Abstract
Background: Body mass index (BMI) is directly related to testosterone (total T and free T) and inversely to SHBG cross-sectionally, but little is known about how changes in body fat and androgen markers affect each other over time.
Methods: Participants included 969 White and Black women from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort, who were ages 18–30 at entry into the study and were pre- or perimenopausal 16 yr later at the time of the CARDIA Women’s Study (CWS). Total T and SHBG were assayed from specimens drawn at the CWS examination and stored serum from the yr 2 and 10 CARDIA exams. Free T was calculated based on total T and SHBG. BMI and waist circumference were measured at yr 2, 10, and 16.
Results: Despite clinically significant increases in BMI and waist circumference, total T and free T tended to decline, whereas SHBG remained relatively constant. BMI and waist circumference were directly correlated with free T and inversely correlated with SHBG in cross-sectional analyses. In longitudinal, multivariable analyses, an annualized increase in BMI was inversely related to a concurrent annualized decrease in SHBG (β = −0.79 ng/dl, and se = 0.22 in Blacks; β = −1.07 ng/dl; and se = 0.31 in Whites). However, early increases in BMI were not related to later decreases in SHBG.
Conclusion: Increases in adiposity are closely tied to decreases in SHBG, but changes in BMI and SHBG may occur concurrently rather than sequentially.
A 14-year longitudinal study of 969 white and black women ages 18-30 finds that increases in body mass index and waist circumference are closely related to increasing adrogenicity and concurrent decreases in SHBG over time.
In both pre- and postmenopausal women, relatively high levels of testosterone (T) and low levels of SHBG are associated with increased risk of type 2 diabetes (1,2,3) and an adverse cardiovascular risk factor profile (4,5,6,7,8). The extent to which these relations are confounded or mediated by overall and/or central adiposity is unclear; some studies find associations are independent of measures of body fat (8,9), whereas other studies do not (6). Separating independent physiological effects of hyperandrogenicity and body fat on disease outcomes is difficult because the factors influence each other in a reciprocal fashion. In vitro studies suggest that visceral adiposity increases pancreatic secretion of insulin and decreases hepatic clearance of insulin. As a result, hepatic SHBG synthesis is inhibited (10), SHBG levels decline, and availability of androgens increase at the tissue level, encouraging further accumulation of visceral fat (11). Understanding the influence of decreased adiposity on hyperandrogenicity is clinically relevant for clarifying the potential benefits of weight loss.
Although direct cross-sectional associations of adiposity with total and/or free T and an inverse association with SHBG have been consistently observed (12,13,14), few population-based data exist that address the issue of how change in one factor relates temporally to change in the other factor. In the Coronary Artery Risk Development in Young Adults (CARDIA) Women’s Study (CWS), measures of androgenicity, body mass index (BMI), and waist circumference were available at three points in time over a 14-yr period. The purpose of this analysis was to examine the temporal relations between changes in those factors in reproductive-age women and to compare those relations in Whites and African-Americans.
Materials and Methods
Study sample
The CWS sample came from participants in the CARDIA study, a prospective, multicenter, observational, population-based investigation of the development of cardiovascular disease risk in White and Black young adults, ages 18–30 yr at baseline (1985–1986). The recruitment procedures and characteristics of the CARDIA cohort have been previously described (15). Briefly, the CARDIA cohort consists of 5115 men and women approximately balanced at baseline by age (45% aged 18–24, 55% aged 25–30 yr), race (52% Black, 48% White), sex (54% women), and education (40% completed ≤12 yr of education). Participants were recruited from Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA, using community-based or list-based sampling frames. The overall response rate was 51%. Follow-up examinations have been conducted 2 (1987–1988), 5 (1990–1991), 7 (1992–1993), 10 (1995–1996), 15 (2000–2001), and 20 (2005–2006) years after baseline, with retention rates of 91, 88, 81, 79, 74, and 72%, respectively.
