Abstract
Context: GH and IGF-I are important regulators of metabolism and body composition. In acromegaly, a state of GH and IGF-I excess, the lipolytic and insulin antagonistic effects of GH may alter adipose tissue (AT) distribution.
Objectives: Our objective was to test the hypothesis that in acromegaly whole-body AT mass is less and to examine for the first time the relationship between GH/IGF-I excess and intermuscular AT (IMAT), an AT depot associated with insulin resistance in other populations.
Design, Setting, and Patients: We conducted a cross-sectional study in 24 adults with active acromegaly compared with predicted models developed in 315 healthy non-acromegaly subjects.
Outcome Measures: Mass of AT in the visceral AT (VAT), sc AT (SAT), and IMAT compartments from whole-body magnetic resonance imaging and serum levels of GH, IGF-I, insulin, and glucose were measured.
Results: VAT and SAT were less in active acromegaly (P < 0.0001); these were 68.2 ± 27% and 79.5 ± 15% of predicted values, respectively. By contrast, IMAT was greater (P = 0.0052) by 185.6 ± 84% of predicted. VAT/trunk AT ratios were inversely related to IGF-I levels (r = 0.544; P = 0.0054). Acromegaly subjects were insulin resistant.
Conclusions: VAT and SAT, most markedly VAT, are less in acromegaly. The proportion of trunk AT that is VAT is less with greater disease activity. IMAT is greater in acromegaly, a novel finding, which suggests that increased AT in muscle could be associated with GH-induced insulin resistance. These findings have implications for understanding the role of GH in body composition and metabolic risk in acromegaly and other clinical settings of GH use.
Acromegaly patients have less visceral adipose tissue and subcutaneous adipose tissue but greater inter-muscular adipose tissue, suggesting that increased muscular fat could be associated with GH-induced insulin resistance.
GH and IGF-I have important roles in the regulation of metabolism and body composition (1,2,3). GH is lipolytic and an important regulator of fat mass (1). At supraphysiological levels, GH induces insulin resistance in liver and muscle. IGF-I, by contrast, is not lipolytic and has significant physiological insulin-sensitizing effects (4). In acromegaly, when both GH and IGF-I levels are high, their interaction is complex, but the GH excess phenotype in terms of insulin resistance and body composition predominates (5).
Although many features of the acromegaly phenotype are well known, few quantitative studies have linked specific changes in adipose tissue (AT) mass and distribution with disease severity assessed by IGF-I. In previous studies, using traditional body composition models, acromegaly patients had low body fat (6) that increased with therapy (7). However, total-body magnetic resonance imaging (MRI), a state-of-the-art technique for measuring AT distribution, had not previously been used to study acromegaly. We undertook our investigation to first characterize AT distribution by MRI in relation to disease severity based on IGF-I levels; given GH’s lipolytic effect, we hypothesized that AT, most prominently visceral, would be reduced in acromegaly in proportion to disease severity. By MRI we also sought to quantify, for the first time, the effect of GH and IGF-I excess on intermuscular AT (IMAT), AT that has accumulated around muscle and its segments. IMAT is recently recognized to be metabolically active and to correlate with insulin resistance in other populations (8,9). Through this study we also aimed to gain some insight into the relationships between GH/IGF-I excess, metabolic abnormalities, and AT distribution in acromegaly.
Subjects and Methods
Acromegaly subjects
We studied 24 subjects with active acromegaly (defined by elevated IGF-I level) (Table 1) including 15 males and nine females of which 19 were Caucasian, four Hispanic, and one African-American, with a mean age 46.2 ± 1.67 yr and body mass index (BMI) of 30.8 ± 1.12 kg/m2. Subjects’ IGF-I levels were elevated for 2.5–14 yr (mean 7.2 ± 3.97 yr) before the study. Nineteen had noncurative transsphenoidal surgery from 6 months to 14 yr previously (mean 4.2 ± 4.26 yr). Four had previous radiotherapy (RT): two γ-knife RT (5 and 8 months previously) and two stereotactic fractionated RT (6 months and 9 yr previously). Seven had previous medical therapy for acromegaly that did not normalize their IGF-I: four dopamine agonists, three long-acting octreotide, one short-acting octreotide, and two pegvisomant, which were last taken a mean of 12.2 months (range 2–36 months) before the study. Five had no previous acromegaly therapy at the time of study but subsequently had surgery. All had a GH-secreting pituitary tumor confirmed pathologically. Six males had secondary hypogonadism; five were on stable testosterone replacement doses for more than 1 yr previously, and one had an untreated mildly low testosterone level. Six females had regular menses, and three were postmenopausal and not on hormone replacement therapy. One had secondary adrenal and thyroid insufficiency treated with stable replacement doses of prednisone (5 mg/d) and Synthroid (100 μg/d). Two had type II diabetes mellitus treated with oral hypoglycemic agents, and one had type I diabetes mellitus treated with an insulin pump; glycosylated hemoglobin levels were 6–7% at the time of study. All were ambulatory with normal renal function and no liver disease.
Table 1.
