Abstract
Obesity has been linked with an increased risk of prostate cancer. The formation of toxic free oxygen radicals has been implicated in obesity mediated disease processes. Leptin is one of the major cytokines produced by adipocytes and controls body weight homeostasis through food intake and energy expenditure. The rationale of the study was to determine the impact of leptin on the metastatic potential of androgen-sensitive (LNCaP) cells as well as androgen-insensitive (PC-3 and DU-145) cells. At a concentration of 200 nm, LNCaP cells showed a significant increase (20% above control; P < .0001) in cellular proliferation without any effect on androgen-insensitive cells. Furthermore, exposure to leptin caused a significant (P < .01 to P < .0001) dose-dependent decrease in migration and invasion of PC3 and Du-145 prostate carcinoma cell lines. At the molecular level, exposure of androgen-independent prostate cancer cells to leptin stimulates the phosphorylation of MAPK at early time point as well as the transcription factor STAT3, suggesting the activation of the intracellular signaling cascade upon leptin binding to its cognate receptor. Taken together, these results suggest that leptin mediates the invasive potential of prostate carcinoma cells, and that this effect is dependent on their androgen sensitivity.
1. INTRODUCTION
Prostate cancer is the second leading cause of cancer related death among American men [1]. Obesity has assumed epidemic proportions in the United States and has been linked with increased risk of prostate, breast, colon, and endometrial cancers [2–7]. More recently, there have been several studies suggesting that obesity may impact upon risk, detection, and outcome with regard to prostate cancer [8].
Leptin, one of the major adipose cytokines controls body weight by regulating food intake and energy expenditure [9, 10]. Adipose tissue leptin mRNA and circulating leptin levels increase in obesity. Clinical studies have demonstrated that, blood leptin levels are associated with clinically relevant prostate cancer [11, 12], although some studies have questioned these results [13–16]. In vitro, leptin has been postulated as a growth promoter for androgen-independent DU-145 and PC-3 cells [17–20], but not in androgen-dependent LNCaP cells [20]. Several reports have demonstrated that leptin induces the formation of toxic oxygen radicals [21–25]. Oxidant damage has been implicated as a major causative factor in obesity mediated disease processes. The rationale of the present study is to confirm the role of leptin on the metastatic potential of prostate carcinoma cells. We tested androgen-sensitive LNCaP cells as well as androgen-resistant PC-3 and DU-145 cells for their ability to metastasize in the presence and absence of leptin. Additionally, we investigated the effect of leptin on mitogen-activated protein kinase (MAPK) phosphorylation, and on the activation of Erk-1 and Erk-2, and STAT-3.
2. MATERIALS AND METHODS
2.1. Reagents
Leptin was obtained from Sigma-Aldrich Inc., St. Louis, Mo. Antibodies used were rabbit polyclonal antiphospho-p44/42 MAP kinase, rabbit polyclonal anti-STAT-3, rabbit polyclonal antiphospho-STAT-3, and horseradish peroxidase-conjugated goat antirabbit antibodies from Cell Signaling Technology, Beverly, Mass.
2.2. Cell culture
The PC-3 and LNCaP cell lines were propagated in RPMI 1640 medium, whereas DU-145 cell line was propagated in EMEM medium (Invitrogen Corporation, Carlsbad, Calif) supplemented with 10% FBS (HyClone, Logan, Utah). For protein studies, cells were grown to subconfluent levels in RPMI or EMEM, respectively, and supplemented with 10% FBS in 6-well culture plates (106 cells/well). The cells were then washed once with basal medium and starved of growth factors by switching them to basal medium containing 1% FBS for 16 hours. Cells were again washed once with basal medium and then exposed to various experimental conditions for different time points. At the end of each time point, cells were washed with ice-cold phosphate-buffered saline (PBS, Invitrogen), lysed in cell lysis buffer containing 50 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 2 mM Na3VO4, 50 mM NaF, 70 mM 2-mercaptoethanol, 1% v/v Triton X-100, 2% w/v SDS, and 1 μg/ml protease inhibitor mixture (Sigma-Aldrich, Mo). Cell lysates were used as indicated below.
