Abstract
A central issue in evolutionary biology is the extent to which complex social organization is under genetic control. We have found that a single genomic element marked by the protein-encoding gene Gp-9 is responsible for the existence of two distinct forms of social organization in the fire ant Solenopsis invicta. This genetic factor influences the reproductive phenotypes and behavioral strategies of queens and determines whether workers tolerate a single fertile queen or multiple queens per colony. Furthermore, this factor affects worker tolerance of queens with alternate genotypes, thus explaining the dramatic differences in Gp-9 allele frequencies observed between the two social forms in the wild. These findings reveal how a single genetic factor can have major effects on complex social behavior and influence the nature of social organization.
Keywords: fire ants/polygyny/social behavior/social evolution/Solenopsis invicta
Organic evolution is marked by a small number of major transitions, one of which is the evolution of complex social behavior (1, 2). Social life can take a variety of forms, each distinguished by features such as group size and the reproductive roles of group members (3, 4–8). A critical need in evolutionary biology is to identify the causes of social behavior and its conspicuous variation and especially to determine the extent to which social organization is under genetic control (9–15). Such information is crucial for reconstructing pathways of social evolution (16, 17) and can lead to improved genetic models for the origin and spread of new social variants (18–20) that may constitute adaptive solutions to changed environments or transitional stages during speciation (3, 21–25). Current views on insect social evolution stress the importance of ecological and behavioral environments in molding what are largely plastic social behaviors (3, 17, 21, 22, 25–30). As a result of this emphasis on behavioral plasticity and an immediate role of the environment, studies of the genetic underpinnings of social behavior have languished. Here we present evidence that major variation in the social organization of fire ant colonies is under simple genetic control, providing a unique demonstration of a strong genetic component to complex social behavior.
The fire ant Solenopsis invicta is an introduced pest species in the southern United States that exists in two distinct social forms. The monogyne form features colonies with a single fertile (egg-laying) queen, whereas the polygyne form features colonies with multiple fertile queens. The two social forms differ in other major aspects of their reproductive biology, including the phenotypes and reproductive strategies of young queens and the modes of colony founding (3). Monogyne nests produce heavy queens that accumulate large fat reserves and exhibit rapid oogenesis, whereas polygyne nests produce mostly lighter queens with lesser reserves and more gradual oogenesis (31). These phenotypic differences are adaptive because young monogyne queens found colonies independently (without workers), a task requiring large energy reserves and rapid reproduction, whereas polygyne queens generally seek adoption as egg layers into existing polygyne nests, a strategy requiring neither attribute. Previously, the two forms were found to exhibit pronounced allele frequency differences at the enzyme-encoding gene Pgm-3 (32), and the phenotypes of young polygyne queens were shown to be associated with their genotype at this locus (33). Furthermore, polygyne queens possessing a particular Pgm-3 genotype were never accepted as new egg layers by polygyne workers but instead were executed as they attempted to initiate reproduction (32, 33). These findings led to the conclusion that Pgm-3 is under different selection regimes in the two social forms of S. invicta and that the gene is important in regulating reproduction and social structure in polygyne colonies (3). More recently, another protein-encoding gene, designated Gp-9, was found to be very tightly linked to Pgm-3 (recombination frequency, rf = 0.0016; ref. 34). Remarkably, Gp-9 is a better predictor of polygyne queen acceptance (35) and exhibits even stronger allele-frequency differences between the forms than Pgm-3 (34). Indeed, a common allelic variant in the polygyne form, Gp-9b, is completely absent in the monogyne form (34). Thus, the aim of the present study was to learn whether Gp-9 (or genes that it marks) controls the form of social organization in S. invicta. This was accomplished by determining whether variation at this gene is associated with the three major social differences between the forms, namely (i) differences in queen phenotype, (ii) differences in the identity of queens accepted as nestmates by workers of the two forms, and (iii) differences in the numbers of queens accepted by workers of the two forms.
EXPERIMENTAL METHODS
We tested whether the different queen phenotypes characteristic of each social form are associated with variation at Gp-9 by taking advantage of the fact that both genotypes BB and Bb are found among young polygyne queens.‡ Entire polygyne colonies (n = 20) collected in northern Georgia were transferred into laboratory rearing units by using standard procedures. All sexuals were removed from these colonies except for adult winged (virgin) queens with unsclerotized cuticles (0–3 days old). Twenty of these queens were collected from each colony after another 8 days (when they were 8–11 days old) and placed individually in small fragments of the source colony containing ≈300 worker brood and adults. This separation of young queens removed them from the pheromonal signals from fertile nestmates that normally act to suppress reproductive development of young queens (36). After 3 days of separation, each of the queens was weighed, and the presence or absence of their wings was noted (wing shedding marks the onset of oogenesis; ref. 37).
