Abstract
Context: Leptin and ghrelin, hormones involved in human energy homeostasis, are both produced in the stomach.
Objective: We sought to determine whether the presence of Helicobacter pylori affects gastric and systemic levels of leptin and ghrelin.
Design, Setting, and Patients: We consecutively enrolled 256 patients referred for upper endoscopy at a Veterans Affairs outpatient endoscopy center.
Outcomes: We obtained fasting serum, fundic and antral biopsies, and gastric juice. Based on histological, biochemical, and serological assays, patients were categorized as H. pylori+ or H. pylori−. Leptin and total ghrelin levels in serum, gastric biopsies, and gastric juice were determined by specific ELISAs.
Results: Of the 256 subjects, 120 were H. pylori+ and 96 were H. pylori−; 40 patients of indeterminate status were excluded. Serum and fundic leptin levels correlated with body mass index in the H. pylori+ (r = 0.35; P < 0.0001 and r = 0.35; P < 0.0001, respectively) and H. pylori− (r = 0.65; P < 0.0001 and r = 0.41; P < 0.0001, respectively) groups, but H. pylori+ subjects had significantly lower serum leptin levels [median 2.2 ng/ml (interquartile range 0.9–4.6) vs. 4.0 ng/ml (1.7–7.2); P = 0.0003]. Serum ghrelin levels were similar in the H. pylori+ and H. pylori− groups [median 1651 pg/ml (interquartile range 845-2247) vs. 1629 pg/ml (992–2886); P = 0.23]. H. pylori status did not significantly affect gastric biopsy leptin and ghrelin levels. Ghrelin levels in gastric juice varied over 4 log10 (<80–776,000 pg/ml) and correlated with gastric juice pH in the H. pylori+ group (r = 0.68; P < 0.0001).
Conclusions: These findings provide evidence that H. pylori status affects leptin and ghrelin homeostasis, presumably via intragastric interactions.
There are lower fasting circulating leptin levels, but unchanged circulating ghrelin levels, in gastric Helicobacter pylori colonization although gastric juice ghrelin varied with pH in the H. pylori-positive subjects, suggesting that H. pylori status may play a role in the regulation of energy metabolism through its effects on gastric physiology.
Energy balance and food intake are functions central to the survival of animals and humans and are partly regulated by the hormones leptin and ghrelin (1). Leptin is the peptide product of the ob gene expressed by adipocytes and by chief and endocrine P cells in the gastric epithelium (2). Serum leptin levels reflect body fat stores and help maintain stable body weight by suppressing food intake and increasing energy metabolism (1). Gastric leptin contributes to serum leptin levels (2), regulates intestinal nutrient absorption, delays gastric emptying, signals short-term satiety via vagal afferent nerves, and is associated with Barrett’s esophagus (3,4).
Ghrelin is a peptide hormone predominantly produced by the X/A cells in the gastric oxyntic mucosa (5). Ghrelin stimulates food intake, decreases energy expenditure, and promotes weight gain (6). In contrast to leptin, serum ghrelin levels are inversely related to adipose tissue mass and may help maintain energy homeostasis when nutrients are scarce (7). Ghrelin also exerts anti-ulcerative effects in the rat stomach, perhaps via the ghrelin type 1b receptor, which has been identified in the human stomach and esophagus (8,9). However, whether ghrelin acts locally in the gastrointestinal lumen via gastric secretions has not been examined.
Helicobacter pylori, a persistent colonizer of the human stomach, is associated with acute gastritis (neutrophil predominance), peptic ulcer disease, and gastric adenocarcinoma. The H. pylori-induced inflammatory response affects many gastric cell types, including those responsible for leptin and ghrelin production (10). Elimination of H. pylori after antimicrobial therapy has been reported to increase nutrition and weight, suggesting that H. pylori could play a role in the regulation of leptin and ghrelin expression (11,12). In previous studies, H. pylori colonization has been associated with high, equivalent, or low circulating leptin levels (11,13,14,15,16); the relationship between H. pylori status and ghrelin levels is also uncertain (14,15,16,17,18,19,20,21,22).
We previously found that eradication of H. pylori in adult males resulted in a significant (2.4%) increase in body mass index (BMI), with a concomitant 25% increase in integrated (mean of fasting and post-meal) leptin levels and a 76% increase in integrated ghrelin levels (41). Therefore, we hypothesized that gastric H. pylori colonization reduces circulating levels of leptin and ghrelin, perhaps by inhibiting their gastric production. We also hypothesized that ghrelin and leptin are present in gastric secretions and that H. pylori colonization may affect their levels in gastric juice. To test these hypotheses, we examined gastric, circulating, and gastric juice levels of leptin and ghrelin in well-defined fasting H. pylori+ and H. pylori− adult male subjects.