Demographic and endocrinological characteristics in the 24 subjects with active acromegaly in this study
| Subject | Age (yr) | Sex | Prior therapy | IGF-I (μg/liter) | GHa (μg/liter) | Leptin (ng/ml) | HOMA | QUICKI | Composite ISI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | S | 500 | 1.8 | 1.3 | 0.420 | 0.45 | 16.01 |
| 2 | 55 | M | S/C/P | 580 | 1.2 | 8.5 | 4.398 | 0.31 | 2.12 |
| 3 | 49 | F | S/C/P | 468 | 1.5 | 24.7 | 5.630 | 0.30 | |
| 4 | 37 | M | S | 551 | 2.2 | 5.2 | |||
| 5 | 43 | M | S/RT | 725 | 10.0 | 1.2 | 1.417 | 0.36 | 6.61 |
| 6 | 51 | F | S | 572 | 1.9 | 3.9 | 0.790 | 0.40 | 13.71 |
| 7 | 40 | F | S | 671 | 3.3 | 12.9 | 5.340 | 0.30 | 2.39 |
| 8 | 46 | M | S/SA | 387 | 2.3 | 24.4 | 4.770 | 0.30 | 1.62 |
| 9 | 35 | M | 799 | 62.0 | 2.6 | 0.878 | 0.39 | 8.53 | |
| 10 | 40 | M | S/SA | 518 | 4.8 | 7.7 | 2.489 | 0.33 | 4.18 |
| 11 | 57 | M | S/RT | 855 | 3.7 | 6.8 | 2.953 | 0.33 | 3.93 |
| 12 | 50 | F | 987 | 3.8 | 35.5 | 5.679 | 0.30 | 1.44 | |
| 13 | 47 | M | 646 | 3.2 | 5.9 | 2.607 | 0.33 | 3.80 | |
| 14 | 50 | F | S/C | 486 | 2.7 | 9.9 | 1.402 | 0.36 | 5.16 |
| 15 | 43 | M | S | 337 | 0.7 | 5.6 | 0.847 | 0.39 | 7.13 |
| 16 | 41 | F | 829 | 52.0 | 4.5 | 2.131 | 0.34 | 3.32 | |
| 17 | 37 | M | S/RT | 660 | 3.5 | 8.9 | 5.986 | 0.30 | 2.06 |
| 18 | 68 | F | S | 540 | 5.6 | 16.3 | 1.293 | 0.37 | |
| 19 | 44 | M | S | 561 | 2.6 | 2.4 | 1.604 | 0.36 | 8.26 |
| 20 | 30 | F | S | 792 | 2.2 | 31.5 | 14.421 | 0.27 | 0.98 |
| 21 | 42 | F | S/RT | 517 | 2 | 38.8 | 6.519 | 0.292 | 1.632 |
| 22 | 49 | M | S/SA | 508 | 1.8 | 2.8 | 0.92 | 0.389 | 7.642 |
| 23 | 56 | M | S/BC | 828 | 8 | 5 | 1.658 | 0.354 | 3.755 |
| 24 | 50 | M | 505 | 2.4 | 3 | 1.053 | 0.38 | 6.507 | |
| Acromegaly | 46 ± 1.67 | 15 M/8 F | 618 ± 33 | 7.7 ± 3.1 | 11.2 ± 2.3 | 3.24 ± 0.65b | 0.34 ± 0.009c | 5.28 ± 0.87d | |
| Healthy | 40.5 ± 2.2 | 23 M/12 F | 1.814 ± 0.26 | 0.37 ± 0.006 | 8.76 ± 1.21 |
Normal IGF-I ranges according to age are as follows: ages 26–30 yr, 117–329 ng/ml; 31–35 yr, 115–307 ng/ml; 36–40 yr, 109–284 ng/ml; 41–45 yr, 101–267 ng/ml; 46–50 yr, 94–252 ng/ml; 51–55 yr, 87–238 ng/ml; 56–60 yr, 81–225 ng/ml; 61–65 yr, 75–212 ng/ml; 66–70 yr, 69–200 ng/ml. HOMA = [fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/liter)/22.5)] (15). QUICKI = 1/[(log(I0) + log(G0)], where I0is the fasting plasma insulin (μU/ml), and G0 is fasting blood glucose (mg/dl) (16). Composite ISI = 10,000/square root of [fasting (glucose (mg/dl) × insulin (μU/ml))] × [mean glucose × mean insulin OGTT] (17). Results in the last two rows are means ± se. SA, Somatostatin analog; BC, bromocriptine; C, cabergoline; F, female; M, male; P, pegvisomant; S, transsphenoidal surgery.
Nadir values after 100-g oral glucose in all patients except nos. 4 and 18.
b–d Acromegaly vs. healthy:
P = 0.025;
P = 0.05;
P = 0.043.
Non-acromegaly comparison groups
Body composition testing comparison group.
A group of 185 females and 130 males, ages 18–84 yr, of different ethnicities were studied to develop a model of predicted body composition. All were ambulatory, nonsmoking, weight stable (±2 kg over the previous 6 months), and not heavy exercisers. Those with a history of untreated diabetes mellitus, malignant/catabolic conditions, or taking medications that could potentially influence body composition were excluded.
Laboratory testing comparison group.
Thirty-five healthy subjects matched to the acromegaly subjects for age, sex, and BMI served as a comparison group for the insulin sensitivity assessments. The group included 23 males and 12 females, mean age 40.5 ± 2.2 yr, mean BMI 28 ± .47 kg/m2, which was comparable to the acromegaly group (Table 1).
The study was approved by the Institutional Review Boards of Columbia University Medical Center and St. Luke’s-Roosevelt Hospital Center. All subjects gave written informed consent before participation.
Study design
Laboratory testing.
Each acromegaly subject and the 35 healthy subjects underwent blood sampling after fasting and at 60, 90, and 120 min after a 100-g oral glucose tolerance test. Serum was frozen at −80 C in multiple aliquots. Fasting samples were assayed for IGF-I and leptin and those at all time points of the OGTT for insulin, glucose, and GH. Each subjects’ samples were run in the same assay and in duplicate. Laboratory and body composition testing were done on separate days within 2 wk.
Body composition testing.
Each acromegaly and body composition comparison group subject underwent the following.
Anthropometric measurements.
Body weight was measured with a digital scale to the nearest 0.01 kg and height with a stadiometer to the nearest 0.5 cm.
MRI.
Total and regional body AT volumes were measured by whole-body multislice MRI on a 1.5-T scanner (6X Horizon; General Electric, Milwaukee, WI) in all comparison and in 16 acromegaly subjects and on a Philips 1.5 T Gyroscan (Philips Medical, Cleveland, OH) in the eight remaining acromegaly subjects. Subjects were placed on the MRI platform with their arms extended above their heads, and about 40 axial images of 10 mm thickness at 40-mm intervals from head to toe were acquired. Abdominal visceral AT (VAT) and sc AT (SAT) volumes were measured using seven abdominal region slices. Trunk AT was defined as all AT in the body from the shoulder (upper limit defined as the separation between the arms and neck) to the pelvis (lower limit defined as the level of separation of the legs). The IMAT compartment was defined as the AT located between muscle groups and beneath the muscle fascia (10,11). The IMAT is distinct from and does not include intra-myocellular lipid, i.e. the lipid within myocytes. Images were analyzed with SliceOmatic image analysis software (TomoVision, Inc., Montreal, Canada) in the Image Reading Center at St. Luke’s-Roosevelt Hospital Center. MRI volume estimates were converted to mass using the assumed density of 0.92 kg/liter for AT. The coefficient of variation for repeated measurements of the same scan by the same observer of MRI-derived AT volumes is 1.7% for SAT, 2.3% for VAT, and 5.9% for IMAT (11,12).
