Abstract
Context: The chance that a thyroid nodule is malignant is higher when there is a history of childhood radiation exposure.
Objective: The objective of the study was to determine how the size of a thyroid nodule, the number of nodules, and the distribution of nodules influence the risk of cancer in irradiated patients.
Patients: From a cohort of 4296 radiation-exposed people, we studied the 1059 that underwent thyroid surgery.
Design and Outcomes: We studied the association between the size, number, distribution, and rank order of thyroid nodules and the chance of malignancy.
Results: There were 612 malignant nodules in 358 patients and 2037 benign ones in 930 patients. There was no change in the risk that a nodule was malignant with increasing size (odds ratio 0.91/cm, P = 0.11) among the 1709 nodules that were 0.5 cm or greater. A solitary nodule had a similar likelihood of being malignant as a nodule that was one of several (18.8 vs. 17.3%), whereas patients with multiple nodules were more likely to have thyroid cancer than those with solitary nodules [30.7 vs. 18.7%; risk ratio 1.64 (1.27–2.13)]. Aspirating only the largest nodule would have missed 111 of the cancers (42%), whereas aspirating the two largest nodules would have missed 45 of the cases (17%), although none would have been 10 mm or greater.
Conclusions: In radiation-exposed patients, the following conclusions were made: 1) the likelihood that a nodule is malignant is independent of nodule number and size; 2) the likelihood of cancer is increased if more than one nodule is present; 3) evaluating the two largest nodules by fine-needle aspiration would have resulted in a significant number of cases being missed but none with large cancers; and 4) more than half of the patients with thyroid cancer had multifocal tumors.
In radiation-exposed patients, the likelihood that a nodule is malignant is independent of its size and how many nodules are present. The chance that a patient has cancer is increased when more than one nodule is present. These findings also support the recommendation in recently published guidelines that radiationexposed patients should receive special attention in terms of diagnostic evaluation.
Childhood radiation exposure increases the chance that a person will develop both benign and malignant thyroid nodules (1,2,3). Recently several endocrine organizations have released guidelines for evaluating and managing thyroid nodules (4,5,6,7). They all recognize that in radiation-exposed patients, the size of a thyroid nodule alone is an inadequate predictor of the risk of malignancy and suggest that all nodules, including the small ones (<10 mm), should undergo fine-needle aspiration (FNA) evaluation. Additionally, they recommend total thyroidectomy when surgery is indicated in a radiation-exposed patient (5,6,7).
We have been studying a cohort of 4296 radiation-exposed patients treated for benign conditions in the head and neck area predominantly in the 1940s and 1950s; 1059 patients subsequently had surgery for nodular thyroid disease and form the basis of the current study. The goal was to use the surgical findings to evaluate the recently promulgated recommendations by determining how the size of a thyroid nodule, the number of nodules, and the distribution of nodules influence the risk of malignancy in radiation-exposed patients.
Patients and Methods
Radiation-exposed cohort
We performed a retrospective analysis of data obtained in a cohort study of 4296 patients who were younger than 16 yr of age when they received external radiation treatments for various benign conditions of the head and neck area from 1939 to 1962 at Michael Reese Hospital in Chicago. The Thyroid Follow-up Program began in 1974 when an effort was made to contact all irradiated patients, inform them about the radiation exposure, and invite them to participate in the study (8,9). The study was approved by the Michael Reese Hospital and University of Illinois at Chicago institutional review boards. During the study patients were invited to the study center at which they underwent a clinical evaluation, which included a physical examination of the head and neck area and thyroid imaging. The imaging was initially thyroid scanning using Tc-pertechnetate with a pinhole collimator and then, after 1993, thyroid ultrasound. When abnormalities were detected, patients were referred to their physicians for further management. Patients also received questionnaires periodically. If a patient was diagnosed with a thyroid-related medical condition or underwent a procedure, the medical records, pathology reports, and pathology specimens were reviewed. A total of 1099 patients underwent thyroid surgery between 1947 and 2004 due to the presence of suspicious thyroid lesions. Most of the surgeries, 96.1%, occurred before 1993 and so were on the basis of physical examination and/or scintigraphy. The indications for surgery at that time were either the presence of a palpable nodule or an abnormal thyroid scan. We were unable to obtain any pathology records for 21 patients and another 19 had no thyroid neoplasms found, leaving 1059 patients with at least one thyroid nodule that were included in the current study. The extent of surgery, number of nodules per patient and their size, location, and histologic type (benign or malignant) were recorded.