We conducted two sets of experiments to determine whether queen genotype at Gp-9 influences the tolerance shown by polygyne workers toward queens when they are introduced into the workers’ colonies. The first set of experiments was designed to mimic the natural process by which polygyne colonies adopt new queens (38). For the first such experiment, entire colonies (n = 29 polygyne and n = 15 monogyne) collected in areas of northern Georgia known to contain only the monogyne or polygyne form were transferred into laboratory rearing units. Forty (polygyne colonies) or 20 (monogyne colonies) winged virgin queens were collected from each colony when 8–11 days old and placed individually in small fragments of the source colony. After 3 days of separation, 20 queens from each source colony were introduced into a foreign polygyne test colony containing four fertile queens and several thousand worker brood and adults (33); the remaining 20 queens from each polygyne source colony were genotyped at Gp-9. All of the introduced queens surviving for 3 days in the test colonies, which were judged to have been accepted, were collected and genotyped. By comparing the genotypes of the surviving queens with those of their nestmates, we could infer the change in Gp-9 genotype frequencies caused by worker discrimination and thus determine the effect of Gp-9 genotype on queen survival in the test colonies. The use of virgin queens in this assay was appropriate because virgin egg-laying queens are common in polygyne S. invicta nests (39).
For a second experiment mimicking natural queen recruitment, newly mated queens of each form were introduced into polygyne test colonies to assess worker tolerance. These queens were collected from the ground immediately after a mating flight in areas of northern Georgia known to contain only one form or the other. Queens from the monogyne-colony area were introduced into 15 test colonies (10 per colony), as were queens from the polygyne-colony area (20 per colony). Gp-9 genotype proportions in queens collected from the polygyne-colony area were estimated by genotyping a subset (n = 450) of these queens. Among the BB queens collected from this area, some portion comprised polygyne queens, whereas the remainder comprised immigrant monogyne queens. These proportions were estimated by determining the mtDNA haplotypes of a subset (n = 81) of BB queens [haplotypes diagnostic for each form exist in Georgia (ref. 40; C. DeHeer, M. Goodisman, & K.R., unpublished data)]. From these proportions we calculated the number of BB queens of each form that were introduced into the test colonies. Surviving queens were genotyped at Gp-9.
A second set of experiments testing whether queen Gp-9 genotype influences polygyne worker tolerance involved introductions of older, fertile queens into the test colonies. Four types of fertile queens were used: normal monogyne mother queens (genotype BB, n = 14 queens originating from N = 14 colonies), normal polygyne queens (genotype Bb, n = 64 and N = 16), food-deprived monogyne mother queens (genotype BB, n = 18 and N = 18), and polygyne queens made physogastric (exhibiting extreme ovarian development; genotype Bb, n = 23 and N = 23). The latter two categories comprised queens that were manipulated to phenotypically resemble queens of the alternate social form. Food-deprived monogyne queens were held individually with <500 workers and provided only one small mealworm on alternate days as food for 1 week before their introduction into the test colonies. Such treatment, previously shown to reduce queen fecundity in S. invicta (41), reduced the average weight of monogyne test queens to 12.1 ± 1.0 mg [mean ± SD; fertile polygyne queens typically weigh <15 mg, whereas monogyne queens typically weigh >17 mg (42, 43)]. Queen weight and fecundity are highly correlated in S. invicta (42–46), so these food-deprived monogyne queens resembled polygyne queens with respect to their fecundity as well as their weight. Fertile polygyne queens were made physogastric by being held individually in laboratory rearing units with several thousand worker adults and brood from their parent colony and being fed ad libitum over a period of 5 months. These queens weighed 18.6 ± 1.9 mg at the time of their testing, and thus they resembled monogyne queens with respect to both their weight and fecundity. All introduced queens of polygyne origin originated from different colonies than those used as test colonies. Four normal polygyne queens were introduced into each of the 16 test colonies; for the remaining three categories of fertile queens, a single queen was introduced into each test colony. Queens accepted by the test colonies were genotyped at Gp-9.