Model development procedure
Prediction equations for the mass of total AT (TAT), VAT, and SAT compartments were developed using generalized linear models from the body composition comparison group data (Table 2). We chose to develop prediction models for AT depot mass with adjustment for factors known to influence AT mass including age, height, race, gender, and weight. For model development, the comparison group was randomly separated into two, model development (two thirds of subjects) and cross-validation (one third of subjects), groups; group characteristics were similar (Table 3). For the multiple regression analysis used to develop the prediction equations, the MRI-measured AT compartment was the dependent variable, gender was a fixed factor, and age, body weight, height, and ethnicity were included as covariates. All main effects for covariates and possible two-way interactions were investigated. Covariates that contributed significantly to the model were initially included to find the best-fitting model with the lowest se. The developed models were then validated by the leave-one-out methods (13). The prediction models were validated in the cross-validation group. TAT, SAT, and VAT values for each subject in the model cross-validation group were calculated using the developed prediction equations. Observed differences between estimated and actual AT masses were tested for significance by Student’s t tests, and the level of agreement was assessed by Bland and Altman (14) methods. Predicted and measured values did not differ significantly (Table 3). Correlation coefficients (r) of the mean measured and predicted tissue masses with the difference between them were as follows: for females, TAT r = 0.003, P = 0.96; SAT r = 0.098, P = 0.21; VAT r = 0.120, P = 0.18; and for males, TAT r = 0.101, P = 0.17; SAT r = 0.13 P = 0.18; VAT r = 0.03, P = 0.71, demonstrating good agreement between the models.
Table 2.
Developed models for prediction of TAT, SAT, VAT, and IMAT in patients with active acromegaly
| Tissue | Model |
|---|---|
| TAT | |
| Female | TAT (kg) = 34.71 − (0.042)age + (0.66)wt − (0.356)ht + (0.001)wt × age |
| Male | TAT (kg) = 29.032 + (0.121)age + (0.489)wt − (0.316)ht |
| SAT | |
| Female | SAT (kg) = 31.956 − (0.048)age + (0.641)wt − (0.334)ht + (0.001)wt × age |
| Male | SAT (kg) = 23.56 + (0.078)age + (0.438)wt − (0.264)ht |
| VAT | |
| Female | VAT (kg) = 3.248 − (0.006)age + (0.02)wt − (0.023)ht + (0.0004)wt × age − (0.110)AA − (0.165)H − (0.275)W |
| Male | VAT (kg) = 6.085 + (0.043)age + (0.051)wt − (0.057)ht |
| IMAT (11) | |
| African-American | IMAT = −0.414 + (0.187)malea + (0.008)age + (0.059)TAT − (0.00006)wt2 |
| Asian | IMAT = −0.148 + (0.187)male + (0.008)age + (0.044)TAT − (0.00006)wt2 |
| White | IMAT = −0.306 + (0.187)male + (0.008)age + (0.047)TAT − (0.00006)wt2 |
Age is in years, height (ht) is in centimeters, and weight (wt) is in kilograms. A, Asian; AA, African-American; H, Hispanic; W, White.
1 = male; 0 = female.
Table 3.
Characteristics of non-acromegaly subjects in model development and validation groups
| Model development
|
Model validation
|
|||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Sample no. | 124 | 87 | 61 | 43 |
| Age (yr) | 45.5 ± 17.2 | 36.6 ± 14.9 | 45.2 ± 16.4 | 40.0 ± 15.2 |
| Weight (kg) | 68.7 ± 14.3 | 80.0 ± 10.2 | 66.5 ± 14.4 | 84 ± 12.3 |
| Height (cm) | 162 ± 7.3 | 176 ± 6.8 | 162 ± 7.2 | 178 ± 6.7 |
| TAT (kg) (actual) | 25.2 ± 11.2 | 18.2 ± 6.79 | 23.0 ± 10.9 | 19.2 ± 7.0 |
| TAT (kg) (predicted) | 22.1 ± 9.67a | 18.2 ± 5.17d | ||
| VAT (kg) (actual) | 1.11 ± 0.75 | 2.22 ± 1.49 | ||
| VAT (kg) (predicted) | 1.22 ± 0.64b | 2.13 ± 1.23e | ||
| SAT (kg) (actual) | 21.33 ± 8.9 | 16.95 ± 6.19 | ||
| SAT (kg) (predicted) | 20.93 ± 9.3c | 16.41 ± 4.67f | ||
a–c Model validation P values for women:
P = 0.88;
P = 0.187;
P = 0.20 vs. actual value.
d–f Model validation P values for men:
P = 0.13;
P = 0.57;
P = 0.42 vs. actual value.
The prediction equations for IMAT were developed as described by Gallagher et al. (11). IMAT was predicted from sex, age, weight squared, TAT, and TAT-by-race interaction in African-American (n = 118), Asian (n = 51), and White (n = 169) women and men (Table 2). The relationship of IMAT to the predictive model was not assessed in the four acromegaly subjects of Hispanic ethnicity because the predictive model was unavailable for this ethnic group.
Hormone assays
GH was measured by a two-site immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX). The standards contain 22-kDa recombinant human GH calibrated to the World Health Organization International Reference Preparation of human GH 88/624. The intraassay coefficient of variation (CV) is 3.1%, and the interassay CV is 5.9%. Assay sensitivity in our laboratory is 0.05 μg/liter.
IGF-I was measured by chemiluminescent immunometric assay (Immulite; Diagnostic Products Corp., Los Angeles, CA). The standard is calibrated against World Health Organization First International Reference Preparation 1988, IGF-I 87/518. Insulin was measured by Immulite (Diagnostic Products). The intraassay CV is 5.3%, interassay CV is 6.1%, and sensitivity is 2 μIU/ml. Glucose was measured by the hexokinase method. Leptin was measured by human RIA kit (LINCO Research, St. Charles, MO).
Estimates of insulin sensitivity
Insulin sensitivity was estimated by homeostasis model assessment (HOMA) scores (15), by the quantitative insulin sensitivity check index (QUICKI) (16), and by a measure of whole-body insulin sensitivity derived from the oral glucose tolerance test (17), the composite insulin sensitivity index (ISI), which correlates highly with the rate for whole-body glucose disposal during the euglycemic insulin clamp (17) (Table 1).
Statistical analysis
Each subject’s VAT, SAT, TAT, and IMAT mass was compared with their respective predicted values by paired t test. Pearson’s correlation tests assessed the relationships between IGF-I or GH and the mass of each AT compartment relative to predicted, to the ratios of compartment masses (e.g. VAT/trunk AT), or to insulin sensitivity indices. Insulin sensitivity indices were compared in acromegaly and healthy subject groups by ANOVA. To define body composition changes in patients with a moderate/severe vs. a mild degree of GH/IGF-I excess, some analyses were also performed separately as indicated in Results for the 22 patients with moderate/severe acromegaly (IGF-I ≥ 1.5 times the upper limit of normal and in the two patients (nos. 8 and 15) who had IGF-I levels between 100 and 150% of the upper limit of normal. P values < 0.05 were significant. Data are given as mean ± sd unless stated otherwise.
Results
AT mass of acromegaly compared with predicted values
TAT.