2.3. Proliferation assay
RPMI or EMEM + 10% FBS was added to each well of a 24-well plate and pretreated for 4 hours with various concentrations of leptin at 37°C in a CO2 incubator. Growth factor starved PC-3, LNCaP, or DU-145 cells were then transferred to each well (2 × 104 cells/well) along with 1 μM [3H]Thymidine, and incubated in the CO2 incubator. After 144 hours of incubation, the cells were then washed three times with ice-cold saline, precipitated with 10% TCA, washed with methanol and air-dried. The labeled DNA was dissolved in 0.05 N NaOH at 37°C, neutralized with 0.05 N HCl, transferred to scintillation vial containing the scintillation cocktail and counted.
2.4. In vitro migration and invasion assay
A modified Boyden chamber method using Transwell membrane inserts (Costar, Corning, NY) was used to measure PC-3 and DU-145 migration and invasion. For invasion studies, Matrigel (BD Biosciences, Bedford, Mass) was added to the upper surface of the Transwell membrane and allowed to solidify in a sterile environment for 24 hours. PC-3 or DU-145 cells (14 × 104 cells/ml) were suspended in basal medium supplemented with 2% FBS, and 0.02% BSA, incubated with various concentrations of leptin, and plated onto the upper surface of the Transwell membrane inserts in a total volume of 100 μL. Basal medium supplemented with 2% FBS and 0.02% BSA was added to the lower chamber in a total volume of 400 μL. The cells were then incubated at 37°C in a CO2 incubator. After 10 hours of incubation, cells present on the upper surface of the membrane were scraped off, and the cells that had migrated to the lower surface of the membrane were fixed, stained and counted using an inverted Axiovert 25 microscope (Carl Zeiss, Thornwood, NY).
2.5. Western blotting
Prostate cancer cells, pretreated for 16 hours with basal medium supplemented with 1% FBS, were treated with 25, 50, 100, and 200 ng of leptin for various time points. After the completion of each time point, the cells were lysed in the cell lysis buffer containing 0.1% w/v bromophenol blue. The samples were boiled for 5 minutes and separated using 7.5% SDS-PAGE (40 μg of total protein/lane). The separated proteins were then transferred onto nitrocellulose membranes (Bio-Rad, Hercules, Calif), blocked with Tris-buffered saline containing 5% nonfat dry milk for 1 hour at room temperature, and incubated with the respective antibodies against the proteins in Tris-buffered saline containing 5% nonfat dry milk and 0.5% Tween 20, overnight at 4°C. After washing and incubation with horseradish peroxidase-conjugated secondary antibody, the proteins were revealed using the enhanced chemiluminescent detection kit (Pierce Biotechnology, Biotechnology, Rockford, Ill).
2.6. Statistical analysis
The data were analyzed using the Statview software to obtain the SE values and the significance (p) values for the various results from 3 separate experiments.
3. RESULTS
3.1. Leptin attenuates cellular invasion of androgen-resistant prostate cancer cells
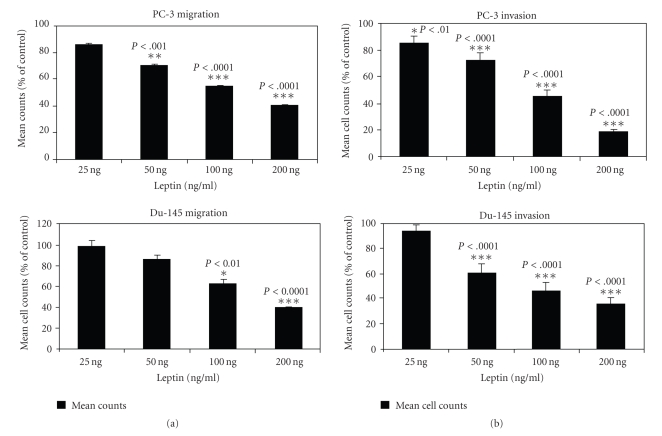
Androgen-resistant PC-3 cells and DU-145 cells were suspended in media along with increasing concentrations of leptin and plated onto the upper surface of the Transwell inserts that were previously coated with Matrigel, as described in Material and Methods section. As seen in Figure 1(a), leptin attenuated the invasion of Matrigel by PC-3 cells as well as DU-145 cells in a concentration dependent manner, with significant attenuation observed at 50 ng, 100 ng, and 200 ng concentrations of leptin, indicating that nanogram concentrations of leptin inhibit the invasive capacity of these androgen-independent prostate carcinoma cell lines.