Patients and Methods
Study population
At the ambulatory endoscopy unit at the Manhattan campus of the Veterans Affairs New York Harbor Healthcare System, we prospectively recruited male adults of at least 18 yr of age referred for routine upper endoscopy for any indication. To exclude gender as a variable, because females have higher average leptin levels, only male patients were studied (23). Participants also were excluded if they had known coagulopathy, history of esophageal or gastric varices, previous gastric surgery, or treatment with antibiotics, steroids, or immunomodulating agents in the month before enrollment. The Institutional Review Board of New York University School of Medicine approved the study protocol, and written informed consent was obtained from all participants.
Clinical evaluation and specimen collection
All patients presented after a 12-h overnight fast. Before the scheduled endoscopy, a physician conducted a preprocedure evaluation, including history and physical examination. Demographic and clinical information was collected via questionnaire administered by trained interviewers at study entry. Participants self-reported ethnic designation as White non-Hispanic, Black non-Hispanic, Hispanic, or Asian. Height and weight was recorded for each participant and BMI calculated as weight (kilograms) divided by height (meters squared). Just before endoscopy, 15 ml blood was collected, centrifuged, and stored as serum at −20 C until examined.
Endoscopy
A complete endoscopic evaluation of the esophagus, stomach, and duodenum up to the second portion was performed with an Olympus GIF-130 or GIF-160 videoendoscope (Olympus America, Melville, NY) after iv administration of meperidine and midazolam. Upon endoscope entry into the stomach, approximately 15 ml gastric secretion was collected for pH and peptide determination. Biopsies, which included epithelium and mucosa, were obtained from the gastric antrum for rapid urease testing (Hpfast; GI Supply, Camp Hill, PA) and from the gastric antrum, fundus, and body for culture, histological examination, and biochemical testing. Two biopsies from each site were studied. The physicians performing endoscopy were blinded to the H. pylori status of the subjects and used identical technique in obtaining biopsies from each subject. Biopsy samples measuring 8 mm3 were rinsed in phosphate buffered saline (pH 7.4) and homogenized. Gastric biopsies were stored at −80 C and gastric juice samples at −20 C.
Determination of H. pylori status
Serum was evaluated for IgG antibodies against H. pylori whole-cell and cagA antigens by ELISA, with results expressed as OD ratios relative to laboratory standards, as previously described (24,25). Values were considered positive if the OD ratio was 1.0 or higher for the whole-cell antigen assay and 0.35 or higher for the cagA antigen assay. Patients were categorized as H. pylori+ if H. pylori was isolated on culture of the gastric biopsy or if positive in at least two of the following tests: rapid urease assay, histological examination of gastric biopsy specimens for bacterial cells with characteristic morphology, or assay for serum IgG antibodies to H. pylori group or cagA antigens. Patients of indeterminate H. pylori status were positive in only one of these four tests.
Pathology
Body and antral biopsies were examined by specialized gastrointestinal pathologists. Each sample was evaluated for the presence and grade of chronic gastritis, acute gastritis, and atrophy/intestinal metaplasia in the body and antrum, according to the updated Sydney System: 0 (none), 1 (mild), 2 (moderate), or 3 (marked) (26).
Tissue and serum peptide determination
An ELISA (R&D systems, Minneapolis MN) was used to measure leptin in biopsy homogenates, serum, and gastric juice samples. Concentrations in biopsies were normalized based on biopsy protein (picograms per milligram protein) (BCA Protein Assay; Pierce Biotechnology, Rockford IL), and in serum or gastric juice per milliliter. As described by the manufacturer, the intraassay and interassay variabilities are 3.2 and 4.1%, respectively, and the minimum detectable leptin level is 0.0078 ng/ml. A competitive enzyme immunoassay (Phoenix Peptide, Burlingame CA) was used to measure ghrelin in the biopsy homogenates, serum, and gastric juice samples. The assay uses an antibody directed against the C-terminal fragment of the ghrelin peptide and detects acyl, des-acyl, and other minor forms of ghrelin. The intraassay and interassay variability of the assay are 5 and 14%, respectively, and the minimum detectable ghrelin level is 80 pg/ml. All tests were performed at least in duplicate. Identical control samples were examined for each immunoassay plate; if values for the controls were outside the interassay variability reported by the manufacturer, all samples in the plate were reassayed.
Statistical analysis
Data are expressed as mean ± sd or median and interquartile range (IQR) (25–75th percentile), depending on the distribution of the values. Continuous variables were compared using Student’s t test on log-transformed data after controlling for possible confounders [age, smoking status, gastric pH, BMI, and alcohol, proton pump inhibitor (PPI), histamine 2 (H2) blocker, or nonsteroidal antiinflammatory drug (NSAID) use] in a multivariate linear regression. We defined the thresholds for significance to maximize statistical rigor. For the t test analyses, we used the Bonferroni adjustment to establish a conservative threshold of P ≤ 0.005 for significance by dividing 0.05 by 10, the number of comparisons performed. Correlations were analyzed using the Spearman rank test, with a value of P ≤ 0.001 considered as significant to allow for 5% overall type I error. Categorical variables were compared using the χ2 test, with a significance threshold of P ≤ 0.008 (0.05 divided by 6, the number of comparisons performed). Statistical analysis was performed using SPSS software version 13.0 for Windows (SPSS Inc., Chicago, IL).