The mass of AT in each compartment for each subject is shown in Table 4. TAT was below predicted in 18 of 22 subjects with moderate/severe acromegaly; on average, TAT was 81.0 ± 16% of predicted values (range 49–113%). TAT was below predicted in all 22 subjects combined (P = 0.0002) and in male (P = 0.0058) and female (P = 0.01) subjects. TAT was above predicted in two of two subjects with mildly active disease.
Table 4.
Anthropometrics and total-body MRI-derived AT masses in 24 patients with active acromegaly
| Subject no. | BMI | W/H | VAT (kg) | SAT (kg) | IMAT (kg) | TrAT (kg) | TAT (kg) |
|---|---|---|---|---|---|---|---|
| 1 | 26.1 | 0.997 | 0.791 | 9.973 | 1.052 | 6.624 | 11.816 |
| 2 | 34.1 | 0.972 | 3.036 | 20.976 | 0.888 | 16.652 | 24.885 |
| 3 | 37.5 | 0.947 | 3.631 | 30.176 | 2.808 | 24.656 | 36.659 |
| 4 | 30.6 | 0.900 | 1.288 | 19.136 | 1.018 | 12.880 | 19.633 |
| 5 | 24.6 | 0.818 | 0.460 | 8.004 | 0.346 | 4.784 | 8.773 |
| 6 | 24.7 | 0.818 | 0.828 | 15.180 | 1.066 | 9.200 | 17.085 |
| 7 | 28.2 | 0.783 | 1.196 | 20.792 | 1.425 | 13.248 | 23.441 |
| 8 | 40.8 | 1.053 | 10.580 | 38.364 | 3.642 | 40.204 | 50.083 |
| 9 | 32.0 | 0.843 | 0.368 | 12.512 | 0.402 | 7.636 | 13.350 |
| 10 | 30.5 | 0.903 | 2.392 | 24.748 | 0.882 | 16.652 | 27.947 |
| 11 | 30.0 | 0.840 | 3.036 | 22.724 | 2.256 | 18.400 | 27.959 |
| 12 | 31.2 | 0.810 | 1.330 | 28.943 | 0.786 | 16.288 | 31.065 |
| 13 | 29.5 | 0.910 | 2.114 | 18.549 | 1.494 | 13.490 | 21.880 |
| 14 | 23.2 | 0.867 | 1.073 | 14.130 | 0.774 | 9.973 | 15.977 |
| 15 | 30.2 | 0.967 | 7.745 | 25.522 | 0.754 | 23.710 | 34.020 |
| 16 | 23.9 | 0.651 | 0.500 | 13.300 | 0.805 | 14.639 | 14.639 |
| 17 | 35.9 | 0.893 | 2.700 | 28.900 | 2.252 | 33.817 | 33.817 |
| 18 | 32.0 | 0.907 | 3.042 | 25.900 | 2.723 | 19.832 | 31.648 |
| 19 | 25.0 | 0.888 | 0.464 | 13.900 | 0.946 | 9.739 | 15.288 |
| 20 | 43.5 | 0.950 | 1.822 | 40.599 | 1.479 | 26.961 | 44.198 |
| 21 | 40.0 | 1.0 | 2.133 | 43.391 | 1.452 | 26.865 | 46.103 |
| 22 | 29.5 | 0.97 | 2.486 | 17.876 | 1.370 | 12.387 | 21.728 |
| 23 | 28.5 | 0.988 | 2.231 | 18.830 | 0.758 | 12.403 | 21.640 |
| 24 | 28.0 | 0.951 | 2.401 | 14.574 | 0.998 | 9.653 | 17.972 |
TrAT, Trunk AT; W/H, waist/hip ratio.
VAT.
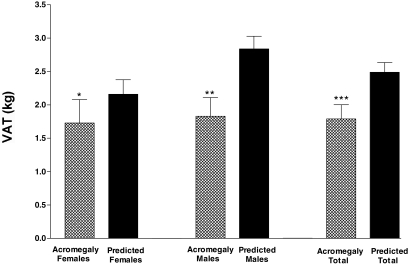
VAT was below predicted in 20 of 22 subjects with moderate/severe disease; on average, VAT was 68.2 ± 27% of predicted (range 13.9–114%) (Fig. 1). VAT was below predicted in these 22 subjects combined (P < 0.0001) and separately in male (P = 0.0002) and female (P = 0.04) subjects. VAT was above predicted in two of two subjects with mildly active disease.
Figure 1.
Mean VAT in subjects with active acromegaly (patterned bar) and their VAT predicted by the model (solid bar) shown separately for females, males, and the total group of subjects with moderate/severe acromegaly (n = 22). VAT was significantly below predicted VAT in the females alone (*, P = 0.04), males alone (**, P = 0.002), and the total group of subjects with active acromegaly (***, P < 0.001).
To examine whether VAT remained below predicted relative to less TAT, predicted VAT was also adjusted for TAT (model not shown). In this adjusted model, VAT remained significantly below predicted in 19 of 22 subjects with moderate/severe acromegaly (P = 0.0002); VAT was 67.5 ± 23% of predicted (range 13.5–160%).
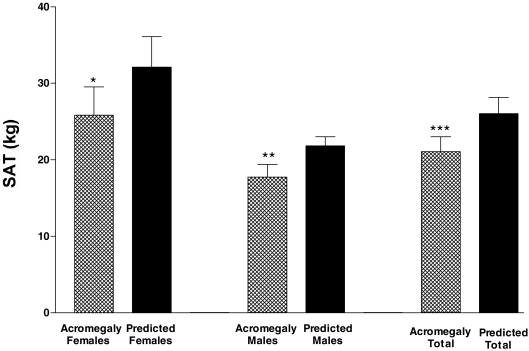
SAT.
SAT was below predicted in 19 of 22 with moderate/severe disease; on average, SAT was 79.5 ± 15% of predicted values (range 52–108%) (Fig. 2). SAT was below predicted in the 22 subjects combined (P < 0.0001) and in male (P = 0.0018) and female (P = 0.006) subjects (Fig. 2). SAT was also below predicted in two of two patients with mildly active disease.
Figure 2.
Mean SAT in subjects with active acromegaly (patterned bar) vs. their SAT predicted by the model (solid bar) shown separately for females, males, and the total group of subjects with moderate/severe acromegaly (n = 22). SAT was significantly below predicted in the females alone (*, P = 0.006), the males alone (**, P = 0.0018), and in the total group of subjects with active acromegaly (***, P < 0.001).
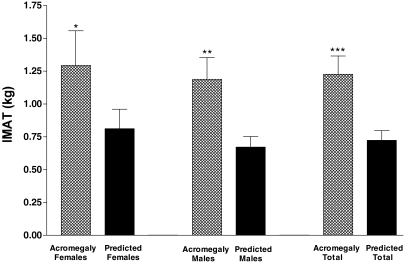
IMAT.