Figure 1.
Cellular invasion and migration of androgen-resistant prostate cancer cells are attenuated by leptin. PC-3 and DU-145 cells were suspended in basal medium supplemented with 2% FBS, and 0.02% BSA, incubated with various concentrations of leptin, and plated onto the upper surface of the Transwell membrane inserts. For invasion studies, Matrigel was added to the upper surface of the Transwell membrane and allowed to solidify in a sterile environment for 24 hours. After 10 hours of incubation at 37°C in a CO2 incubator, cellular invasion (a) and migration (b) were measured by counting the cells that had migrated to the lower surface of the membrane. Values represent the mean ± S.E. of triplicate samples of a representative experiment.
3.2. Migration of PC-3 cells and Du-145 cells is attenuated by leptin
Androgen-independent PC-3 cells and DU-145 cells were incubated with increasing concentrations of leptin (see Figure 1(b)) and allowed to migrate through the pores of the membrane in the modified Boyden chamber containing transwell inserts. Attenuation of migration of both PC-3 and Du-145 cell lines was observed in the presence of increasing concentrations of leptin, with significant attenuation being observed at 100 ng and 200 ng concentrations of leptin for PC-3 cells and at 200 ng concentration of leptin for DU-145 cells, respectively, suggesting that leptin at nanogram concentrations inhibits the mechanism regulating the migration of these cell lines.
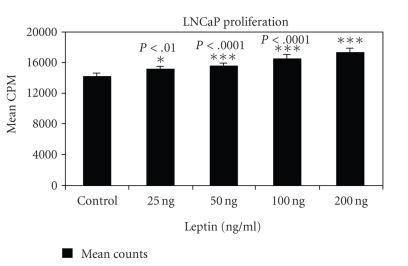
3.3. Leptin induces the proliferation of androgen-dependent prostate cancer cells
Proliferation of androgen-dependent and independent prostate cancer cell lines was observed in the presence of nanogram concentrations of leptin. After achieving the basal level of growth by overnight incubation in basal medium, increasing concentration of leptin was added to the cells, respectively. As seen in Figure 2, a dose-dependent increase in proliferation was observed when the androgen-dependent LNCaP cells were incubated with leptin, with significant growth being observed at 50 ng, 100 ng, and 200 ng concentrations of leptin, respectively. There was no change in growth of androgen-independent PC-3 cells and Du-145 cells in the presence of increasing concentration of leptin (data not shown), suggesting that leptin induces the proliferative capacity of only the androgen-dependent prostate carcinoma cell lines.
Figure 2.
Proliferation of androgen-dependent prostate cancer cells is induced by leptin. Growth factor starved LNCaP cells were incubated with increasing concentrations of leptin, and proliferation was measured by the incorporation of [3H]thymidine after 144 hours. Values represent the mean ± S.E. of triplicate samples of a representative experiment.