Thyroid nodule characteristics
The size (largest dimension in millimeters) and the location (right lobe, left lobe, or isthmus) were recorded for each nodule according to the description in the pathology reports. As a result of the pathology review, some early cases were reclassified from papillary adenomas to papillary cancers. Later, after most of the cases had been reviewed, with the recognition that there is a follicular variant of thyroid cancer, the histological classification of differentiated thyroid cancer changed (10). Because the slides had been returned to the original institutions, cancers were reclassified based on the medical records. Mixed papillary-follicular cancers were reclassified as papillary. Follicular cancers were reclassified as follicular variant of papillary carcinoma when the original pathologist or our pathologist described the nuclear changes characteristic of papillary cancer. Other follicular cancers could not be reclassified due to insufficient information. Due to these limitations, in this study we classified thyroid nodules in two groups, malignant or benign, and did not attempt to differentiate further in their subtypes. We did not find any medullary, anaplastic, or thyroid lymphomas in our cohort.
In 33 patients with thyroid cancer, the largest focus of malignancy was in a lymph node. In 28 of these cases, the lymph node focus was larger than any of the patient’s benign nodules and was therefore considered the presenting feature. The mean size of these was 29.6 mm (range 10–55 mm, sd 13.6 mm, median 25 mm). The lymph node foci of cancer were not counted as nodules and were excluded from the analysis of size vs. malignancy. These 33 patients were excluded from the analysis of the size rank order of nodules and the analysis of focality.
For studying the association between the nodule size and malignancy, only the nodules with a known size were included in the analysis. There were 166 micronodules, described as having a size of approximately 1 mm or as microscopic or an equivalent term. These were excluded and were not used to classify patients as having cancer. This left 1998 nodules in 1059 patients, 399 of them malignant. For analyses that included the number of thyroid nodules, macronodules of unknown size were included.
For the patients with thyroid cancer, we calculated the rank order of the largest malignant nodule in relation with the rest of their nodules. In this analysis we included those benign nodules of unknown size if the pathologist indicated in his report if the nodule was smaller or larger than the biggest malignant nodule for that particular patient. Knowing the rank order of the largest malignant thyroid nodule, we calculated how many cancers would have been missed if the nodule size were the only factor used for determining the need for needle aspiration.
To determine how the number of nodules influenced the cancer risk, we divided our patients in two groups, those with solitary nodules and those with multiple nodules, and compared the prevalence and the distribution of thyroid carcinomas in them. For patients with thyroid cancer, we examined the prevalence and the distribution of additional benign and malignant thyroid nodules.
Statistical analysis
Between-group comparisons for continuous variables were carried out by logistic regression and for binary variables by χ2 analysis. The number of benign and malignant nodules was compared, controlling for covariates, using multiple linear regression. Difference at the level of P < 0.05 was considered significant.
Results
In 1059 patients we determined that there were 612 malignant nodules in 358 of them and 2037 benign ones (a total of 2649 nodules) in 930 of them (Table 1). The sizes of 64 malignant and 421 benign nodules were not known. We were unable to determine the size of any nodule in 62 patients. In 21 patients with cancer, we were unable to determine the size of any malignant nodule.
Table 1.
Thyroid nodule prevalence according to their size, malignancy, and number of patients affected
| Thyroid nodule size | Histology
|
Patients (n) | Patients with cancer (n) | ||
|---|---|---|---|---|---|
| Malignant | Benign | All | |||
| All nodulesa | 612 | 2037 | 2649 | 1059 | 358 |
| Known size | 548 | 1616 | 2164 | 977b | 337b |
| Microscopic | 149c | 17 | 166 | 133 | 54c |
| Unknown size | 64 | 421 | 485 | 62d | 21d |
In 33 patients with cancer, the largest malignant focus was in a lymph node. These foci are not included.
Number of patients with at least one nodule or one malignant nodule of known size.