After the yr-15 exam, the CARDIA women were recruited for a yr-16 exam as part of the CWS, an ancillary study designed to examine the role of androgens and polycystic ovaries (visualized on transvaginal ultrasound) on cardiovascular risk factors and subclinical cardiovascular disease. To be eligible for CWS, women had to have attended the yr-15 exam, have at least one ovary, not be pregnant, and live within 50 miles of a clinical center. A total of 1517 women met these criteria and were contacted about participation in CWS. Of those, 1163 women (76.7%) completed the CWS exam. The current analyses excluded 192 women who were postmenopausal at yr 16 (i.e. FSH > 40 mIU/dl or at least 12 months since a self-reported last menstrual period) plus two additional women with large amounts of missing data, leaving 969 pre- or perimenopausal women on whom this report is based. Study protocols were approved by the Institutional Review Boards of the participating institutions, and all participants signed an informed consent.
Assessment of androgenicity
Three indicators of androgenicity, total T, free T, and SHBG, were measured in serum drawn at the yr-16 exam, conducted on d 2–10 of the menstrual cycle for 64.6% of the sample. All specimens were drawn and processed following a standardized protocol and then frozen and stored for a month before being shipped to the Obstetrics/Gynecology Research and Diagnostic Laboratory at the University of Alabama at Birmingham for batch processing.
Stored specimens from the yr-2 and the yr-10 CARDIA exams for the 969 women in the analytic sample were also used to measure the three markers of androgenicity. These specimens were drawn without regard to menstrual cycle day. Hormone measures for yr 2 and 10 were available for approximately 81.3 and 90% of the analytic sample, respectively, and 74.4% had hormone measures at all three points in time. All available specimens for any specific woman were processed in a single batch to avoid measurement error due to laboratory drift.
Total T was measured with a competitive immunoassay (Beckman-Coulter, Los Angeles, CA) that employs direct chemiluminescent technology on the ACS:180 automated chemiluminescent system. SHBG was determined using equilibrium dialysis, which estimates the amount of T capable of being bound by SHBG in the patient sample. Free T was calculated on the basis of measured total T and SHBG using the method described by Pearlman et al. (16). Because the minimum level of detectability for total T was 10 ng/dl, all values of 10 or less were set to 5.
Measurements of BMI and waist circumference
BMI, defined as weight in kilograms divided by height in meters squared, and waist circumference (centimeters) were measured by trained and certified clinic staff following standardized protocols (17,18) at the yr-2, -10, and -15 CARDIA examinations and the yr-16 CWS examination (BMI only).
Covariates
Covariates examined in this analysis were those known or hypothesized to influence either androgens or body size: oral contraceptive (OC) use, smoking, age, physical activity (19), education, and parity. Information about all covariates was obtained from self-report at yr 2, 10, and 15, except for OC use, which was taken from the yr-16 CWS exam rather than from yr 15.
Data analyses
Descriptive statistics included means and sd or medians and interquartile ranges for continuous variables and proportions for categorical variables. Differences in means, medians, or proportions between Blacks and Whites and between OC users and nonusers were compared using t tests, Wilcoxon rank sum tests, and χ2 tests, respectively. Paired t tests were used to evaluate whether mean within-woman change in a continuous variable between two points in time was statistically different from zero. Spearman correlation coefficients provided unadjusted measures of association between year-specific androgens, BMI, and waist circumference and changes in androgens, BMI, and waist circumference. Given the known influence that pregnancy, lactation, and OC use have on hormone levels, women who were pregnant (yr 2, 10, and 16) or breastfeeding (yr 2 and 10 only because of missing lactation data at yr 16) were excluded from these analyses, and those on OCs were examined separately.
To examine the relation between change in androgenicity over time and concurrent change in BMI and waist circumference, we used repeated-measures linear regression (Proc Mixed, SAS version 9.1). Changes in each androgen, BMI, and waist circumference were expressed as annualized rates of change [(value at time y − value at time x)/number of years between x and y] with a maximum number of two observations per woman (change between yr 2 and 10 and change between yr 10 and 16). All final models were adjusted for yr 2 androgen level, BMI, or waist circumference, age, and OC use. Change in OC use was included as a time-dependent variable (i.e. change between yr 2 and 10 and between yr 10 and 16). Models were either stratified by race or included interaction terms to evaluate whether the associations of baseline androgen level, baseline BMI (or waist), and change in BMI (or waist) with change in androgen differed between Blacks and Whites. Because BMI and waist circumference were highly correlated (r = 0.91 at yr 2) as were change in BMI and change in waist circumference (r = 0.89 and 0.68 between yr 2 and 10 and 10 and 16, respectively), measures of BMI and waist circumference were examined in separate models.
