-type and Kir 6.2 KO mice. n = 4 for all assays.
Discussion
We examined the cardioprotective effects of the A3AR agonist CP-532,903 in two mouse models of ischemia/reperfusion injury. We found that administration of CP-532,903 concentration-dependently improved functional recovery of isolated mouse hearts subjected to global ischemia and reperfusion and reduced the size of myocardial infarction in mice subjected to 30 min of coronary occlusion and 24 h of reperfusion. The protective effects of CP-532,903 were considerable, producing a ~40% reduction in infarct size and significant improvement in all parameters of post-ischemic functional recovery of isolated mouse hearts. In the isolated mouse hearts studies, CP-532,903 was effective when used at low concentrations that did not influence A1AR-mediated bradycardia or A2AAR/A2BAR-mediated coronary dilation. The protection against injury provided by CP-532,903 was not apparent in isolated heart studies using hearts from A3KO mice or from Kir6.2 KO mice lacking expression of the pore-forming subunit of the sarcolemmal KATP channel. These observations demonstrate that CP-532,903 provides a direct cardioprotective effect in the ischemic myocardium by a mechanism involving activation of the A3AR and sarcolemmal KATP channels.
CP-532,903 is a member of a new series of N6-benzylsubstituted adenosine 5’-Nmethylcarboxamide AR agonists developed by Hill and colleagues (DeNinno et al., 2003; Tracey et al., 2003; DeNinno et al., 2006) that resulted from a search for more selective human A3AR agonists. The unique structural feature of CP-532,903 as well as other members within this series is an amino substitution at the 3’ position of the ribose ring rather than a hydroxyl group (Figure 1). Unlike other members in this series including CP-608,039, CP-532,903 has been reported to maintain relatively high A3AR selectivity in some non-human species including the rabbit (90-fold), leading to the selection of this compound as a potentially useful agent for study of the A3AR in preclinical animal models (DeNinno et al., 2003; Tracey et al., 2003; DeNinno et al., 2006). In the present investigation, we confirmed that CP-532,903 also displays high selectivity for the mouse A3AR. In radioligand binding assays, CP-532,903 bound with high affinity (Ki = 9.6 nM) to the mouse A3AR and with 100-fold selectivity compared to its closest pharmacological relative the A1AR. The A3 versus A1AR selectivity of CP-532,903 in the mouse is comparable to that of the first generation A3AR agonists IB-MECA (68-fold) and Cl-IB-MECA (210-fold; Ge et al., 2006). One important finding of our studies is that we observed that CP-532,903 exhibited very weak agonist activity for mouse A2A and A2BARs producing no stimulation up to a concentration of 10 µM (Figure 3). This is a significant observation, since it has been suggested that IB-MECA and Cl-IB-MECA may stimulate A2AARs with relatively high potency in some species (Murphree et al., 2002). Indeed, we have previously shown that IBMECA stimulated cAMP production in HEK 293 cells transfected with the mouse A2AAR with an EC50 value of ~700 nM (Ge et al., 2006). Thus, CP-532,903 exhibits superior A3 versus A2A/A2BAR selectivity compared to currently available first generation A3AR agonists.
Tracey and colleagues (Tracey et al., 2003) have previously reported that CP-532,903 effectively reduced infarct size in an isolated rabbit heart model of regional infarction. In these studies, pretreatment with CP-532,903 was shown to produce a maximal reduction in infarct size of 77% at a concentration of 150 nM and an EC50 value of ~1 nM (Tracey et al., 2003). Pretreatment with CP-532,903 was also shown to reduce infarct size in an in vivo rabbit model of infarction at doses (0.25 and 1 mg/kg) that were devoid of hemodynamic effects (Tracey et al., 2003). Although CP-532,903 was shown to be an effective cardioprotective agent in this study, it remained uncertain whether it reduced ischemic injury via activation of the A3AR or via interaction with the other AR subtypes. This issue could not be addressed directly, since selective A3AR agonists proven to be useful in the rabbit are not currently available. The results of the present investigation clearly demonstrate that the protection provided by CP-532,903 in both the isolated mouse heart model as well as the in vivo mouse model of infarction was completely lost in studies using A3AR KO mice, supporting the theory that CP-532,903 alleviates ischemic injury via the A3AR.