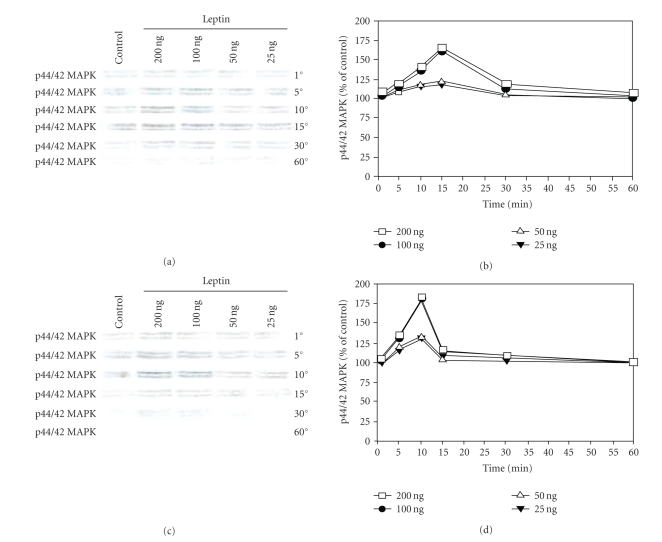
3.4. Leptin stimulated MAPK phosphorylation
We hypothesized that binding of leptin to its receptors in the prostate cancer cell lines would lead to the early phosphorylation of MAPK. To test this hypothesis, androgen- dependent LNCaP cells and androgen-independent PC-3 and DU-145 cells were starved of growth factors overnight (basal medium supplemented with 1% FBS), following which they were exposed to increasing concentration of leptin for various time points. Phosphorylation of MAPK was observed at the end of each time point for all cell lines. Interestingly, leptin did not induce the phosphorylation of MAPK in the androgen-dependent LNCaP cells (data not shown). However, in androgen-independent prostate cancer cell lines, phosphorylation of MAPK was observed to be maximal at 15 minutes (PC-3 cells, see Figures 3(a) and 3(b)) and 10 minutes (DU-145 cells, see Figures 3(c) and 3(d)) upon exposure to leptin. Maximal increase in MAPK phosphorylation was observed upon incubating these cells with 100 ng and 200 ng of leptin, respectively, suggesting that binding of leptin to its receptors induces an intracellular signaling cascade leading to the phosphorylation of MAPK in the androgen-independent prostate cancer cell lines.
Figure 3.
Leptin stimulates MAPK phosphorylation in PC-3 and DU-145 cells. Growth factor-starved PC-3 (a) or DU-145 cells (c) were exposed to increasing concentration of leptin for various time points. Cell lysates obtained after each time point were separated on a 7.5% SDS-PAGE and Western-blotted. Phosphorylated MAPK levels were obtained after incubation with p44/42 MAPK antibody and chemiluminescent detection. (b) and (d), levels of p44/42 MAPK obtained after densitometric analysis and represented as percentage of control against time (control being set at 100%). The results of one of the three independent experiments are shown.
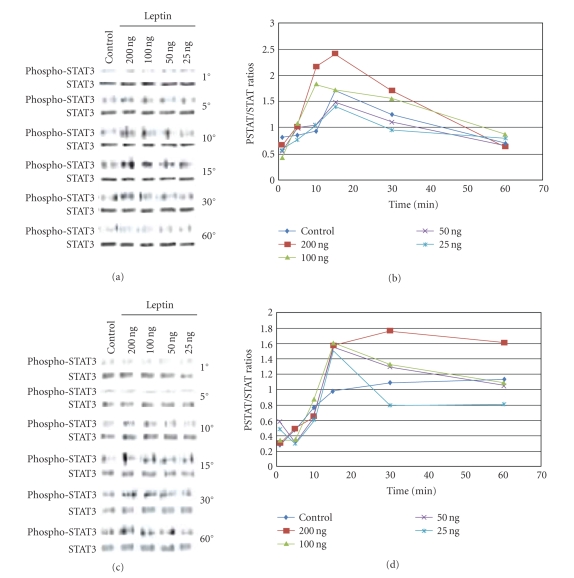
3.5. STAT-3 phosphorylation is induced by leptin
After an overnight incubation in basal medium with 1% FBS, androgen-dependent (LNCaP) and -independent (PC-3) prostate cancer cells were incubated with a dose-dependent increase in leptin levels for various time points. STAT-3 phosphorylation was investigated over time for both cell lines. As seen in Figures 4(a) and 4(b), leptin induced the time-dependent phosphorylation of STAT-3 in PC-3 cells, reaching maximal levels after 15 minutes of exposure, and returning to baseline after 60 minutes of incubation. Exposure of PC-3 cells to increased leptin levels led to a correspondent increase in the level of maximal STAT-3 phosphorylation at the 15 minutes time point. Leptin exposure also induced the phosphorylation of STAT-3 in LNCaP cells (see Figures 4(c) and 4(d)), reaching maximal levels after 30 minutes of stimulation. However, in LNCaP cells, STAT-3 remained phosphorylated even after prolonged (60 minutes) exposure to leptin, suggesting that leptin induces the phosphorylation of STAT-3 only at an early time point in androgen-independent cells, while in androgen-dependent prostate cancer cells, STAT-3 phosphorylation is a delayed but prolonged event.