One hundred twenty patients had one or more microscopic foci of cancer. In 46 of these patients, only microscopic foci were present; in another eight patients, the largest focus of cancer was in a lymph node, and only microscopic foci were in the thyroid.
Sixty-two patients with all of their nodules of unknown size and 21 patients with all of their malignant nodules of unknown size.
Relationship between the size of a nodule and the chance it is malignant
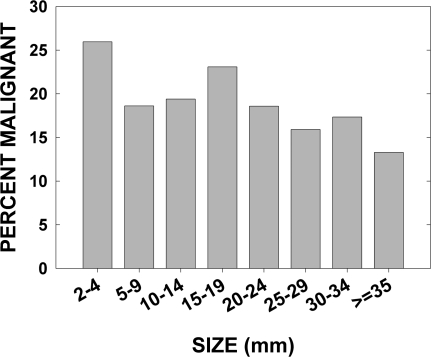
Overall, 23.1% of the 2649 nodules were malignant. We performed logistic regression to determine whether there was a relation between the size of a nodule and whether it was malignant or benign. All nodules of unknown size (n = 485) and micronodules (n = 166) were excluded. Among the micronodules, 149 were malignant and 17 were benign. The evident bias toward diagnosing tiny cancers and overlooking very small benign neoplasms was the reason for excluding them. This left 1998 nodules for this analysis, of which 399 (20.0%) were malignant. There was a small decrease (odds ratio 0.87/cm, P = 0.01) in the malignancy rate with increasing size of a nodule (Fig. 1). The first bar in Fig. 1 indicates that this bias may extend to nodules less than 0.5 cm. Further limiting the analysis to nodules 0.5 cm or greater left 1709, of which 324 (19.0%) were malignant. For this group there was no relation between the nodule size and malignancy rate (odds ratio 0.91/cm, P = 0.10).
Figure 1.
Prevalence of thyroid cancer as a function of nodule size. For nodules 5 mm or greater, there was no correlation between nodule size and thyroid cancer risk (by logistic regression analysis).
Rank order by size of benign and malignant nodules
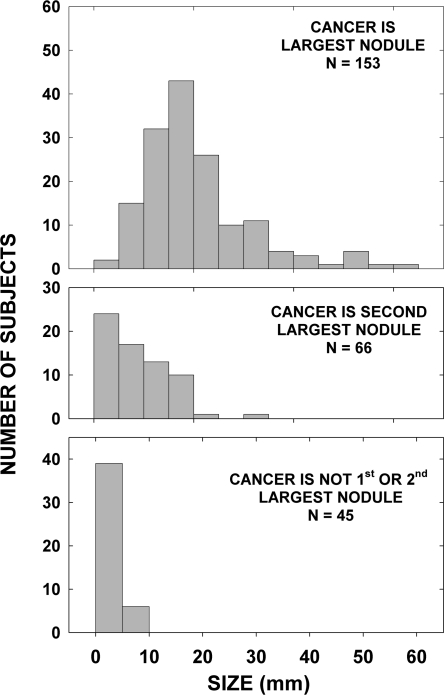
The rank order of the largest malignant nodule was determined for each case. Excluded from this analysis were the patients (n = 44) whose benign nodules and/or the largest cancer was of an unknown size, except for nine individuals in whom it was possible to determine how many benign nodules, if any, were larger than the largest malignant one. Also excluded were the 33 patients whose largest focus of cancer was in a lymph node. Those with a solitary nodule were included. Thyroid cancer was reported to be the largest nodule in 153 patients, the second largest in 66, and the third largest or greater in 45 patients (Fig. 2). In other words, in retrospect, if a strategy of aspirating only the largest nodule had been adopted, 111 of the cancer cases (42%) would have been missed, including 25 that were 10 mm or greater. If the two largest nodules were aspirated, 45 of the thyroid cancer cases (17%) would have been missed, although none with cancers 10 mm or greater.
Figure 2.
Cancer size by rank order of the largest cancer. The figure shows frequency histograms of the distribution of cancer size when the cancer was the largest (upper panel), second largest (middle panel), or the third largest or greater lesion (lower panel) among all of the nodules (benign and malignant) in 297 patients with thyroid cancer.