Results
Patient demographic and clinical characteristics
Among the 256 patients consecutively enrolled, 96 were H. pylori−, and 120 were H. pylori+; 40 patients of indeterminate status were excluded from further analysis. The most common indications for endoscopy in the H. pylori+ and H. pylori− groups were iron-deficiency anemia (n = 52), heme-positive stool (n = 44), gastroesophageal reflux disease (GERD) (n = 32), dyspepsia (n = 26), Barrett’s esophagus follow-up (n = 25), and dysphagia (n = 14) and did not significantly vary according to H. pylori status. The H. pylori+ and H. pylori− groups did not differ significantly in age, BMI, or aspirin, alcohol, or NSAID use; however, as expected, Black and Hispanic persons were overrepresented in the H. pylori+ group (27). Although smoking was more prevalent in the H. pylori+ group than in the H. pylori− group, this difference was not statistically significant. Compared with H. pylori− subjects, H. pylori+ subjects used antisecretory (PPI or H2 blocker) medications at lower rates, although this difference (P = 0.03) did not meet our standard for significance (P < 0.008). As expected, H. pylori+ subjects had higher gastric juice pH (10), a phenomenon observed only in antisecretory medication nonusers (Table 1).
Table 1.
Characteristics of the 216 subjects examined in this study
| Characteristic | H. pylori status | P value | |
|---|---|---|---|
| Negative (n = 96) | Positive (n = 120) | ||
| Age, yr (mean ± sd) | 64 ± 11 | 65 ± 12 | 0.50a |
| Indications for endoscopy [n (%)]c | 0.07b | ||
| Iron-deficiency anemia | 20 (21) | 32 (27) | |
| Barrett’s esophagus follow-up | 19 (20) | 6 (5) | |
| Heme-positive stool | 16 (17) | 28 (23) | |
| GERD | 15 (16) | 17 (14) | |
| Dyspepsia | 11 (11) | 15 (13) | |
| Dysphagia | 5 (5) | 9 (8) | |
| Nausea/vomiting | 3 (3) | 4 (3) | |
| Other | 9 (9) | 7 (6) | |
| Race/ethnicity [n (%)]c | 0.001b | ||
| White, non-Hispanic | 55 (57) | 33 (28) | |
| Black, non-Hispanic | 23 (24) | 51 (43) | |
| Hispanic | 15 (16) | 32 (27) | |
| Asian | 0 (0) | 2 (2) | |
| Other | 2 (2) | 1 (1) | |
| BMI, kg/m2 (mean ± sd) | 28.4 ± 5.3 | 27.6 ± 4.9 | 0.30a |
| PPI or H2 blocker use, [n (%)] | 50 (52) | 42 (35) | 0.03b |
| NSAID or aspirin use, [n (%)] | 48 (50) | 70 (58) | 0.32b |
| Smoking [n (%)] | 15 (16) | 32 (27) | 0.10b |
| Alcohol use [n (%)] | 35 (36) | 42 (35) | 0.88b |
| Gastric juice pH [median (IQR)] | 3 (2–6) | 5 (2–7) | 0.003a |
| pH in PPI users only, median (IQR) (n) | 4 (2–7) (45) | 7 (3–8) (37) | 0.05a |
| pH in H2 blocker users only, median (IQR) (n) | 2.5 (1–4.8) (4) | 2 (1–3) (9) | 0.10a |
| pH in users of both H2 blockers and PPI, median (IQR) (n) | 5 (2–5) (4) | (0) | |
| pH in antisecretory non-users, median (IQR) (n) | 2 (1–5) (43) | 4 (2–7) (74) | 0.001a |
GERD, Gastroesophageal reflux disease.
Student’s t test (based on normalized data) after controlling for possible confounders (age, smoking status, gastric pH, BMI, and alcohol, PPI, H2 blocker, or NSAID use) in a multivariate linear regression. For the t test analyses, we used the Bonferroni adjustment to establish a conservative threshold of P ≤ 0.005 for significance by dividing 0.05 by 10, the number of comparisons performed. Significant results are in bold.
χ2 test, P < 0.008 (Bonferroni adjustment: 0.05 divided by 6). Significant results are in bold.
Percent values do not add to 100 due to rounding.
Of the 216 enrolled H. pylori+ and H. pylori− subjects, 189 body and 204 antral specimens were adequate for pathological grading. Atrophy/intestinal metaplasia was more common in the H. pylori+ compared with H. pylori− group in the antrum (37 vs. 16%; P = 0.001) but not in the body (14 vs. 9%; P = 0.38). As expected, antral chronic gastritis, acute gastritis, and atrophy/intestinal metaplasia grades were significantly higher in H. pylori+ compared with H. pylori− subjects. Body acute gastritis grade was significantly higher in H. pylori+ compared with H. pylori− subjects (0.75 ± 0.98 vs. 0.04 ± 0.19, P < 0.0001) (Table 2).