Figure 4.
Leptin stimulates early phosphorylation of STAT-3 in PC-3 cells. PC-3cells (a) or LNCaP cells (c), devoid of growth factors by an overnight exposure to basal medium, were exposed to increasing concentrations of leptin for various time points. 25 μg of the cell lysates obtained after each time point were separated on a 7.5% SDS-PAGE and Western-blotted, and the phosphorylated STAT-3 proteins were revealed by incubating with phospho-STAT-3 antibody followed by chemiluminescent detection. The membranes were stripped and reprobed with STAT-3 antibodies to verify the protein levels. In (b) and (d), densitometric analysis of phospho-STAT-3 and STAT levels was determined. A time-course, dose-response curves of phosphor-STAT-3/STAT-3 (PSTAT/STAT) ratios for the effect of increasing leptin concentrations are depicted for both (b) PC3 and (d) LNCaP. The original western blots are shown in (a) and (c). Results shown are representative of three separate experiments.
4. DISCUSSION
It has been shown that leptin influences the proliferation and migration of endothelial and epithelial cells and is capable of promoting angiogenesis [21]. In vitro studies on prostate cancer cell lines showed that leptin promotes proliferation of androgen-independent prostate cancer [20]. Leptin also induces cell migration and the expression of growth factors in prostate cancer cells, suggesting that studies undertaken to ascertain leptin and/or obesity association to prostate cancer should differentiate patients according to androgen resistance [19]. Leptin has been demonstrated to potently induce the invasion of endometrial cancer cells [22] and also contributes to the invasive phenotype of colonic and kidney epithelial cells at various stages of the neoplastic progression [23]. Our results demonstrate that the invasive and migratory capabilities of androgen-insensitive prostate cancer cells are attenuated upon exposure to nanogram concentrations of leptin for a short duration of 10 hours, and that this attenuation increases in a dose-dependent manner. Our observations are in contrast to those of Frankenberry et al. [19] who demonstrated that a longer 24 hour exposure of leptin increased the migration of androgen-independent prostate cancer cells. We did not observe any change in the proliferation of androgen-independent prostate cancer cells upon exposed to nanogram concentrations of leptin (data not shown). In contrast, the proliferation of androgen-sensitive prostate cancer cells is increased in a dose-dependent manner when they are exposed to nanogram concentrations of leptin, suggesting leptin's ability to differentiate between the androgen-sensitive and -insensitive prostate carcinoma cells. Our results are in contrast to those observed by Onuma et al. [20] who showed an increased proliferation of androgen-independent prostate cancer cells using high (microgram) levels of leptin. Our contrasting observations using very low (nanogram) levels of leptin suggest that prostate cancer cells are very sensitive and behave differently at varying levels of leptin exposure.
As prostate cancer progresses, decreased androgen levels promote the growth of androgen-resistant prostate cells. These cells are hypersensitive to growth factors and cell regulators, especially mitogenic factors. The source of these growth factors and cytokines, in addition to outside factors affecting their expression, is important to understanding the progression of prostate cancer disease [21]. The leptin signal is transduced through the leptin receptor (LEPR). The different isoforms of this receptor arise from alternate splicing of the precursor mRNA [24]. The “long form” LEPRb leptin receptor is the only isoform that forms an IL-6-type homodimer and associates with the Janus kinase 2 (JAK2) tyrosine kinase to mediate intracellular signaling. JAK2 phosphorylates itself and the tyrosine residue (Tyr 985) on the intracellular tail of the LEPRb receptor. This phosphorylated tyrosine residue on the receptor then recruits the adaptor protein Grb-2 and p21ras, ultimately leading to the phosphorylation and activation of MAPK [25]. Our observations demonstrate that exposure of androgen-independent prostate cancer cells to leptin does indeed stimulate the phosphorylation of MAPK at an early time point, suggesting the stimulation of the intracellular signaling cascade upon leptin binding to its cognate receptor on the cell surface of these cells. Our observations are in accordance with those reported earlier by Banks et al. [25] suggesting involvement of MAPK activation upon binding of leptin to its surface receptor. Interestingly, we did not observe MAPK phosphorylation when the androgen-sensitive prostate cancer cells were exposed to leptin, suggesting the involvement of a different signaling cascade in these cells.