IMAT was above predicted in 17 of 20 subjects; on average, IMAT was 185.6 ± 84% of predicted values (range 71–424%) (Fig. 3). IMAT was above predicted overall (P = 0.0003) and in males alone (P = 0.002) and showed a trend in females (P = 0.067). The IMAT study group included 18 patients without diabetes and two with type II diabetes; IMAT was above predicted in 15 of 18 patients without diabetes and two of two patients with type II diabetes. IMAT remained significantly above predicted among the 18 nondiabetic subjects (P = .001, paired t test, IMAT vs. predicted).
Figure 3.
Mean IMAT in subject with active acromegaly (patterned bar) shown in relation to their IMAT predicted by the model (solid bar) shown separately for females, males and the total group of subjects with acromegaly (n = 20 non-Hispanic subjects). Females alone showed a trend for IMAT to be above predicted (*, P = 0.067). IMAT was significantly elevated above predicted in the males alone (**, P = 0.002) and in the total group (***, P = 0.003).
AT distribution and serum IGF-I and GH levels
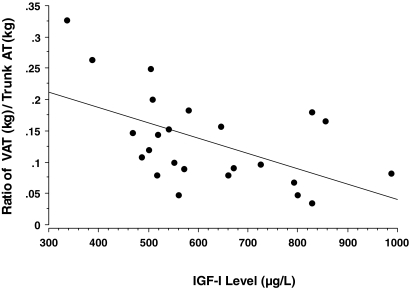
Increasing GH/IGF-I excess, as reflected in increasing IGF-I levels, was associated with a progressively smaller proportion of total trunk AT that was VAT (for the ratio of VAT to trunk AT vs. IGF-I, r = 0.544; P = 0.006) (Fig. 4). Increasing disease severity correlated with the degree of deviation of measured VAT from predicted VAT (for IGF-I levels vs. VAT/VAT predicted, r = 0.55; P = 0.0054). However, IGF-I did not correlate with the ratio of IMAT/IMAT predicted (P = 0.7253), SAT/SAT predicted (P = 0.596), or TAT/TAT predicted (P = 0.2212). Expressing IGF-I as a percentage of the upper limit of normal did not improve these correlations. Fasting and glucose-suppressed GH levels did not correlate significantly with these AT mass or distribution measures.
Figure 4.
Ratio of VAT to trunk AT vs. serum IGF-I levels in subjects with active acromegaly. Rising serum IGF-I levels are inversely related to the proportion of trunk AT that is VAT (r = 0.544; P = 0.006).
AT distribution, insulin sensitivity, and other hormone levels
Insulin sensitivity was impaired in the acromegaly vs. healthy comparison group. HOMA score for acromegaly was 3.27 ± 0.654 (mean ± se) vs. 1.814 ± 0.26 for healthy subjects (P = 0.025). QUICKI for acromegaly was 0.34 ± 0.009 vs. 0.37 ± 0.006 for healthy subjects (P = 0.05). Composite ISI was 5.28 ± 0.87 vs. 8.76 ± 1.21 (P = 0.043). Insulin sensitivity did not correlate with VAT mass. However, the composite ISI was negatively correlated with SAT (r = 0.645; P = 0.0016); TAT (r = 0.621; P = 0.0027), and trunk AT (r = 0.600; P = 0.004). Total-body IMAT mass showed a trend toward a negative correlation with QUICKI (r = 0.40; P = 0.0589) and with the composite ISI (r = 0.342; P = 0.1291) and a positive correlation with HOMA (r = 0.391; P = 0.0649), all suggesting that greater IMAT mass may be associated with reduced insulin sensitivity or increased insulin resistance in acromegaly. Correlations of the components of total IMAT, limb IMAT, or body IMAT with insulin sensitivity did not differ from those with total IMAT.
Leptin levels did not correlate with GH or IGF-I levels. Leptin correlated significantly with SAT (r = 0.865; P < 0.0001), TAT (r = 0.698; P = 0.0038), and trunk AT (r = 0.622; P = 0.0012). Leptin levels did not correlate with IMAT (r = 0.413, P = 0.451) or VAT (r = 0.249; P = 0.2415).
Discussion
The present study demonstrates that patients with GH and IGF-I excess due to acromegaly have less VAT and SAT than predicted. VAT is most reduced, and the proportion of VAT in central or trunk AT falls as disease activity, gauged by IGF-I levels, rises. A further novel finding is that unlike other AT depots, IMAT is greater than predicted, which may occur in conjunction with insulin resistance in acromegaly. Increased IMAT may be a previously unrecognized consequence of or contributor to insulin resistance in muscle associated with acromegaly.
GH directly modulates AT metabolism and causes lipolysis (1,2,18), a mechanism for the reduced VAT in acromegaly. GH-excess-enhanced AT lipolysis occurs in part through stimulation of β-adrenergic receptors, the adenylate cyclase system, and hormone-sensitive lipase expression (1,19,20). GH also inhibits lipoprotein lipase decreasing triglyceride accumulation in AT (19,21,22). Acromegaly decreases plasma and AT lipoprotein lipase activity (23) and increases lipid oxidation rates that normalize with treatment (24).
We demonstrated a more pronounced lowering of VAT than SAT mass, which is consistent with regional differences in GH effects. Although the mechanisms for preferential reduction of VAT by GH are unclear (19), catecholamine and β-adrenergic-dependent lipolysis may be greater in VAT than SAT (25,26,27). VAT is also preferentially reduced with GH therapy in GH deficiency (28,29) and in other populations (30,31). It is unknown whether VAT reduction in acromegaly affects intra- and/or retroperitoneal AT depots because we did not separately quantify these. We also demonstrate that VAT contributes less to trunk or central AT as GH/IGF-I excess increases. Similarly, VAT to SAT ratio reduction occurs as GH and IGF-I levels increase with GHRH therapy in centrally obese HIV patients (32), suggesting that this AT redistribution pattern is characteristic of increasing GH/IGF-I. Importantly, also, although both GH and IGF-I are elevated in acromegaly, the AT changes reflect those due to GH and not IGF-I, which is anti-lipolytic and has few receptors on mature adipocytes (33,34). Although IGF-I’s effects contrast with those of GH on adipocyte development, stimulating differentiation and reducing apoptosis (35,36), and suggest it may somewhat counteract GH, the combined effect of GH and IGF-I excess on AT reflects clinically just that of GH. The mechanisms for this warrant further elucidation.