Table 2.
Gastric pathology according to H. pylori status
| Pathology | H. pyloristatus | P valuea | |
|---|---|---|---|
| Negative | Positive | ||
| Body (n)b | 81 | 108 | |
| Chronic gastritis | |||
| Present (%) | 53 | 61 | 0.32 |
| Grade (mean ± sd) | 0.63 ± 0.68 | 1.02 ± 1.04 | 0.25 |
| Acute gastritis | |||
| Present (%) | 4 | 44 | <0.0001 |
| Grade (mean ± sd) | 0.04 ± 0.19 | 0.75 ± 0.98 | <0.0001 |
| Atrophy or intestinal metaplasia | |||
| Present (%) | 9 | 14 | 0.38 |
| Grade (mean ± sd)
|
0.12 ± 0.46
|
0.27 ± 0.74
|
0.25
|
| Antrum (n)b | 89 | 115 | |
| Chronic gastritis | |||
| Present (%) | 60 | 56 | 0.67 |
| Grade (mean ± sd) | 0.65 ± 0.60 | 1.03 ± 1.13 | 0.002 |
| Acute gastritis | |||
| Present (%) | 2 | 57 | <0.0001 |
| Grade (mean ± sd) | 0.02 ± 0.15 | 0.91 ± 0.98 | <0.0001 |
| Atrophy or intestinal metaplasia | |||
| Present (%) | 16 | 37 | 0.001 |
| Grade (mean ± sd) | 0.22 ± 0.60 | 0.51 ± 0.82 | 0.004 |
Based on the updated Sydney System, samples were categorized as pathology present or absent and graded according to degree of pathology: 0 (none), 1 (mild), 2 (moderate), or 3 (marked).
Presence of pathology was compared using χ2 test (P < 0.008), and gastritis grade was compared using Student’s t test (P < 0.005). Significant results are in bold.
Number (n) of adequate body and antrum specimens are provided according to H. pylori status.
Serum, gastric, and gastric juice leptin
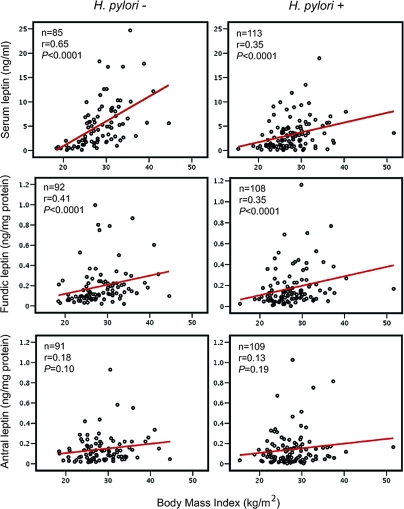
As expected, serum leptin levels were correlated with BMI in all subjects (r = 0.51; P < 0.0001), and in the H. pylori+ and H. pylori− subsets (r = 0.35; P < 0.0001 and r = 0.65; P < 0.0001, respectively) (Figure 1). Adjusted circulating leptin levels were significantly lower in H. pylori+ than H. pylori− subjects [median 2.2 ng/ml (IQR 0.90–4.6) vs. 4.0 ng/ml (1.7–7.2); P = 0.0003] (Table 3) but among the H. pylori+ subjects did not significantly vary by cagA status (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Reflecting the slope differences in Fig. 1, the serum leptin/BMI ratio was somewhat lower in the H. pylori+ group than in the H. pylori− group [median 0.08 ng·m2/ml·kg (IQR 0.04–0.16) vs. 0.13 pg-m2/ml-kg (0.06–0.26); P = 0.008] in a multivariate analysis (Table 3).
Figure 1.
Relationships of serum, fundic, and antral leptin and BMI (kilograms per square meter), according to H. pylori status. R-values represent Spearman rank coefficients in multivariate linear regressions, and P values were calculated based on the regressions.
Table 3.