Activated JAK2 induces the phosphorylation of another tyrosine residue (Tyr 1138) on the intracellular tail of the LEPRb receptor, which binds and mediates the phosphorylation-dependent activation of signal transducer and activator of transcription 3 (STAT3). STAT proteins regulate gene expression by affecting transcription. They are part of the signal transduction pathway of many growth factors and cytokines and are activated by phosphorylation of tyrosine and serine residues by upstream kinases [26]. In benign cells, the signaling by STAT3 is under tight regulation so that the signal is transient. However, aberrant signaling by STAT3 is observed in many types of malignancies, such as myeloma, head and neck cancer, breast cancer, and prostate cancer [27]. Malignant cells expressing persistently activated STAT3 become dependent on it for survival; disruption of activation or expression of STAT3 resulted in apoptosis [28]. Lou, et al. have shown that STAT3 signaling is critical for the growth and survival of prostate cancer cells [29]. We observed the involvement of STAT3 phosphorylation in prostate cancer cells, which is concurrent with earlier published reports, thus suggesting that this transcription factor is an integral part in the leptin signal-transduction cascade. Our observations further suggest that STAT3 phosphorylation can differentiate between the androgen sensitivity of prostate cancer cells when they are exposed to nanogram levels of leptin. In androgen-independent PC-3 prostate cancer cells, leptin induces an early phosphorylation of STAT-3, which then decreases with time to the baseline level, whereas in androgen-sensitive LNCaP cells, a delayed and prolonged STAT3 phosphorylation is observed upon exposure to leptin. It has been previously demonstrated that part of leptin action is mediated through the generation of toxic oxygen radicals [30]. Although, in this study we did not measure a direct cause-effect relationship between free radical generation, and the changes in metastatic potential of prostate cancer cells, indirect evidence of the role of free radicals in this process is available in studies investigating the use of antioxidants in prostate cancer [31].
In conclusion, binding of leptin to its surface receptor induces a signal transduction cascade that has slight variations depending on the androgen sensitivity of the prostate cancer cells. These variations in the intracellular signaling events are translated into biological consequences such as the invasive, migratory, and proliferative capabilities of prostate carcinoma cells that are drastically different between the androgen-independent and -sensitive prostate cancer cells. Thus, differentiation of the metastatic potential of prostate cancer cells on the basis of their androgen sensitivity can be observed when they are exposed to low levels of leptin. Since a majority of leptin's effects (at least on metabolism) are centrally mediated, the in vivo effects of leptin on the metastatic potential of prostate cancer cells may differ from the in vitro observations. However, mechanism-dependent variations in the effects of leptin treatment on the basis of androgen sensitivity of prostate cancer cells can give us a vital insight into the possible in vivo effects of leptin.
ACKNOWLEDGMENTS
The work was supported in part by National Cancer Institute Grant no. CA 918858 (MR). Dr. Ouhtit is supported by the Louisiana Cancer Research Consortium in New Orleans.