In previous studies, body composition changes in acromegaly patients were generally compared with small numbers of normal subjects by bioelectrical impedance analysis (37), dual-energy x-ray absorptiometry (6), or a four-compartment model (38) that could be influenced by the greater total body weight in acromegaly (6,37,38,39). These studies, similar to ours, demonstrated lower body fat (6,38,40,41). In our study, we compared AT mass, assessed by state-of-the-art measurement technique (MRI), to predicted values developed in a large non-acromegaly group and that controlled for height, weight, age, gender, and race differences. Predictive models are advantageous to matching on one or two of the parameters or comparing group means because studying acromegaly cohorts with heterogeneous body compositions is unavoidable in this very rare disease. We considered the potential effects of other hormone changes on our results. Some men had hypogonadism, but because this increases central adiposity, it was unlikely to have lowered VAT in acromegaly. We cannot exclude that other factors unaccounted for by our models contributed to the body composition changes we detected.
We also examined the effect of GH excess on IMAT, a recently recognized and metabolically active AT depot (8,9). Higher IMAT mass has been associated with reduced insulin sensitivity in a number of populations. In lean and obese diabetic and nondiabetic populations, the IMAT compartment in muscle is negatively associated with insulin sensitivity (8,9) and positively correlated with fasting insulin (42) and glucose levels (43). In obese women with HIV, IMAT was independently associated with low insulin sensitivity (44). Recently, it was pointed out that studies to date “highlight the importance of IMAT accumulation as a critical factor regulating glucose trafficking” (45). IMAT had not previously been studied in relation to GH status.
Our data demonstrate, for the first time, that GH excess is associated with greater than predicted amounts of IMAT. Because the molecular features of IMAT have not yet been elucidated, we can only speculate as to why it is greater in acromegaly. Because AT depots are metabolically diverse in terms of GH and other effects (25,27), IMAT may be metabolically distinct and less susceptible to GH’s lipolytic effect (46). IMAT may also be relatively increased in conjunction with a GH-induced accumulation of lipid within muscle. Flux of free fatty acid (FFA) (generated by lipolysis of other depots) into muscle is increased in acromegaly (47,48). Furthermore, short-term GH administration was recently shown to increase intra-myocellular triglyceride content concomitantly with increases in circulating FFA levels (49). We did not assess intra-myocellular lipid, which would have required 1H MR spectroscopy, and it is unknown how intra-myocellular and IMAT are related, but IMAT and intramuscle lipid deposition in association with FFA flux into muscle could lead to IMAT accumulation. Additional studies are needed to define the relationship between IMAT, intra-myocellular lipid, and FFA flux into muscle in acromegaly.
IMAT may also be relatively increased in acromegaly in association with insulin resistance, as it is in other populations. The cause of insulin resistance in acromegaly is multifactorial and includes impaired insulin-stimulated glucose uptake and utilization in skeletal muscle (4,24,50,51,52,53) that may in part be due to increases in systemic or local FFA levels (54) that interfere with insulin signaling in muscle (55,56,57,58). GH has direct effects on muscle for which signaling via the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway may be important (59). Although it is unclear how IMAT could reduce insulin sensitivity, hypotheses are that it may impair muscle blood flow, reduce insulin diffusion capacity, or increase local FFA concentrations within skeletal muscle (8,60). Other studies have demonstrated the relationship of muscle lipid accumulation and insulin resistance to short-term GH administration (61,62,63,64,65), but that of long term GH exposure to IMAT and insulin resistance has not been investigated.
We examined the relationships between regional AT mass and markers of insulin resistance in acromegaly. It is likely that the degree of GH excess and total body fat as well as the distribution of AT all factor in a complex way into the clinical presentation of insulin resistance in this disease. Although our data are cross-sectional, they do suggest that total adiposity and central SAT are more contributory than VAT mass to the degree of insulin resistance in acromegaly. In addition, we found only a trend for IMAT mass to correlate with insulin resistance possibly because our study was relatively small. Thus, although our data only suggest that lipid accumulation around muscle is contributory to insulin resistance in acromegaly, further investigations are warranted to establish the relationship between IMAT, AT redistribution, and insulin resistance in acromegaly.
We also examined AT distribution and biochemical markers in acromegaly. Higher IGF-I levels, a reflection of greater integrated 24-h GH secretion, correlated with greater deviation of AT from predicted values as well as with the proportion of VAT in central AT depots. In previous studies, body fat correlated negatively with mean GH (38) and with IGF-I in some (40) but not other studies (6,38), and hormone level reductions correlated with increases in AT as measured by other techniques (6,7,66,67,68,69). When measured with a modern assay, as in our study, IGF-I levels correlated with AT changes, whereas glucose-suppressed GH levels did not. This apparent paradox, i.e. GH is responsible for the body composition changes, yet IGF-I is the better marker of them, may be explained by the fact that random or nadir GH levels are poorly predictive of the overall degree of GH excess, which is well represented by the serum IGF-I level, a reflection of integrated 24-h GH secretion (70). For this reason, IGF-I levels also correlate better with clinical disease activity and insulin sensitivity than GH levels (71,72). In acromegaly, because both GH and IGF-I are high, the interaction of these two hormones on insulin action is complex. Despite the elevated IGF-I and its important physiological role in increasing insulin sensitivity in skeletal muscle (4), which could potentially offset some of GH’s insulin-antagonistic effect (4), the GH phenotype of insulin resistance predominates in acromegaly as it also seems to with regard to body composition changes.
In conclusion, we have demonstrated that combined GH and IGF-I excess reduces total AT mass, but not all depots are similarly modulated by these hormones; VAT and SAT are decreased, but IMAT is increased by GH/IGF-I excess. Thus, fat accumulation around skeletal muscle occurs in acromegaly and could be a previously unrecognized contributor to or marker of GH-induced insulin resistance. These data have implications for understanding the effects of GH on insulin action in acromegaly as well as other clinical settings of GH use. Despite the outward apparent reduction of fat mass, an increase in IMAT could contribute to the development of unrecognized insulin resistance in muscle that could increase cardiovascular risk in acromegaly as well as in other settings of GH administration. Additional studies are warranted to further elucidate the relationship between GH excess, AT distribution, in particular IMAT, and insulin resistance.
Acknowledgments
We thank Mark Punyanitya of the Image Analysis Lab of the New York Obesity Research Center for assistance with MRI analysis and Dr. Jaime Cruz-Lobo and Dr. Fernando Arias-Mendoza of the Hatch Center for NMR Research for performing some of the MRI scans.
Footnotes
This work was funded by National Institutes of Health Grants R01 DK 064720 and K24 DK 073040 to P.U.F, P30-DK-26687 and P01-DK42618 to the Obesity Research Center, and RR 00645 to the Columbia University General Clinical Research Center.
Disclosure summary: P.U.F, W.S., S.B.H., C.M.R.-V., E.B.G., J.N.B., and D.G. have nothing to declare.