The inward rectifying K+ channel Kir6.2 is the pore-forming subunit of the KATP channel (Inagaki et al., 1995; Seino, 1999; Alekseev et al., 2005; Kane et al., 2005). In cardiac myocytes, Kir6.2 associates with the glibenclamide-sensitive sulfonylurea SUR2A subunit to form functional KATP channels in the sarcolemmal membrane (Inagaki et al., 1996; Alekseev et al., 2005; Kane et al., 2005). KATP channels, which are tightly coupled to the metabolic state of the cell, open in response to various stresses functionally shortening the duration of the action potential lessening time for calcium influx (Alekseev et al., 2005; Kane et al., 2005). Thus, the KATP channel serves to adjust cellular excitability to match metabolic demand. With the aid of Kir6.2 KO mice, the sarcolemmal KATP channel has been shown to importantly participate in normal stress responses, including IPC (Suzuki et al., 2002; Hanley and Daut, 2005; Kane et al., 2005). Although it remains controversial, a related isoform of the KATP channel has also been proposed to be expressed in mitochondria (Hanley and Daut, 2005). Similar to the sarcolemmal KATP channel, the mitochondrial KATP channel is thought to open in response to metabolic stress, thereby preserving mitochondrial integrity and reducing apoptosis secondary to changes caused by increased potassium influx. We and others have previously observed that protection provided by A3AR agonists is blocked by co-administration of glibenclamide, which equally inhibits both isoforms of the KATP channel (Tracey et al., 1998; Thourani et al., 1999; Auchampach et al., 2003). However, the relative importance of the two KATP channel isoforms in A3AR-mediated cardioprotection remained unknown. In the present investigation, we have shown that, like IPC, administration of CP-532,903 does not provide cardioprotection in the isolated heart model using Kir6.2 KO mice confirmed to lack functional sarcolemmal IKATP. We have also provided evidence suggesting that the A3AR is expressed in cardiomyocytes and that it couples to opening of the sarcolemmal KATP channel using electrophysiological techniques.
Suzuki and colleagues (Suzuki et al., 2002) have previously shown that the mitochondrial KATP channel is functional in Kir6.2 KO mice, based on diazoxideinduced changes in flavoprotein oxidation. We have also shown in the present investigation that mitochondrial respiration and ATP-synthesizing capacity of mitochondria from Kir6.2 KO mice are normal. Thus, our data suggest that CP- 532,903 provided a direct cardioprotective effect in the isolated heart model via activation of the sarcolemmal KATP channel, rather than via the putative mitochondrial KATP channel.
The data from the isolated heart experiments indicate that CP-532,903 produced a direct cardioprotective effect involving the sarcolemmal KATP channel. However, it is possible that CP-532,903 reduced infarct size in vivo by additional or alternative mechanisms. Administration of CP-532,903 increased plasma histamine levels and produced a 30% decrease in mean arterial blood pressure, responses that were not observed in A3AR KO mice. Thus, improvement in the oxygen supplydemand balance or depletion of mast cell contents may have contributed to the reduction in infarct size in the in vivo model, although we think these potential mechanisms are unlikely based on previous work preformed by our laboratory (Ge et al., 2006). Since it has been shown that A3AR agonists including CP-532,903 reduce infarct size when administered at the time of reperfusion (Auchampach et al., 2003; Tracey et al., 2003), it is also possible that CP-532,903 protected against infarction in the present investigation by reducing reperfusion-mediated injury. Even though CP-532,903 was administered 10 min prior to the ischemic period in our studies, it was likely present during the initial phase of reperfusion since hemodynamic responses to CP-532,903 persisted for over 45 min. Numerous studies in various models of inflammation have suggested that A3AR activation suppresses inflammatory responses and may regulate neutrophilmediated injury (Hasko and Cronstein, 2004). Thus, CP-532,903 may have acted, in part, by suppressing injury caused by reperfusion-induced inflammation. In support of this theory, we have shown in preliminary experiments that Cl-IB-MECA does not reduce infarct size when administered at the time of reperfusion in bone marrow chimeric mice lacking the expression of A3ARs in bone marrow-derived cells (Ge et al., 2004). Although the results of the present investigation clearly implicate the involvement of the A3AR, additional studies are required to investigate the multiple different mechanisms by which CP-532,903 effectively limits infarct size under in vivo conditions and to determine the relative contribution of the sarcolemmal KATP channel.