To examine the relative extent to which the tolerance displayed by polygyne workers toward multiple queens is influenced by worker Gp-9 genotype and by the social environment in which workers develop, we established test colonies in which fertile polygyne (Bb) queens were held individually in laboratory rearing units for 5 months. All adult workers were removed from each unit twice during the first 2 months of the rearing period, so that the workers present at the time of testing had experienced only a single queen as immatures and as adults. We introduced five fertile polygyne (Bb) queens into each unit to test the tolerance of these workers. As a control, we conducted the same test on monogyne colonies containing only BB queens and BB workers that were kept under identical conditions and were subjected to the same manipulations. Mother queens heading the single-queen test units in this and the subsequent experiments were removed 3 days before the introduction of the foreign queens to extinguish recognition cues associated with the mother (47, 48) and thereby favor the acceptance of the introduced queens (49). Results qualitatively similar to those presented were obtained when the mother queens were present during the introductions.
For another series of experiments designed to test the effects of Gp-9 genotype on worker tolerance toward Bb queens, newly mated queens collected in northern Georgia immediately after a mating flight were allowed to found colonies individually in the laboratory. These colonies developed for a period of 6 months, at which point they contained many hundred worker adults and brood that had experienced only a single fertile queen (their mother). Five fertile polygyne (Bb) queens were then introduced into each colony to determine worker responses. Gp-9 genotypes were obtained for each foundress queen and 20 of her worker offspring to infer the genotype of the single haploid mate of the queen and to determine the genotypic makeup of the colony worker force (34, 50). The social form of BB foundresses was ascertained by determining their mtDNA haplotypes, with only queens bearing a haplotype diagnostic for one of the forms being included in these experiments (ref. 40; C. DeHeer, M. Goodisman, & K.R., unpublished data). Colonies with four different queen/worker compositions were tested: (i) colonies with a monogyne (BB) queen and BB workers, (ii) colonies with a polygyne BB queen and BB workers, (iii) colonies with a polygyne BB queen and Bb workers, and (iv) colonies with a polygyne Bb queen and Bb (and other) workers.
To test the effect of Gp-9 genotype on worker tolerance toward BB queens, we introduced such queens into different types of test colonies started individually by newly mated queens in the laboratory (the workers therefore had experienced only a single queen, their mother). The social form of each foundress queen and the Gp-9 genotype composition of her worker offspring were determined as described above. In a first variant of this experiment, single polygyne BB queens were introduced into colonies that were founded by heterozygous polygyne queens and thus contained Bb workers. In a second variant, single BB queens were introduced into colonies that were founded by BB queens of the alternate form and that contained only BB workers (the queens had mated with B males). Seven trials of this latter type were conducted; five involved queens of polygyne origin being introduced into test colonies headed by monogyne queens, and two involved monogyne queens being introduced into colonies headed by queens of polygyne origin. Finally, two BB queens were simultaneously introduced into each of two colonies that were founded by polygyne BB queens and contained only BB workers to determine whether such colonies would tolerate multiple queens.
RESULTS AND DISCUSSION
We first tested the hypothesis that the different queen phenotypes characteristic of each social form of S. invicta are associated with variation at Gp-9.§ This hypothesis stems from the observation that young (nonfertile) queens of the monogyne form, which are heavy individuals, all are BB homozygotes at Gp-9, whereas young queens of the polygyne form, which are generally light, are mostly Bb heterozygotes (34). ANOVA showed that the weight of young polygyne queens [which correlates with fat content (31)] is significantly influenced by their Gp-9 genotype (F = 55.13, df = 1, P < 0.001), with BB homozygotes significantly heavier than other queens (15.2 ± 2.0 and 11.5 ± 1.5 mg, respectively; mean ± SD; n = 348).¶ Moreover, young polygyne queens with genotype BB are similar in weight (15.7 ± 1.2 mg) to young monogyne queens (31), all of which possess this same genotype. Gp-9 genotype also influences the rate of oogenesis in young polygyne queens, with BB queens initiating oogenesis significantly sooner than other polygyne queens (G = 42.56, df = 1, P < 0.001; n = 348). Thus, queen reproductive phenotype, a complex individual trait closely tied to colony social organization, is under genetic control in this ant, with the gene(s) involved associated with Gp-9.‖ Importantly, these genetic effects on queen phenotype explain the earlier finding that the B allele is fixed in the monogyne form (34) because the independent mode of colony founding practiced by monogyne queens requires large energy reserves and rapid oogenesis.