First Published Online March 18, 2008
Abbreviations: AT, Adipose tissue; BMI, body mass index; CV, coefficient of variation; FFA, free fatty acid; HOMA, homeostasis model assessment; IMAT, intermuscular AT; ISI, insulin sensitivity index; MRI, magnetic resonance imaging; QUICKI, quantitative insulin sensitivity check index; RT, radiotherapy; SAT, sc AT; TAT, total AT; VAT, visceral AT.
References
- Ho KK, O'Sullivan AJ, Hoffman DM 1996 Metabolic actions of growth hormone in man. Endocr J 43(Suppl):S57–S63 [DOI] [PubMed] [Google Scholar]
- Russell-Jones DL, Weissberger AJ 1996 The role of growth hormone in the regulation of body composition in the adult. Growth Regul 6:247–252 [PubMed] [Google Scholar]
- Bengtsson BA, Brummer RJ, Bosaeus I 1990 Growth hormone and body composition. Horm Res 33(Suppl 4):19–24 [DOI] [PubMed] [Google Scholar]
- Clemmons DR 2004 The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest 113:25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR 2002 Roles of insulin-like growth factor-I and growth hormone in mediating insulin resistance in acromegaly. Pituitary 5:181–183 [DOI] [PubMed] [Google Scholar]
- O'Sullivan AJ, Kelly JJ, Hoffman DM, Freund J, Ho KK 1994 Body composition and energy expenditure in acromegaly. J Clin Endocrinol Metab 78:381–386 [DOI] [PubMed] [Google Scholar]
- Brummer RJ, Lonn L, Kvist H, Grangard U, Bengtsson BA, Sjostrom L 1993 Adipose tissue and muscle volume determination by computed tomography in acromegaly, before and 1 year after adenomectomy. Eur J Clin Invest 23:199–205 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE 2000 Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71:885–892 [DOI] [PubMed] [Google Scholar]
- Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D 2005 Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 82:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D 2004 Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 79:874–880 [DOI] [PubMed] [Google Scholar]
- Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB 2005 Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 81:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Heshka S, Gallagher D, Kotler DP, Mayer L, Albu J, Shen W, Freda PU, Heymsfield SB 2004 Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy x-ray absorptiometry in adults. J Appl Physiol 97:655–660 [DOI] [PubMed] [Google Scholar]
- Efron B 1983 Estimating the error rate of a prediction rule: improvements on crossvalidation. J Am Stat Assoc 78:316–331 [Google Scholar]
- Bland JM, Altman DG 1986 Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310 [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ 2000 Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Nam SY, Lobie PE 2000 The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev 1:73–86 [DOI] [PubMed] [Google Scholar]
- Richelsen B 1997 Action of growth hormone in adipose tissue. Horm Res 48(Suppl 5):105–110 [DOI] [PubMed] [Google Scholar]
- Yang S, Mulder H, Holm C, Eden S 2004 Effects of growth hormone on the function of β-adrenoceptor subtypes in rat adipocytes. Obes Res 12:330–339 [DOI] [PubMed] [Google Scholar]
- Richelsen B, Pedersen SB, Kristensen K, Borglum JD, Norrelund H, Christiansen JS, Jorgensen JO 2000 Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism 49:906–911 [DOI] [PubMed] [Google Scholar]
- Ottosson M, Vikman-Adolfsson K, Enerback S, Elander A, Bjorntorp P, Eden S 1995 Growth hormone inhibits lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab 80:936–941 [DOI] [PubMed] [Google Scholar]
- Simsolo RB, Ezzat S, Ong JM, Saghizadeh M, Kern PA 1995 Effects of acromegaly treatment and growth hormone on adipose tissue lipoprotein lipase. J Clin Endocrinol Metab 80:3233–3238 [DOI] [PubMed] [Google Scholar]
- Moller N, Schmitz O, Joorgensen JO, Astrup J, Bak JF, Christensen SE, Alberti KG, Weeke J 1992 Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J Clin Endocrinol Metab 74:1012–1019 [DOI] [PubMed] [Google Scholar]
- Lafontan M, Berlan M 2003 Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci 24:276–283 [DOI] [PubMed] [Google Scholar]
- Hoffstedt J, Arner P, Hellers G, Lonnqvist F 1997 Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J Lipid Res 38:795–804 [PubMed] [Google Scholar]
- Richelsen B, Pedersen SB, Moller-Pedersen T, Bak JF 1991 Regional differences in triglyceride breakdown in human adipose tissue: effects of catecholamines, insulin, and prostaglandin E2. Metabolism 40:990–996 [DOI] [PubMed] [Google Scholar]
- Bengtsson BA, Eden S, Lonn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tolli J, Sjostrom L, Isaksson OG 1993 Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab 76:309–317 [DOI] [PubMed] [Google Scholar]
- Snel YE, Brummer RJ, Doerga ME, Zelissen PM, Bakker CJ, Hendriks MJ, Koppeschaar HP 1995 Adipose tissue assessed by magnetic resonance imaging in growth hormone-deficient adults: the effect of growth hormone replacement and a comparison with control subjects. Am J Clin Nutr 61:1290–1294 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjorntorp P, Sjostrom L, Bengtsson BA 1997 Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab 82:727–734 [DOI] [PubMed] [Google Scholar]
- Engelson ES, Glesby MJ, Mendez D, Albu JB, Wang J, Heymsfield SB, Kotler DP 2002 Effect of recombinant human growth hormone in the treatment of visceral fat accumulation in HIV infection. J Acquir Immune Defic Syndr 30:379–391 [DOI] [PubMed] [Google Scholar]
- Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S 2004 Growth hormone-releasing hormone in HIV-infected men with lipodystrophy: a randomized controlled trial. JAMA 292:210–218 [DOI] [PubMed] [Google Scholar]
- Bluher S, Kratzsch J, Kiess W 2005 Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Pract Res Clin Endocrinol Metab 19:577–587 [DOI] [PubMed] [Google Scholar]
- Clemmons DR 2006 Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol 6:620–625 [DOI] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Tornqvist H, Debatin KM, Wabitsch M 2004 Inhibition of death-receptor mediated apoptosis in human adipocytes by the insulin-like growth factor I (IGF-I)/IGF-I receptor autocrine circuit. Endocrinology 145:1849–1859 [DOI] [PubMed] [Google Scholar]
- Scavo LM, Karas M, Murray M, Leroith D 2004 Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J Clin Endocrinol Metab 89:3543–3553 [DOI] [PubMed] [Google Scholar]
- Brummer RJ, Bengtsson BA, Bosaeus I 1992 Validation of body composition determination by bioelectrical impedance analysis in acromegaly. Eur J Clin Nutr 46:47–52 [PubMed] [Google Scholar]