Relationship between the number of nodules and the risk of cancer
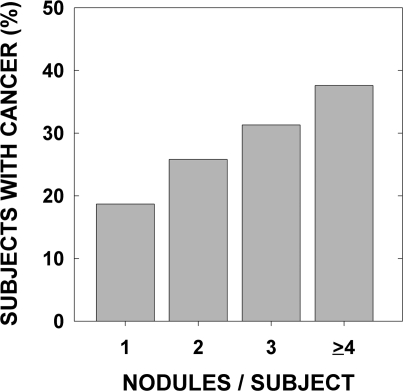
The effect of the number of nodules on the risk of cancer in each patient was analyzed in two ways. First, we studied whether the total number of nodules influenced the probability that a patient had cancer (Table 2). Of the 1026 patients with thyroid nodules (1059 patients less the 33 with the largest focus of cancer in a lymph node), 299 had isolated and 727 had multiple nodules. The number of thyroid cancer cases was 56 among the patients with solitary nodules (18.7%) and 223 in patients with multiple nodules (30.7%) (risk ratio 1.64; 95% confidence interval 1.27–2.13) and the risk increased as the number of nodules increased (odds ratio 1.35 per nodule; 96% confidence interval 1.27–1.43) (Fig. 3).
Table 2.
Frequency of thyroid cancer in patients with single and multiple nodules
| Nodules per patienta | Patients (n) | Total nodules (n)a | Malignant nodules (n)a | Patients with cancer (n)a | Patients with microcancers (n) |
|---|---|---|---|---|---|
| Solitary | 299 | 299 | 56 | 56 (18.7%)b | 37 (12.4%) |
| 2 | 306 | 612 | 116 | 79 (25.8%) | 43 (14.2%) |
| 3 | 224 | 672 | 111 | 70 (31.3%) | 41 (18.3%) |
| 4 or more | 197 | 842 | 141 | 74 (37.6%) | 15 (7.6%) |
| All multiples | 727 | 2126 | 368 | 223 (30.7%)b | 99 (13.6%) |
Excluding microscopic nodules (see Table 1).
P < 0.001 by χ2 analysis for single vs. multiple nodules.
Figure 3.
Thyroid cancer rate according to the number of nodules per patient. The risk of thyroid cancer increased with the number of nodules.
The results were similar if the assignment of patients to the cancer category also included the presence of microcarcinomas. In that case, the number of thyroid cancer cases was 68 among the patients with solitary nodules (22.7%) and 257 in patients with multiple neoplasms (35.4%) (P < 0.001). In addition, excluding all nodules less than 5 mm from consideration, rather than just the microcarcinomas, gave very similar results to those in Fig. 3. For cases with one, two, three, or four or more nodules the proportions with cancer were 19.6, 23.2, 26.5, and 36.4%, respectively.
The second analysis was whether the number of nodules influenced the probability that a nodule was cancer. A solitary nodule had a likelihood of being malignant that was the same as a nodule that was one of several. Specifically, 56 of 299 solitary nodules (18.8%) were cancerous, compared with 368 of 2126 nonsolitary nodules (17.3%). To eliminate the bias introduced by the preponderance of malignancy in small nodules, we repeated the analysis excluding nodules less than 0.5 cm. This resulted in a nonsignificant higher risk of cancer in a solitary nodule (18.7 vs. 15.6%, P = 0.08).
Cancer laterality
To describe cancer laterality, we excluded 33 cases in whom the largest malignant focus was in a lymph node and 13 cases in whom the largest focus was in the isthmus. This left 862 nodules in 287 patients with cancer in whom the exact locations were known. More than half of these patients (152 of the 287, 51.9%) had multifocal thyroid cancers. Both lobes were affected in 84 of the patients (55%), counting a malignant focus in the isthmus as in the opposite lobe. With respect to the largest cancer nodule, 68 patients (45%) had additional ipsilateral cancer, 57 (38%) had additional contralateral cancers, and 27 (18%) had additional cancers in both lobes.