To examine associations of annualized change in androgenicity between yr 10 and 16 as a function of earlier changes in BMI (between yr 2 and 10), multiple linear regression was used, following an approach identical to that described above except for having only one observation of the dependent and independent variable per woman.
Finally, multiple linear regression models, adjusted for baseline age, baseline BMI, and race, provided estimates of the mean change in androgens as a function of weight change (categorized as loss of more than 4.9 lb, stable weight between −4.9 and 4.9 lb, moderate weight gain of between 5 and 15 lb, and large gain of more than 15 lb), during each of the two time periods (i.e. between yr 2 and 10 and between yr 10 and 16). Separate models were run for each of the androgens and each of the time periods of interest. These analyses excluded women on OCs or pregnant at baseline, yr 10, or yr 16, or breastfeeding at baseline or yr 10.
Results
As shown in Table 1, the Black women had a higher BMI and larger waist circumference and greater increases in BMI and waist circumference early in follow-up than the Whites, although both groups experienced substantial increases. The Black women were also slightly younger, considerably less physically active, more likely to be cigarette smokers and to have only a high school education, and less likely to be OC users at yr 10 and 16.
Table 1.
Characteristics of CWS cohort by race
| Characteristic | Blacks (n = 489) | Whites (n = 480) | P valueb |
|---|---|---|---|
| Agea (yr) | 39.3 (3.8) | 40.6 (3.3) | <0.0001 |
| BMI (kg/m2) | 32.7 (8.0) | 27.5 (7.2) | <0.0001 |
| Change in BMI mean (kg/m2) | |||
| yr 15 − yr 10 | 1.5 (3.2) | 1.2 (2.5) | 0.12 |
| yr 10 − yr 2 | 3.3 (3.8)c | 1.9 (3.5)c | 0.0001 |
| Waist circumferencea (cm) | 91.1 (16.2) | 81.9 (14.7) | <0.0001 |
| Change in waist circumference (cm) | |||
| yr 15 − yr 10 | 3.2 (7.8) | 3.7 (7.7) | 0.35 |
| yr 10 − yr 2 | 7.9 (8.7)c | 4.5 (8.4)c | <0.0001 |
| High school diploma or lessa, n (%) | 129 (26.4) | 68 (14.2) | <0.0001 |
| OC user, n (%) | |||
| yr 16 | 60 (12.6) | 91 (19.4) | 0.004 |
| yr 10 | 58 (13.2) | 91 (19.9) | 0.007 |
| yr 2 | 135 (29.7) | 138 (30.0) | 0.95 |
| Cigarette smoker, n (%) | |||
| yr 16 | 117 (24.0) | 81 (16.9) | <0.0001 |
| yr 10 | 113 (25.3) | 80 (17.6) | <0.0001 |
| yr 2 | 131 (28.4) | 109 (23.5) | <0.0001 |
| Paritya, n (%) | 0.09 | ||
| None | 25 (5.7) | 37 (9.7) | |
| 1 | 94 (21.3) | 80 (21.0) | |
| 2 or more | 322 (73.0) | 264 (69.3) | |
| Physical activitya, median exercise units (interquartile range) | 176 (76–327) | 277 (132–476) | <0.0001 |
Results are shown as mean (sd) unless indicated otherwise.
Characteristics at time of yr 15 core CARDIA examination.
P value for racial difference in means, medians, or proportions; t test or Wilcoxon rank sum test for continuous variables, χ2 test for categorical variables.
Mean within-woman change in BMI and waist significantly different from zero (P < 0.0001) by paired t test.
Despite the large increases in BMI and waist circumference (Table 1), total T and free T tended to decline, whereas SHBG remained relatively constant (Table 2). Despite the racial differences in baseline BMI and change in BMI between yr 2 and 10, there were few differences between Black and White women in either baseline androgen levels or change in androgen levels (Table 2). Year 2 SHBG was slightly higher in Whites compared with Blacks (in the non-OC users), but there were no differences in yr-2 total T or free T, and the 14-yr decrease in total T and free T in the Black women (−16.9, sd = 20.6 ng/dl; −0.12, sd = 0.18 ng/dl, respectively) did not differ significantly from the Whites (−17.5, sd = 17.5 ng/dl; −0.12, sd = 0.15 ng/dl, respectively). There were no racial differences in changes in SHBG, and the mean within-woman change was small and nonsignificant.