- Bengtsson BA, Brummer RJ, Eden S, Bosaeus I 1989 Body composition in acromegaly. Clin Endocrinol (Oxf) 30:121–130 [DOI] [PubMed] [Google Scholar]
- Brummer RJ, Lonn L, Bengtsson BA, Kvist H, Bosaeus I, Sjostrom L 1996 Comparison of different body composition models in acromegaly. Growth Regul 6:191–200 [PubMed] [Google Scholar]
- Hu HY, Yamamoto H, Sohmiya M, Abe T, Murakami Y, Kato Y 1994 Body composition assessed by bioelectrical impedance analysis (BIA) and the correlation with plasma insulin-like growth factor I (IGF-I) in normal Japanese subjects and patients with acromegaly and GH deficiency. Endocr J [Erratum (1996) 43:following 130] 41:63–69 [DOI] [PubMed] [Google Scholar]
- Kaji H, Sugimoto T, Nakaoka D, Okimura Y, Kaji H, Abe H, Chihara K 2001 Bone metabolism and body composition in Japanese patients with active acromegaly. Clin Endocrinol (Oxf) 55:175–181 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB 2003 Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26:372–379 [DOI] [PubMed] [Google Scholar]
- Yim JE, Heshka S, Albu J, Heymsfield S, Kuznia P, Harris T, Gallagher D 2007 Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 31:1400–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albu JB, Kenya S, He Q, Wainwright M, Berk ES, Heshka S, Kotler DP, Engelson ES 2007 Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am J Clin Nutr 86:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy T, Grinspoon SK 2007 Adipose compartmentalization and insulin resistance among obese HIV-infected women: the role of intermuscular adipose tissue. Am J Clin Nutr 86:5–6 [DOI] [PubMed] [Google Scholar]
- Richelsen B 1999 Effect of growth hormone on adipose tissue and skeletal muscle lipoprotein lipase activity in humans. J Endocrinol Invest 22:10–15 [PubMed] [Google Scholar]
- Rabinowitz D, Klassen GA, Zierler KL 1965 Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest 44:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz D, Zierler KL 1963 Differentiation of active from inactive acromegaly by studies of forearm metabolism and response to intra-arterial insulin. Bull Johns Hopkins Hosp 113:211–224 [PubMed] [Google Scholar]
- Krag MB, Gormsen LC, Guo Z, Christiansen JS, Jensen MD, Nielsen S, Jorgensen JO 2007 Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab 292:E920–E927 [DOI] [PubMed] [Google Scholar]
- Fineberg SE, Merimee TJ, Rabinowitz D, Edgar PJ 1970 Insulin secretion in acromegaly. J Clin Endocrinol Metab 30:288–292 [DOI] [PubMed] [Google Scholar]
- Hansen I, Tsalikian E, Beaufrere B, Gerich J, Haymond M, Rizza R 1986 Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. Am J Physiol 250:E269–E273 [DOI] [PubMed] [Google Scholar]
- Luger A, Prager R, Gaube S, Graf H, Klauser R, Schernthaner G 1990 Decreased peripheral insulin sensitivity in acromegalic patients. Exp Clin Endocrinol 95:339–343 [DOI] [PubMed] [Google Scholar]
- Foss MC, Saad MJ, Paccola GM, Paula FJ, Piccinato CE, Moreira AC 1991 Peripheral glucose metabolism in acromegaly. J Clin Endocrinol Metab 72:1048–1053 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Moller N, Christiansen JS, Jorgensen JO 2001 Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 50:2301–2308 [DOI] [PubMed] [Google Scholar]
- Boden G 2006 Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep 6:177–181 [DOI] [PubMed] [Google Scholar]
- Dominici FP, Turyn D 2002 Growth hormone-induced alterations in the insulin-signaling system. Exp Biol Med (Maywood) 227:149–157 [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI 2002 Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- Randle PJ 1998 Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14:263–283 [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Jessen N, Pedersen SB, Vestergaard E, Gormsen L, Lund SA, Billestrup N 2006 GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am J Physiol Endocrinol Metab 291:E899–E905 [DOI] [PubMed] [Google Scholar]
- Torriani M, Grinspoon S 2005 Racial differences in fat distribution: the importance of intermuscular fat. Am J Clin Nutr 81:731–732 [DOI] [PubMed] [Google Scholar]
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Haring HU 1999 Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48:1113–1119 [DOI] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI 1999 Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42:113–116 [DOI] [PubMed] [Google Scholar]
- Boden G, Lebed B, Schatz M, Homko C, Lemieux S 2001 Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50:1612–1617 [DOI] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO, Alberti KG, Flyvbjerg A, Schmitz O 1990 Short-term effects of growth hormone on fuel oxidation and regional substrate metabolism in normal man. J Clin Endocrinol Metab 70:1179–1186 [DOI] [PubMed] [Google Scholar]
- Moller N, Butler PC, Antsiferov MA, Alberti KG 1989 Effects of growth hormone on insulin sensitivity and forearm metabolism in normal man. Diabetologia 32:105–110 [DOI] [PubMed] [Google Scholar]
- Bengtsson BA, Brummer RJ, Eden S, Bosaeus I, Lindstedt G 1989 Body composition in acromegaly: the effect of treatment. Clin Endocrinol (Oxf) 31:481–490 [DOI] [PubMed] [Google Scholar]
- Landin K, Petruson B, Jakobsson KE, Bengtsson BA 1993 Skeletal muscle sodium and potassium changes after successful surgery in acromegaly: relation to body composition, blood glucose, plasma insulin and blood pressure. Acta Endocrinol (Copenh) 128:418–422 [DOI] [PubMed] [Google Scholar]
- McLellan AR, Connell JM, Beastall GH, Teasdale G, Davies DL 1988 Growth hormone, body composition and somatomedin C after treatment of acromegaly. Q J Med 69:997–1008 [PubMed] [Google Scholar]
- Tominaga A, Arita K, Kurisu K, Uozumi T, Migita K, Eguchi K, IIda K, Kawamoto H, Mizoue T 1998 Effects of successful adenomectomy on body composition in acromegaly. Endocr J 45:335–342 [DOI] [PubMed] [Google Scholar]
- Barkan AL, Beitins IZ, Kelch RP 1988 Plasma insulin-like growth factor-I/somatomedin-C in acromegaly: correlation with the degree of growth hormone hypersecretion. J Clin Endocrinol Metab 67:69–73 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Van Wyk JJ, Ridgway EC, Kliman B, Kjellberg RN, Underwood LE 1979 Evaluation of acromegaly by radioimmunoassay of somatomedin-C. N Engl J Med 301:1138–1142 [DOI] [PubMed] [Google Scholar]
- Puder JJ, Nilavar S, Post KD, Freda PU 2005 Relationship between disease-related morbidity and biochemical markers of activity in patients with acromegaly. J Clin Endocrinol Metab 90:1972–1978 [DOI] [PubMed] [Google Scholar]