References
- 1.Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochemical and Biophysical Research Communications. 2006;340(4):1158–1166. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 2.Amling CW, Kane CJ, Riffenburgh RH, et al. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58(5):723–728. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 3.Furuya Y, Akimoto S, Akakura K, Ito H. Smoking and obesity in relation to the etiology and disease progression of prostate cancer in Japan. International Journal of Urology. 1998;5(2):134–137. doi: 10.1111/j.1442-2042.1998.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiology Biomarkers & Prevention. 2001;10(4):345–353. [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England Journal of Medicine. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids. 1998;33(11):1055–1059. doi: 10.1007/s11745-998-0305-8. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowska M, Golaszewska J, Wincewicz A, Koda M, Baltaziak M, Sulkowski S. Leptin—from regulation of fat metabolism to stimulation of breast cancer growth. Pathology Oncology Research. 2006;12(2):69–72. doi: 10.1007/BF02893446. [DOI] [PubMed] [Google Scholar]
- 8.Strom SS, Wang X, Pettaway CA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clinical Cancer Research. 2005;11(19 I):6889–6894. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 9.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 10.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in OB/OB mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 11.Stattin P, Söderberg S, Hallmans G, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. Journal of Clinical Endocrinology & Metabolism. 2001;86(3):1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 12.Chang S, Hursting SD, Contois JH, et al. Leptin and prostate cancer. Prostate. 2001;46(1):62–67. doi: 10.1002/1097-0045(200101)46:1<62::aid-pros1009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Hsing AW, Chua S, Jr., Gao Y-T, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. Journal of the National Cancer Institute. 2001;93(10):783–789. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 14.Stattin P, Kaaks R, Johansson R, et al. Plasma leptin is not associated with prostate cancer risk. Cancer Epidemiology Biomarkers & Prevention. 2003;12(5):474–475. [PubMed] [Google Scholar]
- 15.Freedland SJ, Sokoll LJ, Mangold LA, et al. Serum leptin and pathological findings at the time of radical prostatectomy. Journal of Urology. 2005;173(3):773–776. doi: 10.1097/01.ju.0000152619.96795.b2. [DOI] [PubMed] [Google Scholar]
- 16.Baillargeon J, Platz EA, Rose DP, et al. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiology Biomarkers & Prevention. 2006;15(7):1331–1335. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- 17.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. Journal of Surgical Research. 2004;116(2):337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Somasundar P, Frankenberry KA, Skinner H, et al. Prostate cancer cell proliferation is influenced by leptin. Journal of Surgical Research. 2004;118(1):71–82. doi: 10.1016/j.jss.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. The American Journal of Surgery. 2004;188(5):560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. Journal of Biological Chemistry. 2003;278(43):42660–42667. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 21.Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281(5383):1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 22.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocrine-Related Cancer. 2006;13(2):629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attoub S, Noe V, Pirola L, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, Rho-, and Rac-dependent signaling pathways. The FASEB Journal. 2000;14(14):2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 24.Tartaglia LA. The leptin receptor. Journal of Biological Chemistry. 1997;272(10):6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 25.Banks AS, Davis SM, Bates SH, Myers MG., Jr. Activation of downstream signals by the long form of the leptin receptor. Journal of Biological Chemistry. 2000;275(19):14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 26.Ihle JN. STATs and MAPKs: obligate or opportunistic partners in signaling. BioEssays. 1996;18(2):95–98. doi: 10.1002/bies.950180204. [DOI] [PubMed] [Google Scholar]
- 27.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clinical Cancer Research. 2002;8(4):945–954. [PubMed] [Google Scholar]
- 28.Niu G, Shain KH, Huang M, et al. Overexpression of a dominant-negative signal transducer and activator of transcription 3 variant in tumor cells leads to production of soluble factors that induce apoptosis and cell cycle arrest. Cancer Research. 2001;61(8):3276–3280. [PubMed] [Google Scholar]
- 29.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of STAT3 signaling pathway. The Prostate. 2000;42(3):239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Katiyar SK, Meeran SM. Obesity increases the risk of UV radiation-induced oxidative stress and activation of MAPK and NF-κB signaling. Free Radical Biology and Medicine. 2007;42(2):299–310. doi: 10.1016/j.freeradbiomed.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoque A, Albanes D, Lippman SM, et al. Molecular epidemiologic studies within the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Cancer Causes and Control. 2001;12(7):627–633. doi: 10.1023/a:1011277600059. [DOI] [PubMed] [Google Scholar]