Relationship between the number of malignant and benign nodules
Related to our ongoing interest in evidence for radiation susceptibility factors, we analyzed the concordance of benign and malignant nodules. We hypothesized that susceptible individuals would have more benign and malignant foci, so we determined the relationship between the number of cancer foci and benign nodules. For this analysis we omitted the cases of cancer presenting in lymph nodes, 17 cases in whom there was no information about the size of the cancer, and 46 cases in whom the only cancers were microscopic, leaving 262 cancer cases. The latter 46 cases would bias the outcome because, by definition, they must have had benign nodules as the reason for surgery. Among the remaining 262 cases, there were 62 with 77 microscopic foci. The initial analyses presented here excluded them, and then they were confirmed by analyses that included them.
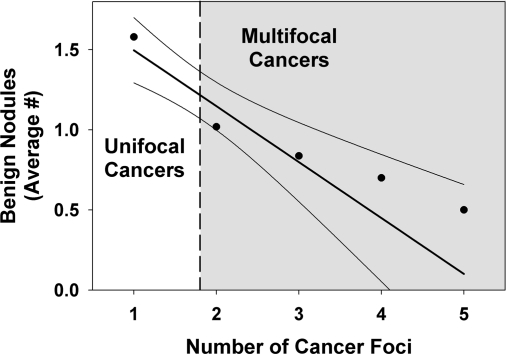
There were a total of 235 benign nodules in the 161 patients with unifocal cancers and 81 benign nodules in the 101 patients with multifocal cancers. Thus, patients with unifocal cancer had, on average, more benign nodules than patients with multifocal cancers (1.50 vs. 0.80, P < 0.01 by rank sum test). Furthermore, the number of benign nodules decreased as the number of malignant ones increased whether microscopic cancers were included (Fig. 4) or not (1.46, 0.86, 0.70, 0.63 benign nodules/patient for one, two, three, or four or more cancer foci/patient).
Figure 4.
Distribution of additional benign nodules in patients with unifocal and multifocal thyroid cancer. The slope was statistically significant (P < 0.01).
We looked at latency, surgery age, and gender as a potential explanations for the inverse relationship between the number of malignant and benign nodules. It seemed possible that a benign nodule may have led to earlier surgery and fewer malignant foci. In fact, there was a direct relationship between latency and the number of benign nodules (0.58 more nodules per 10 yr of latency, P < 0.001) but no relationship for cancer (0.04 fewer foci per 10 yr of latency, P = 0.44). The number of benign nodules remained inversely related to cancer number, controlling for latency and gender.
Discussion
The increasing prevalence of thyroid nodules and thyroid cancer in the general population had been amply documented (11). The increase is due predominantly to the escalating use of increasingly sensitive imaging modalities (12). However, radiation exposure undoubtedly contributes to this trend because, even though the effects of treatments administered in the 1940s and 1950s have lessened, they continue and the number of childhood cancers survivors treated with radiation continues to increase (2,13,14). Diagnostic x-rays with substantial exposure are frequently used in children, but no direct evidence has been presented to link this to thyroid cancer (14). Whereas routine ultrasound of the thyroid is not generally recommended for people with these exposures, it is often used (15). As a result, the management of nodules in the setting of radiation exposure remains an important issue, one addressed by the guidelines issued by the professional organizations cited above. Using the data accumulated in our long-term cohort study, we evaluated these guidelines.
The first finding of this study is that the likelihood that a radiation-related nodule is malignant is essentially independent of its size and the presence of other nodules. The decrease in cancer risk with increasing size is too small to be of any practical consequence and was seen only when we included very small (2–4 mm) nodules. It is likely that many of these small nodules would not have been detected clinically before surgery. The American Thyroid Association guidelines recommend FNA evaluation of nodules larger than 1–1.5 cm with suspicious sonographic appearance or of the largest nodule only if none has suspicious sonographic features (5). The American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi recommendations for evaluating patients with multiple nodules differ because they consider the ultrasound features the principal indication for evaluating nodules irrespective of their size (7). However, in the case of radiation-exposed patients, the guidelines from both organizations are concordant, recommending FNA evaluation of small, i.e. less than 1 cm thyroid nodules as well. Our results provide additional evidence to support the latter recommendation because we did not find any relation between the number of nodules and the risk of malignancy and only a very small effect of nodule size. The results reported by Imaizumi et al. (16) in atomic bomb survivors, in which both nodule volume and nodule volume change over time were not predictive of thyroid cancer, support this conclusion.