Hormone concentration according to H. pylori status
| Hormone | Median hormone concentration (IQR) (n), by H. pyloristatus
|
P valuea | |
|---|---|---|---|
| Negative | Positive | ||
| Serum leptin (ng/ml) (n) | 4.0 (1.7–7.2) (85) | 2.2 (0.90–4.6) (113) | 0.0003 |
| Serum leptin/BMI (ng·m2/ml·kg) (n) | 0.13 (0.06–0.26) (85) | 0.08 (0.04–0.16) (113) | 0.008 |
| Fundic leptin (ng/mg protein) (n) | 0.13 (0.08–0.23) (92) | 0.12 (0.07–0.21) (108) | 0.21 |
| Antral leptin (ng/mg protein) (n) | 0.12 (0.05–0.19) (91) | 0.09 (0.04–0.02) (109) | 0.085 |
| Serum ghrelin (pg/ml) (n) | 1629 (992–2886) (94) | 1651 (845–2247) (119) | 0.23 |
| Fundic ghrelin (pg/mg protein) (n) | 11450 (5327–23837) (89) | 8633 (4399–16969) (107) | 0.44 |
| Antral ghrelin (pg/mg protein) (n) | 7114 (4036–12136) (91) | 8520 (3699–12446) (107) | 0.75 |
| Gastric juice ghrelin (pg/ml) (n) | 711 (323–1851) (81) | 1101 (390–23413) (106) | 0.02 |
The number (n) of specimens adequate for measurement of each hormone is provided, according to H. pylori status. To convert serum leptin values to nanomoles per milliliter, multiply by 0.08. To convert serum ghrelin values to picomoles per liter, multiply by 0.296.
Student’s t test (based on normalized data) after controlling for possible confounders (age, smoking status, gastric pH, BMI, and alcohol, PPI, H2 blocker, or NSAID use) in a multivariate linear regression. For the t test analyses, we used the Bonferroni adjustment to establish a conservative threshold of P ≤ 0.005 for significance by dividing 0.05 by 10, the number of comparisons performed. Significant results are in bold.
Fundic leptin levels also correlated with BMI (r = 0.36; P = 0.0001), with significant trends for both H. pylori+ (r = 0.35; P < 0.001) and H. pylori− (r = 0.41; P < 0.0001) subjects (Fig. 1); the relationship between antral leptin levels and BMI was not statistically significant. The significant correlations between serum and fundic leptin and BMI in the H. pylori+ subjects were driven by the relationships in those carrying cagA+ strains (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Antral and fundic leptin levels were not significantly different between the H. pylori+ and H. pylori− cohorts (Table 3). However, in the H. pylori+ subjects, antral and fundic leptin levels were directly related (r = 0.51; P < 0.0001); for H. pylori− subjects, the relationship was in the same direction but did not reach the predetermined level (P < 0.001) of significance for correlations (r = 0.30; P = 0.004).
We were unable to detect the presence of leptin in gastric juice samples from any of 10 subjects with known high gastric and circulating leptin levels; gastric pH ranged from 2–8. In addition, leptin added to gastric juice samples with pH values ranging from 2–8 could not be detected, and measured levels of exogenously added leptin progressively increased as gastric juice samples were sequentially diluted (data not shown).
Serum, gastric, and gastric juice ghrelin
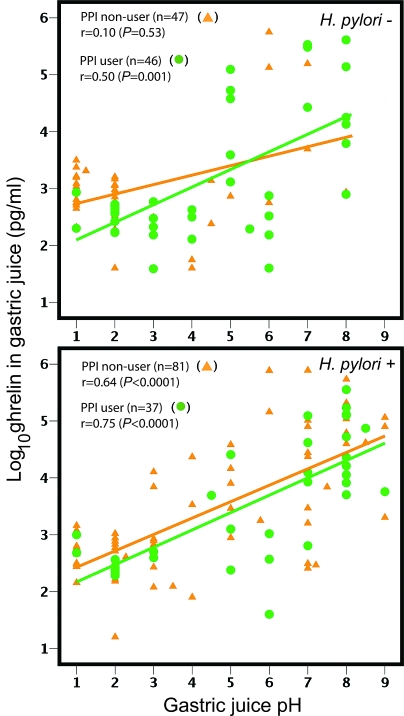
Circulating ghrelin levels obtained from the fasting subjects varied over a relatively small range (2- to 3-fold) and were similar in the H. pylori+ and H. pylori− groups [median 1651 pg/ml (IQR 845-2247) vs. 1629 pg/ml (992-2886); P = 0.23]; gastric tissue ghrelin levels also did not significantly differ according to H. pylori status. In contrast, gastric juice ghrelin levels varied over four orders of magnitude (<80 to 776,000 pg/ml). Values were somewhat higher in H. pylori+ than in H. pylori− subjects [median 1101 pg/ml (IQR 390–23413) vs. 711 pg/ml (323-1851); P = 0.02] (Table 3). Log10 ghrelin levels in gastric juice correlated with pH in the H. pylori+ group (r = 0.68; P < 0.0001), regardless of PPI use, and in the H. pylori− group for PPI users (r = 0.51; P = 0.001) but not for PPI nonusers (Fig. 2). The relationship was present in persons with cagA+ (both PPI users and nonusers) and cagA− (only PPI users) H. pylori strains (supplemental Figs. 2 and 3, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Figure 2.
Relationships of gastric juice levels of ghrelin and gastric juice pH. Log10 ghrelin (picograms per milliliter) was correlated with gastric juice pH, and relationships were stratified based on H. pylori status and PPI use. Spearman rank coefficients are given; P values were calculated using multivariate linear regression.