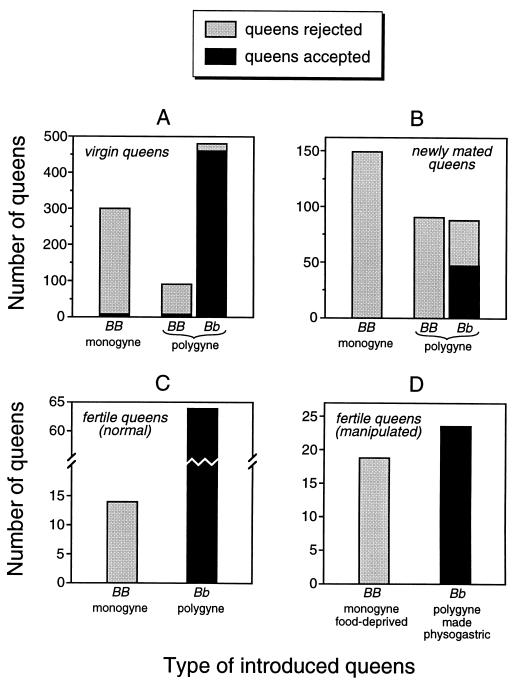
The phenotype of the queen not only determines whether queens can successfully found new colonies independently, it also influences which queens, and how many, are accepted as new egg layers in existing S. invicta nests (33, 41). Given that Gp-9 affects queen phenotype, it is of interest to learn whether a queen’s genotype at this locus predicts her acceptability to workers. Therefore, we compared the responses of polygyne workers toward queens with different Gp-9 genotypes when these queens were introduced into the workers’ colonies. The first such experiment, which mimicked the natural process of queen adoption by polygyne colonies, revealed that worker acceptance of virgin queens ready to begin egg laying is strongly associated with queen Gp-9 genotype. Virtually all virgin monogyne queens (genotype BB) were destroyed by workers, as were most virgin polygyne queens with genotype BB (Fig. 1A; the proportions of colonies accepting these two types of queens did not differ significantly, P > 0.33; all analyses used Fisher’s exact test). The few BB queens accepted by polygyne colonies in this experiment might have been individuals that were not fully mature, as polygyne worker aggression toward BB queens is known to intensify with queen age (35). In contrast to the results for BB queens, virgin polygyne queens with genotype Bb invariably were accepted in multiples by each of the polygyne test colonies [Fig. 1A; the proportion of colonies accepting such queens was significantly greater than the proportion accepting either monogyne (BB) or polygyne BB queens, both P < 0.0001]. In a second experiment mimicking natural queen adoption, newly mated queens were introduced into polygyne test colonies. A strong bias against BB queens of either social form again was evident: no BB queens originating from either monogyne or polygyne nests survived introduction, whereas about half of the Bb (polygyne) queens survived (Fig. 1B; the proportion of colonies accepting Bb queens was significantly greater than the proportions accepting BB queens of either form, both P < 0.0001). The results of these experiments show that young BB queens ready to begin reproduction are discriminated against by polygyne workers, regardless of the social form of their origin.
Figure 1.
Rejection and acceptance of S. invicta queens introduced into foreign polygyne test colonies. The different types of queens tested were young, nonfertile queens, including both virgin queens (A) and newly mated queens (B), and fertile queens, including both normal fertile queens (C) and fertile queens manipulated to resemble queens of the alternate form (D). The Gp-9 genotype and social form of origin of introduced queens is indicated under each bar.
Similar responses of polygyne workers toward queens of different Gp-9 genotypes were observed in a second set of experiments in which older, fertile queens were introduced into the test colonies. All introduced monogyne queens (genotype BB) were killed by the workers (Fig. 1C). In contrast, all polygyne queens (which possessed genotype Bb; ref. 34) were accepted (Fig. 1C), confirming tolerance of multiple Bb queens per nest by polygyne workers (the proportion of colonies accepting these queens was significantly greater than the proportion accepting monogyne queens, P < 0.0001). Because fertile polygyne queens generally weigh less and have lower fecundity than fertile monogyne queens (51), we wished to rule out the possibility that the different responses of polygyne workers toward queens of the two forms were caused by differences in queen weight or fecundity. Therefore, we manipulated the phenotypes of fertile queens to resemble those of queens of the alternate form, thus uncoupling Gp-9 genotype from some aspects of queen phenotype. This manipulation was done by depriving monogyne (BB) queens of food, so that their low weight and fecundity resembled that of polygyne (Bb) queens, and by rearing polygyne queens in a monogyne state, so that they attained the extreme ovarian development (physogastry) characteristic of fertile monogyne queens. Reducing the weight and fecundity of monogyne queens had no effect on their acceptability to polygyne workers, as all queens so treated were nonetheless killed (Fig. 1D; the proportion of colonies accepting these queens did not differ from the proportion accepting unmanipulated monogyne queens, P > 0.99). Similarly, increasing the weight and fecundity of polygyne queens did not alter their acceptability, as all physogastric polygyne queens were accepted as supernumerary reproductives by the polygyne test colonies (Fig. 1D); the proportion of colonies accepting these queens did not differ from the proportion accepting unmanipulated polygyne queens but was significantly greater than the proportion accepting manipulated or unmanipulated monogyne queens (P > 0.99, P < 0.0001, and P < 0.0001, respectively). Thus, some phenotypic correlate of Gp-9 genotype other than weight or fecundity (most likely queen odor; ref. 35) primarily determines the acceptability of queens to polygyne workers. The discrimination against BB queens demonstrated by polygyne workers in these experiments explains why genotype Bb but not BB is found among egg-laying queens of this social form (34). In fact, such discrimination, coupled with apparent age-dependent lethal effects of genotype bb in all females (34), results in virtually all polygyne egg-laying queens bearing the heterozygous genotype at Gp-9.