The second finding, which follows from the first, is that in radiation-exposed patients, the likelihood that a patient has thyroid cancer is increased if more than one nodule is present. Several recent studies have shown that patients with multiple nodules are not less likely to have thyroid cancer, as was once thought (17,18,19,20). These observations were made for patients who had no history of radiation exposure. Our results show that the risk of thyroid cancer in a given nodule is the independent of the fact that the nodule is solitary or one of several nodules. We found that the cancer risk in a patient increased progressively with the number of nodules present. As far as we know, this is the first time an increased thyroid cancer risk in irradiated patients with multinodular thyroid glands has been noted. Why this is true in irradiated patients and not in other patients probably reflects the pathogenesis; the pathogenesis of nontoxic multinodular goiters, although incompletely understood, tends to favor the induction of benign nodules, whereas radiation induces both benign and malignant nodules.
The next finding is that evaluating only the largest or the two largest nodules in patients with multiple thyroid nodules would have resulted in a significant number of small cancers being missed. This finding suggests that in radiation-exposed patients with multinodular thyroid glands, one should attempt to aspirate as many nodules as possible, including those smaller than 10 mm. Moreover, if nodule size alone was used as an indication for FNA, a significant number of cancers would have been missed, depending of the number of nodules considered for aspiration. Although it is not entirely clear how important small malignancies are in irradiated patients, it should be noted that in our cohort about 10% of patients with cancers less than 1.0 cm subsequently had recurrences (21). All of the recurrences were in the cervical area and seven of 11 of them were confirmed by surgery. Therefore, for patients with benign nodules by FNA with additional small nodules and for patients whose only nodules are too small for FNA evaluation, the findings emphasize the importance of careful follow-up.
The next finding is that more than half of the patients with thyroid cancer had multifocal tumors, and in 55% of them, both thyroid lobes were affected. These data support the recommendation of performing total thyroidectomy in all patients undergoing surgery for differentiated carcinoma when there is history of radiation exposure (recommendation 25 of the American Thyroid Association guidelines) (5).
The final finding is that among 262 patients who developed thyroid cancer, there was an inverse relationship between the number of benign and malignant nodules. The number of additional benign nodules in patients with multifocal cancers was smaller than in patients with unifocal thyroid cancer. These findings are contrary to the hypothesis that there are common susceptibility factors for developing radiation-induced benign and malignant thyroid nodules. The exact explanation for the inverse relationship is unclear but may be due, in part, to the fact that some multifocal cancers arose as intrathyroidal metastases, i.e. not independent events, and that benign and malignant nodules have different susceptibility factors.
The major strength of the study is the large number of patient exposed to radiation and then subjected to surgery and the large number of benign and malignant nodules documented by pathology reports and slide reviews. There are, however, several limitations in using the data presented here. First, they were analyzed retrospectively and were not accumulated to answer the specific questions posed. To the extent that surgery was not performed uniformly in those with abnormal examinations or scans, the findings may be biased. However, this was probably minimized by the fact that surgery was carried out in the large majority of cases. When most of the patients were treated, before ultrasound and FNA were widely used, this was the prevailing practice when there was a history of radiation exposure. Second, a number of decisions were required to define which cases were appropriate for each analysis. However, alternate choices of cases did not affect the nature of the conclusions. Because many of the surgeries were performed before thyroid ultrasonography and FNA were used, we could not make a direct correlation between ultrasound and FNA findings and pathology results.
In summary, we analyzed the findings from 1059 radiation-exposed patients who had thyroid surgery to evaluate the current recommendations for managing thyroid nodules in patients with radiation exposure. The results support the special attention, in terms of the aggressiveness of evaluation and treatment that is accorded to patients with a history of radiation exposure (22).
Acknowledgments
We thank Dr. Barbara Collins for her continued participation in these studies and Dr. David Sarne for his valuable input.
Footnotes
This work was supported in part by National Cancer Institute Grant CA21518 (to A.B.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 1, 2008
Abbreviation: FNA, Fine-needle aspiration.