Relationship between leptin and ghrelin levels
In the fundus, leptin and ghrelin levels correlated in the H. pylori+ group (r = 0.34; P < 0.0001) but not significantly in the H. pylori− group (r = 0.20; P = 0.07).
Discussion
Circulating leptin levels and BMI are strongly correlated because adipose tissue, the principal variable affecting BMI, is the major source of leptin (28). However, gastric leptin release also contributes to circulating leptin, as shown from experiments in which feeding fasted animals substantially depleted gastric leptin and increased serum leptin (2). Because gastric H. pylori colonization is known to affect other components of gastric hormonal physiology (e.g. gastrin, somatostastin, and pepsinogens) (10), we sought to examine the influence of H. pylori status on leptin levels. After controlling for potential confounding factors, we found that, as hypothesized, circulating leptin levels were significantly lower in H. pylori+ than in H. pylori− subjects. These results suggest that circulating leptin levels, in addition to reflecting adiposity, are modulated by H. pylori.
One explanation for our findings is that H. pylori colonization inhibits gastric leptin production, perhaps by inducing atrophic changes in leptin-producing tissues, consistent with our finding of a greater degree of antral atrophy/intestinal metaplasia in H. pylori+ compared with H. pylori− subjects. The presence of H. pylori may also modulate gastric cytokine function, thereby reducing circulating levels. Because we did not find an effect of H. pylori status on gastric leptin levels, another possibility is that H. pylori colonization, instead of affecting leptin production, alters the balance of leptin release into the luminal and systemic compartments. These hypotheses should be examined in future studies. Alternatively, H. pylori+ persons may have less adiposity relative to BMI and therefore lower serum leptin levels compared with H. pylori− persons. A recent study of indigenous Amerindians provided evidence that H. pylori+ subjects had more athletic body morphometry, consistent with this hypothesis (29).
We found that H. pylori status did not affect antral, fundic, or circulating ghrelin levels, contrary to our hypothesis and to previous reports that found lower (14,15,19,20) circulating ghrelin levels in H. pylori+ compared with H. pylori− individuals; three other previous studies also found no significant difference according to H. pylori status (16,18,21). One explanation for our findings is that the effect of long-term carriage of H. pylori on ghrelin production is minimal due to host adaptation; in contrast, eradication of H. pylori may acutely induce production of ghrelin (12). Another possibility is that any effect of H. pylori on gastric hormone production may be obscured by confounding variables that are minimized when patients serve as their own controls in eradication studies.
The levels of circulating leptin and ghrelin in the blood reflect their usually reciprocal roles in body weight regulation (7,28), but the relationship between gastric production of the two hormones in the stomach has not been studied. The positive correlation we observed between fundic leptin and ghrelin levels in H. pylori+ persons suggests that H. pylori may be involved in the coordinate regulation of gastric leptin and ghrelin production; conversely, lack of correlation in H. pylori− subjects suggest an absence of such coordination.
Despite a growing body of literature demonstrating that ghrelin regulates local gastrointestinal function, we are not aware of previous studies examining for the presence of ghrelin in gastric juice. We found that ghrelin is present in gastric juice and that levels vary over a large (4 log10) range, much greater than that for either gastric or serum ghrelin. We performed experiments demonstrating that exogenous ghrelin is stable after incubation with gastric juice samples with pH ranging from 2–8 and that endogenous ghrelin levels in gastric juice samples are consistently measured by immunoassay over a range of dilutions (data not shown). In contrast, we were unable to detect leptin in gastric juice, although other investigators have reported detecting gastric juice leptin using RIA (30).
Long-term H. pylori carriage results in progressive gastric atrophy with loss of acid-producing parietal cells (10), and our observation in these older men that gastric juice pH was higher in H. pylori+ than in H. pylori− subjects is consistent with this trend. Previous literature shows that exogenous administration of ghrelin may stimulate (31), inhibit (32), or have no effect (33) on gastric acid secretion, possibly depending on route of administration. Our finding that gastric juice ghrelin levels were related to gastric juice pH, regardless of PPI use (Figure 2), suggests that gastric juice ghrelin inhibits gastric acid secretion or, alternatively, that gastric acidity inhibits release of ghrelin into gastric juice. Another hypothesis is that H. pylori, by decreasing gastric acidity, stimulates ghrelin secretion into the gastric lumen. If this newly discovered relationship is confirmed, new mechanisms of gastric acid regulation involving ghrelin should be explored.