The invariable presence of Bb queens in polygyne nests means that these nests always contain Bb workers (34), and it is primarily workers with this genotype that destroy BB queens (35). To learn whether the corresponding tolerance shown by Bb workers toward multiple Bb queens can be extinguished by manipulating the social environment in which these workers develop (thus uncoupling worker genotype from social environment), we created test colonies that were headed for an extended period by single fertile polygyne (Bb) queens. The workers produced in these test units, at least half of which possessed genotype Bb,** always accepted multiple polygyne (Bb) queens introduced into their units, despite having experienced only monogyny during their entire immature and adult lives (Table 1). Workers in monogyne control colonies, in contrast, invariably killed all introduced polygyne (Bb) queens (Table 1); the proportion of monogyne control colonies accepting Bb queens was significantly less than the proportion of colonies headed by single polygyne queens accepting them (P < 0.0001). These results imply that the tendency of polygyne workers to retain multiple Bb queens does not depend on their social environment (number of queens in their colony), but rather on the workers’ genotypes at Gp-9. Colonies containing Bb workers accept multiple queens, but only if those queens have genotype Bb, whereas colonies containing only BB workers invariably reject Bb queens. The fact that colonies containing any Bb workers accept multiple Bb queens suggests that the presence of such workers in mixed worker groups confers their characteristic behavior (tolerance of Bb queens) on the entire group. Such dominant behavioral effects creating colony-level phenotypes in mixed worker groups have been postulated to occur in social insects in the context of worker control of colony sex allocation (52) and have been demonstrated empirically in the context of hygienic behavior in honey bees (53).
Table 1.
Acceptance of fertile polygyne queens of S. invicta (genotype Gp-9Bb) when introduced into foreign test colonies headed by a fertile monogyne queen or by a fertile polygyne queen kept in monogynous condition for five months
| Genotypes of workers in test colony | Type of queen heading test colony
|
|
|---|---|---|
| Monogyne queen (BB) | Polygyne queen (Bb) made monogynous | |
| BB | 0 (0) | — |
| n = 85, N = 17 | ||
| Bb + another | — | 0.92 (1.0) |
| n = 150, N = 30 | ||
The proportions of total introduced queens that were accepted are shown, followed in parentheses by the proportions of test colonies in which any queens were accepted. Gp-9 genotypes of the queens heading each test colony and the workers constituting it are indicated. The numbers of queens introduced are indicated by n and the numbers of colonies into which they were introduced by N (five queens were introduced into each test colony). The composition of worker genotypes in each test colony depends on the genotype of the queen and of the single haploid male with which she mated (50). All monogyne (BB) queens mated with B males, so only BB workers were produced. The polygyne (Bb) queens mated with either B males or b males, yielding colonies containing a mixture of heterozygous workers and workers with one of the two homozygous genotypes.
To completely disentangle the effects of Gp-9 and social form on worker tolerance toward fertile Bb queens, multiple fertile polygyne queens were introduced into colonies founded in the laboratory by single newly mated queens of each social form. Colonies with four different queen/worker compositions were tested: (i) colonies with a monogyne (BB) queen and BB workers, (ii) colonies with a polygyne BB queen and BB workers, (iii) colonies with a polygyne BB queen and Bb workers, and (iv) colonies with a polygyne Bb queen and Bb (and other) workers. If worker genotype determines the type and number of queens accepted independently of the social environment, then the first two types of colonies (i.e., those lacking Bb workers) but not the latter two types (i.e., those containing at least some Bb workers) are predicted to reject Bb queens. These predictions were confirmed (Table 2). No colonies lacking Bb workers accepted any of the introduced queens, whereas colonies containing such workers always accepted several (P < 0.0001 for the difference in proportions of these two classes of colonies accepting Bb queens). Thus, worker social behavior and resulting social organization are influenced by worker Gp-9 genotype but not by the social environment in which workers or their mothers develop.