References
- Schneider AB, Ron E, Lubin J, Stovall M, Gierlowski TC 1993 Dose-response relationships for radiation-induced thyroid cancer and thyroid nodules: evidence for the prolonged effects of radiation on the thyroid. J Clin Endocrinol Metab 77:362–369 [DOI] [PubMed] [Google Scholar]
- Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice Jr JD 1995 Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 141:259–277 [PubMed] [Google Scholar]
- Schneider AB, Ron E 2003 Radiation and thyroid cancer: lessons from a half century of study. In: Braverman LE, ed. Contemporary endocrinology: diseases of the thyroid. 2nd ed. Totowa, NJ: Humana Press, Inc.; 239–262 [Google Scholar]
- Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI, Tessier FN 2005 Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 237:794–800 [DOI] [PubMed] [Google Scholar]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM 2006 Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 16:109–142 [DOI] [PubMed] [Google Scholar]
- Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W 2006 European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 154:787–803 [DOI] [PubMed] [Google Scholar]
- AACE/AME Task Force on Thyroid Nodules 2006 American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract 12:63–102 [DOI] [PubMed] [Google Scholar]
- Schneider AB, Favus MJ, Stachura ME, Arnold J, Arnold MJ, Frohman LA 1978 Incidence, prevalence and characteristics of radiation-induced thyroid tumors. Am J Med 64:243–252 [DOI] [PubMed] [Google Scholar]
- Favus MJ, Schneider AB, Stachura ME, Arnold JE, Ryo UY, Pinsky SM, Colman M, Arnold MJ, Frohman LA 1976 Thyroid cancer occurring as a late consequence of head-and-neck irradiation. Evaluation of 1056 patients. N Engl J Med 294:1019–1025 [DOI] [PubMed] [Google Scholar]
- Hedinger C, Williams ED, Sobin LH 1988 International histological typing of thyroid tumours. 2nd ed. Berlin, Springer-Verlag. International histological classification of tumours. No. 11. Geneva: World Health Organization [Google Scholar]
- Ron E, Schneider AB 2006 Thyroid cancer. In: Schottenfeld D, Fraumeni Jr JF, eds. Cancer epidemiology and prevention. 3rd ed. New York: Oxford University Press; 975–994 [Google Scholar]
- Zhang Y, Zhu Y, Risch HA 2006 Changing incidence of thyroid cancer. JAMA 296:1350 [DOI] [PubMed] [Google Scholar]
- Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, Mertens AC, Liu Y, Hammond S, Land CE, Neglia JP, Donaldson SS, Meadows AT, Sklar CA, Robison LL, Inskip PD 2006 Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res 166:618–628 [DOI] [PubMed] [Google Scholar]
- Kleinerman RA 2006 Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol 36(Suppl 14):121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden K, Mahon S, Helfand M 2001 Screening high-risk populations for thyroid cancer. Med Pediatr Oncol 36:583–591 [DOI] [PubMed] [Google Scholar]
- Imaizumi M, Usa T, Tominaga T, Akahoshi M, Ashizawa K, Ichimaru S, Nakashima E, Ishii R, Ejima E, Hida A, Soda M, Maeda R, Nagataki S, Eguchi K 2005 Long-term prognosis of thyroid nodule cases compared with nodule-free controls in atomic bomb survivors. J Clin Endocrinol Metab 90:5009–5014 [DOI] [PubMed] [Google Scholar]
- Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, Mandel SJ 2000 Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med 133:696–700 [DOI] [PubMed] [Google Scholar]
- Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella CM 2002 Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 87:1941–1946 [DOI] [PubMed] [Google Scholar]
- Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore Jr FD, Larsen PR, Marqusee E, Alexander EK 2006 Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab 91:3411–3417 [DOI] [PubMed] [Google Scholar]
- Barroeta JE, Wang H, Shiina N, Gupta PK, Livolsi VA, Baloch ZW 2006 Is fine-needle aspiration (FNA) of multiple thyroid nodules justified? Endocr Pathol 17:61–65 [DOI] [PubMed] [Google Scholar]
- Bucci A, Shore-Freedman E, Gierlowski T, Mihailescu D, Ron E, Schneider AB 2001 Behavior of small thyroid cancers found by screening radiation-exposed individuals. J Clin Endocrinol Metab 86:3711–3716 [DOI] [PubMed] [Google Scholar]
- Schneider AB, Sarne DH 2005 Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Nat Clin Pract Endocrinol Metab 1:82–91 [DOI] [PubMed] [Google Scholar]