In this study, we chose to assess total ghrelin levels in fasted patients. Although ghrelin levels vary throughout the day according to caloric intake, Cummings et al. (34) found that prebreakfast fasting ghrelin levels strongly correlated with 24-h integrated area under the curve ghrelin values (r = 0.87; P < 0.0004). Thus, almost all studies of ghrelin physiology have relied on a single determination of fasting ghrelin as a surrogate for overall ghrelin levels. Ghrelin circulates in acyl, des-acyl, and other minor forms. We measured total ghrelin levels for the following reasons. 1) Most studies on ghrelin physiology have assessed total, not acyl, ghrelin. 2) Measurement of total ghrelin levels reflects the complex functions of the various ghrelin isoforms, including acyl and des-acyl ghrelin. 3) Accurate measurement of acyl-ghrelin in serum requires acidification of samples at the time of collection to pH 4, which we plan to do in future studies to add this dimension to the analyses (35). Similarly, we measured fasting morning leptin levels as a surrogate marker for 24-h leptin levels, because fasting morning leptin levels strongly correlate with BMI (28) and in accordance with numerous publications examining other aspects of human physiology (36).
In the present study, we describe associations between H. pylori colonization and leptin and ghrelin levels in stomach, circulation, and gastric juice, but our study design does not permit examining the mechanisms for these findings. Our study is limited because all of our subjects were men, not permitting conclusions about women. Because the majority of the patients we studied were overweight, although we controlled for potential confounders, it is possible that unanticipated variables, including other medications or medical conditions, may have influenced our results. Variation among H. pylori strains also may contribute to the differences in study results.
Our study population consisted of largely overweight, older American men of diverse ethnicities, whereas several previous studies have examined Japanese patients, mostly with BMI less than 30 (17,19,20,22). However, we believe that our obese subjects are representative of the generally obese older U.S. male population; mean BMI in our study was 27, whereas in 2002, American males had a mean BMI of 28 (37). The prevalence of H. pylori in our cohort was 47%, similar to a seroprevalence of 32.5% in a sample of the general U.S. adult population and 56.9% in those over age 70 (27). Although Hispanic and Black subjects were overrepresented in the H. pylori+ group, adjustment for ethnicity did not affect our analyses. These findings are consistent with two recent studies demonstrating no relationship between ethnicity and serum leptin levels (38,39).
Strengths of our study include our ability to control for confounding variables, particularly BMI, age, alcohol, PPI or NSAID use, smoking status, and gastric pH. We also used five validated tests to determine H. pylori status (40) and removed patients of indeterminate status to maximize case ascertainment; most previous studies have used much more limited criteria to assess H. pylori status (13,14,15,16,17,18,19,20,21,22).
In conclusion, H. pylori colonization was associated with reduced circulating leptin levels, independent of BMI, and fundic ghrelin and leptin levels were directly related. Ghrelin is present in gastric juice over a large range of concentrations and is strongly correlated with gastric pH. Our findings are consistent with the hypothesis that H. pylori, a persistent resident of the human stomach, may have an impact on human health and disease by its involvement in the regulation of leptin and ghrelin expression.
Supplementary Material
Footnotes
This work was supported in part by National Institutes of Health Grants R01 GM63270, R01CA97946, and K23CA107123, by the Michael Saperstein Medical Scholars Fund, and by the Diane Belfer Program for Human Microbial Ecology in Health and Disease.
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 8, 2008
Abbreviations: BMI, Body mass index; H2, histamine 2; IQR, interquartile range; NSAID, nonsteroidal antiinflammatory drug; PPI, proton pump inhibitor.
References
- Cummings DE, Overduin J 2007 Gastrointestinal regulation of food intake. J Clin Invest 117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ 1998 The stomach is a source of leptin. Nature 394:790–793 [DOI] [PubMed] [Google Scholar]
- Guilmeau S, Buyse M, Bado A 2004 Gastric leptin: a new manager of gastrointestinal function. Curr Opin Pharmacol 4:561–566 [DOI] [PubMed] [Google Scholar]
- Francois F, Roper J, Goodman AJ, Pei Z, Ghumman M, Mourad M, de Perez AZ, Perez-Perez GI, Tseng CH, Blaser MJ 2008 The association of gastric leptin with oesophageal inflammation and metaplasia. Gut 57:16–24 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML 2001 Circulating ghrelin levels are decreased in human obesity. Diabetes 50:707–709 [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Konturek SJ, Kwiecien S, Drozdowicz D, Bielanski W, Pajdo R, Ptak A, Nikiforuk A, Pawlik WW, Hahn EG 2004 Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept 120:39–51 [DOI] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M 2002 The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 87:2988 [DOI] [PubMed] [Google Scholar]