Table 2.
Acceptance of fertile polygyne queens of S. invicta (genotype Gp-9Bb) when introduced into foreign test colonies headed by single fertile queens of each social form
| Genotypes of workers in test colony | Type of queen heading test colony
|
||
|---|---|---|---|
| Monogyne queen (BB) | Polygyne queen
|
||
| BB | Bb | ||
| BB | 0 (0) | 0 (0) | — |
| n = 25, N = 5 | n = 15, N = 3 | ||
| Bb or Bb + another | — | 0.80 (1.0) | 0.97 (1.0) |
| n = 15, N = 3 | n = 35, N = 7 | ||
The proportions of total introduced queens that were accepted are shown, followed in parentheses by the proportions of test colonies in which any queens were accepted. Gp-9 genotypes of the queens heading each test colony and the workers constituting it are indicated. The numbers of queens introduced are indicated by n and the numbers of colonies into which they were introduced by N (five queens were introduced into each test colony). All monogyne (BB) queens mated with B males, so only BB workers were produced. The polygyne BB queens mated with either B males or b males, yielding colonies containing exclusively BB workers or Bb workers. The polygyne Bb queens also mated with either B males or b males, yielding colonies containing a mixture of heterozygous workers and workers with one of the two homozygous genotypes.
A final set of experiments tested the effect of Gp-9 on worker tolerance toward BB queens. To compare the acceptability of such queens to BB and Bb workers while eliminating any effect of social environment, we introduced BB queens into different types of queenless test colonies started individually by newly mated queens in the laboratory. When single polygyne BB queens were introduced into colonies that were founded by heterozygous polygyne queens and thus contained Bb workers, they were killed in 5 of the 10 trials despite their having originated from the same social form as the foundress of the test colony. The equivocal responses of workers in this test, combined with the observation that virgin polygyne BB queens were not attacked in queenless polygyne colony fragments in which they were isolated (but only when they were returned to their parent colony; see above), suggest that Bb queens must be present as referents in polygyne nests in order for worker discrimination against BB queens to be fully manifested. In contrast to the preceding results, when single BB queens were introduced into queenless colonies that were founded by BB queens of the alternate form and that contained only BB workers, they were accepted in all seven trials (P < 0.05 for acceptance-rate differences in this and the previous experiment). Moreover, when two BB queens were introduced into each of two colonies that were founded by polygyne BB queens and contained only BB workers, only one queen was accepted, whereas the other was executed. This latter result is important because previous studies have shown that queenless monogyne colonies (which contain only BB workers) similarly retain only a single monogyne (BB) queen when several are offered (41). The combined results from these and the previous experiments reveal a strong tendency for colonies lacking Bb workers to tolerate only a single queen, which also must not possess the b allele, whereas colonies possessing Bb workers tolerate multiple queens, which also must bear the b allele.
This study shows that three fundamental social characteristics distinguishing the two social forms of introduced S. invicta are under the control of a single genetic factor marked by Gp-9. First, genotype at Gp-9 influences weight and rapidity of oogenesis in young queens, traits linked to the contrasting reproductive strategies of queens of the two forms. Second, the acceptability of queens as replacement or supernumerary reproductives in nests of the two forms is influenced by queen Gp-9 genotype. Finally, preferences of workers for the number and type of fertile queens are controlled by worker Gp-9 genotypes. These results are important because they explain the remarkable patterns of variation seen at Gp-9 in wild S. invicta populations (34): the b allele is absent in the monogyne form because the low queen weight associated with it is incompatible with independent colony founding, but it is common in the polygyne form because it is required in both castes for the stable expression of polygyny. More generally, our results reveal how a single genetic factor can have major effects on complex social behavior and thus determine the form of social organization. This demonstration of a simple heritable basis to social organization shows that natural selection can act on single genes (or single linkage groups) to dramatically alter the course of social evolution, a conclusion that contrasts with the widely held view that social behavior is a complex syndrome of traits strongly influenced by the environment and only diffusely determined by simple genotypic variation (21–23, 54). Future studies will focus on native South American populations, in which additional complexity in the genetic determinants of social organization exists (34, 55), to unravel the underlying genetic architecture and gain a more complete understanding of the genetic basis of social evolution in these ants.