In summary, CP-532,903 has been shown to be a highly selective agonist for the mouse A3AR with improved selectivity versus the A2AR subtypes. Thus, CP-532,903 should be useful for discerning the biological role of the A3AR in mice. The results further confirm that CP-532,903 exerts a direct protective effect on the ischemic myocardium by activation of the A3AR by a mechanism that involves opening the sarcolemmal KATP channel. The importance of this direct cardioprotective action under in vivo circumstances awaits further investigation. Finally, the results of the present investigation support the contention that A3AR agonists may be useful agents for treating acute ischemia/reperfusion injury.
Acknowledgments
We thank Dr. Aaron Fisher (University of Pennsylvania) for providing Kir6.2 KO mice and Dr. Marlene Jacobson (Merck Laboratories) for providing A3KO mice. We acknowledge the Cardiovascular Center HPLC Core Facility at the Medical College of Wisconsin for purification of radioligands.
Nonstandard Abbreviations
- [125I]AB-MECA
N6-(4-amino-3-[125I]iodobenzyl)adenosine-5’-N-methylcarboxamide
- AR
adenosine receptor
- cAMP
cyclic adenosine monophosphate
- CCPA
2-chloro-N6-cyclopentyladenosine
- Cl-IB-MECA
2-chloro-N6-(3-iodobenzyl)adenosine-5’-N-methylcarboxamide
- CP-532,903
N6-(2,5-dichlorobenzyl)-3’-aminoadenosine-5’-N-methylcarboxamide
- CP-608,039
N6-[2-(3-methylisoxazol-5-ylmethoxy)-5-chloro]benzyl-3’-aminoadenosine-5’-N-methylcarboxamide
- CPX
1,3-dipropyl-8-cyclopentylxanthine
- DMSO
dimethylsulfoxide
- DP
developed pressure
- HEK 293
human embryonic kidney 293
- IB-MECA
N6-(3-iodobenzyl)adenosine-5’-N-methyluronamide
- IPC
ischemic preconditioning
- KATP
ATP sensitive potassium cahnnel
- LAD
left anterior descending
- NECA
adenosine-5’-N-ethylcarboxamide
- PBS
phosphate buffered saline
- Ro-20,1724
4-[(3-butoxy-4-methoxyphenyl)-methyl]-2-imidazolidinone
- RCR
respiratory control ratio
- TTC
tripohenyltetrazolium chloride
- ZM 241385
4-[2-[7-amino-2-(2-furyl)[1,2,4]triazolo-[2,3-a][1,3,5]triazin-5-ylamino]ethyl]phenol
Footnotes
This work was supported by NIH grants R01 HL60051, R01 HL07707, and T32 HL73643, and by American Heart Association research fellowship grants 0320019Z and 0225454Z.
Address for reprint requests: John A. Auchampach, Ph.D., Department of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226
References
- Alekseev AE, Hodgson DM, Karger AB, Park S, Zingman LV, Terzic A. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J Mol Cell Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchampach JA, Ge ZD, Wan TC, Moore J, Gross GJ. A3 adenosine receptor agonist IB-MECA reduces myocardial ischemia-reperfusion injury in dogs. Am J Physiol Heart Circ Physiol. 2003;285:H607–H613. doi: 10.1152/ajpheart.01001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol. 1997a;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, Bolli R. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5'-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res. 1997b;80:800–809. doi: 10.1161/01.res.80.6.800. [DOI] [PubMed] [Google Scholar]
- Black RG, Jr, Guo Y, Ge ZD, Murphree SS, Prabhu SD, Jones WK, Bolli R, Auchampach JA. Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res. 2002;91:165–172. doi: 10.1161/01.res.0000028007.91385.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNinno MP, Masamune H, Chenard LK, DiRico KJ, Eller C, Etienne JB, Tickner JE, Kennedy SP, Knight DR, Kong J, Oleynek JJ, Tracey WR, Hill RJ. 3'-Aminoadenosine-5'-uronamides: discovery of the first highly selective agonist at the human adenosine A3 receptor. J Med Chem. 2003;46:353–355. doi: 10.1021/jm0255724. [DOI] [PubMed] [Google Scholar]
- DeNinno MP, Masamune H, Chenard LK, DiRico KJ, Eller C, Etienne JB, Tickner JE, Kennedy SP, Knight DR, Kong J, Oleynek JJ, Tracey WR, Hill RJ. The synthesis of highly potent, selective, and water-soluble agonists at the human adenosine A3 receptor. Bioorg Med Chem Lett. 2006;16:2525–2527. doi: 10.1016/j.bmcl.2006.01.088. [DOI] [PubMed] [Google Scholar]