- Blaser MJ, Atherton JC 2004 Helicobacter pylori persistence: biology and disease. J Clin Invest 113:321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T 2001 Gastric leptin and Helicobacter pylori infection. Gut 49:324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS 2003 Plasma ghrelin following cure of Helicobacter pylori. Gut 52:637–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Blaser MJ, Francois F, Mathew JP, Ye XY, Goldberg JD, Bini EJ 2005 Helicobacter pylori and overweight status in the United States: data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 162:579–584 [DOI] [PubMed] [Google Scholar]
- Salles N, Menard A, Georges A, Salzmann M, de Ledinghen V, de Mascarel A, Emeriau JP, Lamouliatte H, Megraud F 2006 Effects of Helicobacter pylori infection on gut appetite peptide (leptin, ghrelin) expression in elderly inpatients. J Gerontol A Biol Sci Med Sci 61:1144–1150 [DOI] [PubMed] [Google Scholar]
- Konturek PC, Czesnikiewicz-Guzik M, Bielanski W, Konturek SJ 2006 Involvement of Helicobacter pylori infection in neuro-hormonal control of food intake. J Physiol Pharmacol 57(Suppl 5):67–81 [PubMed] [Google Scholar]
- Jun DW, Lee OY, Lee YY, Choi HS, Kim TH, Yoon BC 2007 Correlation between gastrointestinal symptoms and gastric leptin and ghrelin expression in patients with gastritis. Dig Dis Sci 52:2866–2872 [DOI] [PubMed] [Google Scholar]
- Shiotani A, Miyanishi T, Uedo N, Iishi H 2005 Helicobacter pylori infection is associated with reduced circulating ghrelin levels independent of body mass index. Helicobacter 10:373–378 [DOI] [PubMed] [Google Scholar]
- Gokcel A, Gumurdulu Y, Kayaselcuk F, Serin E, Ozer B, Ozsahin AK, Guvener N 2003 Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol 148:423–426 [DOI] [PubMed] [Google Scholar]
- Isomoto H, Nakazato M, Ueno H, Date Y, Nishi Y, Mukae H, Mizuta Y, Ohtsuru A, Yamashita S, Kohno S 2004 Low plasma ghrelin levels in patients with Helicobacter pylori−associated gastritis. Am J Med 117:429–432 [DOI] [PubMed] [Google Scholar]
- Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K 2005 Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab 90:10–16 [DOI] [PubMed] [Google Scholar]
- Cindoruk M, Yetkin I, Deger SM, Karakan T, Kan E, Unal S 2007 Influence of H. pylori on plasma ghrelin in patients without atrophic gastritis. World J Gastroenterol 13:1595–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C 2004 Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol 99:2121–2127 [DOI] [PubMed] [Google Scholar]
- Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V 1997 Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab 82:579–584 [DOI] [PubMed] [Google Scholar]
- Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ 1988 Campylobacter pylori antibodies in humans. Ann Intern Med 109:11–17 [DOI] [PubMed] [Google Scholar]
- Cover TL, Glupczynski Y, Lage AP, Burette A, Tummuru MK, Perez-Perez GI, Blaser MJ 1995 Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol 33:1496–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MF, Genta RM, Yardley JH, Correa P 1996 Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20:1161–1181 [DOI] [PubMed] [Google Scholar]
- Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G 2000 Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis 181:1359–1363 [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL 1996 Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- Marini E, Maldonado-Contreras AL, Cabras S, Hidalgo G, Buffa R, Marin A, Floris G, Racugno W, Pericchi LR, Castellanos ME, Groschl M, Blaser MJ, Dominguez-Bello MG 2007 Helicobacter pylori and intestinal parasites are not detrimental to the nutritional status of Amerindians. Am J Trop Med Hyg 76:534–540 [PubMed] [Google Scholar]
- Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M, Lewin MJ 2000 Leptin secretion and leptin receptor in the human stomach. Gut 47:178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K 2000 Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 276:905–908 [DOI] [PubMed] [Google Scholar]
- Sibilia V, Pagani F, Guidobono F, Locatelli V, Torsello A, Deghenghi R, Netti C 2002 Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric acid secretion in conscious rats. Neuroendocrinology 75:92–97 [DOI] [PubMed] [Google Scholar]
- Dornonville de la Cour C, Lindstrom E, Norlen P, Hakanson R 2004 Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept 120:23–32 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS 2001 A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K 2004 Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem 50:1077–1080 [DOI] [PubMed] [Google Scholar]
- Zhang F, Chen Y, Heiman M, Dimarchi R 2005 Leptin: structure, function and biology. Vitam Horm 71:345–372 [DOI] [PubMed] [Google Scholar]
- Flegal KM, Troiano RP 2000 Changes in the distribution of body mass index of adults and children in the US population. Int J Obes Relat Metab Disord 24:807–818 [DOI] [PubMed] [Google Scholar]
- Widjaja A, Stratton IM, Horn R, Holman RR, Turner R, Brabant G 1997 UKPDS 20: plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J Clin Endocrinol Metab 82:654–657 [DOI] [PubMed] [Google Scholar]
- Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M 1996 Radioimmunoassay of leptin in human plasma. Clin Chem 42:942–946 [PubMed] [Google Scholar]
- Cutler AF, Havstad S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT 1995 Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology 109:136–141 [DOI] [PubMed] [Google Scholar]
- Kinkhabwala A, Roper J, Shak J, Olivares AZ, Tseng CH, Perez-Perez GI, Blaser MJ, Francois F2007 Prospective evaluation of Helicobacter pylorieradication effects on meal-induced changes in plasma ghrelin and leptin. Gastroenterology 132:A208 (Abstract S1265d) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.