Acknowledgments
We thank M. Chapuisat, P. Christe, C. DeHeer, M. Goodisman, J. Goudet, M. Mescher, R. Page, G. Robinson, and one anonymous reviewer for comments on the manuscript, and C. DeHeer, M. Goodisman, and M. Mescher for important insights and help in obtaining samples. This work was funded by grants from the U.S. and Swiss National Science Foundations and the National Geographic Society.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
To whom reprint requests should be addressed. e-mail: kenross@arches.uga.edu.
The third genotype at this biallelic locus, bb, is largely absent in adult queens and workers, apparently because of age-dependent lethal effects associated with it (34). The few bb individuals detected in this study were excluded from all analyses.
Evidence suggests that the product of Gp-9 may be directly responsible for at least some of the phenotypic effects we report (34, 55), but it is also possible that Gp-9 is simply a marker for a linkage group including one or more other genes of major effect.
Queen weight is significantly influenced not only by Gp-9 genotype but also by colony of origin (F = 4.51, df = 19, P < 0.001); no significant interaction effect was found for these two variables.
Queen genotype at the linked gene Pgm-3 has no effect on queen weight and onset of oogenesis once the effect of Gp-9 is taken into account (55).
Queens of both social forms of S. invicta mate with only a single haploid male (50); thus, the female offspring of heterozygous mothers should segregate in a 1:1 ratio of heterozygotes and homozygotes of one type at loci with two alleles. Because of the apparent age-dependent lethality of genotype bb in females, Bb daughters often are overrepresented among the daughters of Bb queens that mated with b males (34).
References
- 1.Wilson E O. The Insect Societies. Cambridge, MA: Harvard Univ. Press; 1971. [Google Scholar]
- 2.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. New York: Freeman; 1995. [Google Scholar]
- 3.Ross K G, Keller L. Annu Rev Ecol Syst. 1995;26:631–656. [Google Scholar]
- 4.Emlen S T. In: Behavioral Ecology: An Evolutionary Approach. 2nd Ed. Krebs J R, Davies N B, editors. Oxford: Blackwell; 1991. pp. 301–335. [Google Scholar]
- 5.Ross K G, Matthews R W, editors. The Social Biology of Wasps. Ithaca, NY: Cornell Univ. Press; 1991. [Google Scholar]
- 6.Keller L, editor. Queen Number and Sociality in Insects. Oxford: Oxford Univ. Press; 1993. [Google Scholar]
- 7.Keller L, Reeve H K. Trends Ecol Evol. 1994;9:98–102. doi: 10.1016/0169-5347(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 8.Choe J C, Crespi B J, editors. The Evolution of Social Behavior in Insects and Arachnids. Cambridge, U.K.: Cambridge Univ. Press; 1997. [Google Scholar]
- 9.Moritz R F A, Hillesheim E. Behav Ecol Sociobiol. 1985;17:87–89. [Google Scholar]
- 10.Oldroyd B P, Smolenski A J, Cornuet J, Crozier R H. Nature (London) 1994;371:749. [Google Scholar]
- 11.Page R E, Robinson G E. Behav Ecol Sociobiol. 1994;35:99–107. [Google Scholar]
- 12.Heinze J, Tsuji K. Res Popul Ecol. 1995;37:135–149. [Google Scholar]
- 13.Moritz R F A, Kryger P, Allsopp M H. Nature (London) 1996;384:31. [Google Scholar]
- 14.Page R E. Endeavor. 1997;21:114–120. doi: 10.1016/s0160-9327(97)80220-7. [DOI] [PubMed] [Google Scholar]
- 15.Robinson G E, Fahrbach S E, Winston M L. BioEssays. 1997;19:1099–1108. doi: 10.1002/bies.950191209. [DOI] [PubMed] [Google Scholar]
- 16.West-Eberhard M J. In: Animal Societies: Theories and Facts. Itô Y, Brown J L, Kikkawa J, editors. Tokyo: Japan Sci. Soc. Press; 1987. pp. 35–51. [Google Scholar]
- 17.Wcislo W T, Danforth B N. Trends Ecol Evol. 1997;12:468–474. doi: 10.1016/s0169-5347(97)01198-1. [DOI] [PubMed] [Google Scholar]