- Gallo-Rodriguez C, Ji XD, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q, Olah ME, van Galen PJ, et al. Structure-activity relationships of N6-benzyladenosine-5'-uronamides as A3-selective adenosine agonists. J Med Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, Auchampach JA. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine- 5'-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319:1200–1210. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- Ge ZD, Wan TC, Auchampach JA. The A3 adenosine receptor agonist Cl-IB-MECA reduces myocardial infarct size in mice when administered during reperfusion: Mechanistic studies with A3AR gene 'knock-out' and bone marrow chimeric mice. Circulation. 2004;110:III–I29. [Google Scholar]
- Hanley PJ, Daut J. KATP channels and preconditioning: a re-examination of the role of mitochondrial KATP channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hannon JP, Pfannkuche HJ, Fozard JR. A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br J Pharmacol. 1995;115:945–952. doi: 10.1111/j.1476-5381.1995.tb15902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hill RJ, Oleynek JJ, Hoth CF, Kiron MA, Weng W, Wester RT, Tracey WR, Knight DR, Buchholz RA, Kennedy SP. Cloning, expression and pharmacological characterization of rabbit adenosine A1 and A3 receptors. J Pharmacol Exp Ther. 1997;280:122–128. [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JPt, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A3 adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999;277:H1895–H1905. doi: 10.1152/ajpheart.1999.277.5.H1895. [DOI] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji XD, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–C1590. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree LJ, Marshall MA, Rieger JM, MacDonald TL, Linden J. Human A2A adenosine receptors: high-affinity agonist binding to receptor-G protein complexes containing Gβ4. Mol Pharmacol. 2002;61:455–462. doi: 10.1124/mol.61.2.455. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A1 or A3 adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- Thourani VH, Nakamura M, Ronson RS, Jordan JE, Zhao ZQ, Levy JH, Szlam F, Guyton RA, Vinten-Johansen J. Adenosine A3-receptor stimulation attenuates postischemic dysfunction through KATP channels. Am J Physiol. 1999;277:H228–H235. doi: 10.1152/ajpheart.1999.277.1.H228. [DOI] [PubMed] [Google Scholar]
- Tracey WR, Magee W, Masamune H, Kennedy SP, Knight DR, Buchholz RA, Hill RJ. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc Res. 1997;33:410–415. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- Tracey WR, Magee W, Masamune H, Oleynek JJ, Hill RJ. Selective activation of adenosine A3 receptors with N6-(3-chlorobenzyl)-5'-N-methylcarboxamidoadenosine (CB-MECA) provides cardioprotection via KATP channel activation. Cardiovasc Res. 1998;40:138–145. doi: 10.1016/s0008-6363(98)00112-6. [DOI] [PubMed] [Google Scholar]
- Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, Knight DR. Novel N6-substituted adenosine 5'-N-methyluronamides with high selectivity for human adenosine A3 receptors reduce ischemic myocardial injury. Am J Physiol Heart Circ Physiol. 2003;285:H2780–H2787. doi: 10.1152/ajpheart.00411.2003. [DOI] [PubMed] [Google Scholar]
- Van Schaik EA, Jacobson KA, Kim HO, IJzerman AP, Danhof M. Hemydynamic effects and histamine release elicited by the selective adenosine A3 recetpor agonist 2-Cl-IB-MECA in conscious rats. Eur J Pharmacol. 1996;308:311–314. doi: 10.1016/0014-2999(96)00373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Troostenburg AR, Clark EV, Carey WD, Warrington SJ, Kerns WD, Cohn I, Silverman MH, Bar-Yehuda S, Fong KL, Fishman P. Tolerability, pharmacokinetics and concentration-dependent hemodynamic effects of oral CF101, an A3 adenosine receptor agonist, in healthy young men. Int J Clin Pharmacol Ther. 2004;42:534–542. doi: 10.5414/cpp42534. [DOI] [PubMed] [Google Scholar]
- Yang Z, Marshal M, Xu Y, French BA, Linden J. Opposing effects of the adenosine receptor agonist IB-MECA on myocardial infarct size in mice are mediated by A3 proinflammatory receptors and anti-inflammatory A2A receptors. Circulation. 2003;110:III–300. [Google Scholar]