- 18.Crozier R H, Pamilo P. Evolution of Social Insect Colonies: Sex Allocation and Kin Selection. Oxford: Oxford Univ. Press; 1996. [Google Scholar]
- 19.Wolf J B, Brodie E D, Cheverud J M, Moore A J, Wade M J. Trends Ecol Evol. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]
- 20.Young L J, Wang Z, Insel T R. Trends Neurosci. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- 21.Herbers J M. In: Queen Number and Sociality in Insects. Keller L, editor. Oxford: Oxford Univ. Press; 1993. pp. 262–293. [Google Scholar]
- 22.Bourke A F G, Heinze J. Philos Trans R Soc London B. 1994;345:359–372. [Google Scholar]
- 23.Bourke A F G, Franks N R. Social Evolution in Ants. Princeton: Princeton Univ. Press; 1995. [Google Scholar]
- 24.Baird R W, Dill L M. Behav Ecol. 1996;7:408–416. [Google Scholar]
- 25.Richards M H, Packer L. Oikos. 1996;77:68–76. [Google Scholar]
- 26.Herbers J M. Behav Ecol Sociobiol. 1986;19:115–122. [Google Scholar]
- 27.Satoh T. Insectes Soc. 1989;36:277–292. [Google Scholar]
- 28.West-Eberhard M J. In: Natural History and Evolution of Paper-Wasps. Turillazzi S, West-Eberhard M J, editors. Oxford: Oxford Univ. Press; 1996. pp. 290–317. [Google Scholar]
- 29.Schwarz M P, Silberbauer L X, Hurst P S. In: The Evolution of Social Behavior in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 333–346. [Google Scholar]
- 30.Wcislo W T. In: The Evolution of Social Behavior in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 316–332. [Google Scholar]
- 31.Keller L, Ross K G. Behav Ecol Sociobiol. 1993;33:121–129. [Google Scholar]
- 32.Ross K G. Nature (London) 1992;355:347–349. [Google Scholar]
- 33.Keller L, Ross K G. Science. 1993;260:1107–1110. doi: 10.1126/science.260.5111.1107. [DOI] [PubMed] [Google Scholar]
- 34.Ross K G. Genetics. 1997;145:961–974. doi: 10.1093/genetics/145.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller L, Ross K G. Nature (London) 1998;394:573–575. [Google Scholar]
- 36.Fletcher D J C, Blum M S. Science. 1981;212:73–75. doi: 10.1126/science.212.4490.73. [DOI] [PubMed] [Google Scholar]
- 37.Vargo E L, Laurel M. J Insect Physiol. 1994;40:601–610. [Google Scholar]
- 38.Glancey B M, Lofgren C S. Fla Entomol. 1988;71:581–587. [Google Scholar]
- 39.Ross K G, Vargo E L, Keller L. Proc Natl Acad Sci USA. 1996;93:3021–3025. doi: 10.1073/pnas.93.7.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross K G, Shoemaker D D. Heredity. 1997;78:590–602. [Google Scholar]
- 41.Fletcher D J C, Blum M S. Science. 1983;219:312–314. doi: 10.1126/science.219.4582.312. [DOI] [PubMed] [Google Scholar]
- 42.Vargo E L, Fletcher D J C. Physiol Entomol. 1989;14:223–232. [Google Scholar]
- 43.Vander Meer R K, Morel L, Lofgren C S. Physiol Entomol. 1992;17:384–390. [Google Scholar]
- 44.Ross K G. Behav Ecol Sociobiol. 1988;23:341–355. [Google Scholar]
- 45.Tschinkel W R. Physiol Entomol. 1988;13:327–334. [Google Scholar]
- 46.Vargo E L. Behav Ecol Sociobiol. 1992;31:205–210. [Google Scholar]
- 47.Obin M S, Morel L, Vander Meer R K. J Ins Behav. 1993;6:579–587. [Google Scholar]
- 48.Sundström L. Anim Behav. 1997;53:499–510. [Google Scholar]
- 49.Fletcher D J C. Adv Invert Reprod. 1986;4:305–316. [Google Scholar]
- 50.Ross K G, Fletcher D J C. Behav Ecol Sociobiol. 1985;17:349–356. [Google Scholar]
- 51.Fletcher D J C, Blum M S, Whitt T V, Temple N. Ann Entomol Soc Am. 1980;73:658–661. [Google Scholar]
- 52.Craig R. Am Nat. 1980;116:311–323. [Google Scholar]
- 53.Trump R F, Thompson V C, Rothenbuhler W C. J Apic Res. 1967;6:127–131. [Google Scholar]
- 54.Wilson E O. Sociobiology: The New Synthesis. Cambridge, MA: Harvard Univ. Press; 1975. [Google Scholar]
- 55.Keller, L. & Ross, K. G. (1998) J. Evol. Biol., in press.