Table 2.
Year 2 hormone levels and change in hormones by race and OC use
| Non-OC users
|
OC users
|
|||
|---|---|---|---|---|
| n | Value | n | Value | |
| Blacks | ||||
| Total Ta, median (interquartile range), ng/dl | 251 | 41.0 (29.0–57.0) | 106 | 26.5 (16.0–43.0) |
| Change in total Tb, mean (sd), ng/dl | ||||
| yr 10 − yr 2 | 149 | −6.4 (16.4)c | 16 | −9.1 (14.1) |
| yr 16 − yr 10 | 255 | −10.3 (15.9)c | 15 | −4.7 (12.9) |
| Free Ta, median (interquartile range), | 250 | 0.31 (0.21–0.43) | 106 | 0.12 (0.06–0.23) |
| Change in free Tb, mean (sd), ng/dl | ||||
| yr 10 − yr 2 | 147 | −0.04 (0.15) | 16 | −0.08 (0.16) |
| yr 16 − yr 10 | 255 | −0.07 (0.14) | 15 | −0.03 (0.08) |
| SHBGa, median (interquartile range), ng/dl | 250 | 24.0 (19.0–33.0)c | 107 | 44.0 (32.0–58.0) |
| Change in SHBGb, mean (sd), ng/dl | ||||
| yr 10 − yr 2 | 148 | −1.15 (9.0) | 16 | −0.81 (16.4) |
| yr 16 − yr 10 | 256 | −0.93 (9.0) | 15 | 3.93 (15.0) |
| Whites | N | N | ||
| Total Ta, median (interquartile range), ng/dl | 241 | 44.0 (31.0–55.0) | 121 | 23.0 (16.0–36.0) |
| Change in total Tb, mean (sd), ng/dl | ||||
| yr 10 − yr 2 | 134 | −10.3 (16.8)c | 19 | −8.8 (10.0) |
| yr 16 − yr 10 | 208 | −6.3 (14.1)c | 25 | −3.0 (10.9) |
| Free Ta, median (interquartile range), ng/dl | 241 | 0.30 (0.19–0.44) | 121 | 0.11 (0.06–0.19) |
| Change in free Tb, mean (sd), ng/dl | ||||
| yr 10 − yr 2 | 134 | −0.07 (0.14) | 19 | −0.04 (0.08) |
| yr 16 − yr 10 | 209 | −0.05 (0.13) | 25 | 0.00 (0.05) |
| SHBGa, median (interquartile range), ng/dl | 245 | 27.0 (20.0–36.0)c | 122 | 45.0 (33.0–62.0) |
| Change in SHBGb, mean (sd), ng/dl | ||||
| yr 10 − yr 2 | 136 | 0.13 (8.8) | 19 | −0.42 (19.0) |
| yr 16 − yr 10 | 210 | −0.79 (10.2) | 25 | −5.4 (18.2) |
From yr 2; excludes women who were pregnant or breastfeeding at yr 2.
Excludes women who were pregnant at either point in time or breastfeeding at either yr 2 or 10 or who were not consistently non-OC users or OC users at both points in time.
Significant difference in means between Blacks and Whites within stratum of non-OC users (P < 0.05) based on nonparametric test.
Compared with non-OC users, women on OCs had lower levels of total T and free T and a higher level of SHBG. There were no significant racial differences in baseline androgen levels among the OC users, and meaningful comparisons between Black and White women in change in androgen levels were not possible due to the small numbers who used OCs at more than one point in time.
Table 3 summarizes the correlations between yr-2 androgens and change in androgens between yr 2 and 10 and yr 10 and 16 with yr-2 BMI and waist circumference and change in BMI and waist circumference. As expected, both yr-2 BMI and waist circumference were moderately and directly correlated with yr-2 free T and inversely correlated with yr-2 SHBG. The direction and magnitude of these cross-sectional correlations for the yr-10 and -16 variables were similar to those at yr 2 (data not shown).
Table 3.
Spearman correlations of yr-2 BMI and waist circumference and change in BMI and waist circumference with yr-2 hormonesa and change in hormonesb
| yr 2
|
Change, yr 2–10
|
Change, yr 10–16
|
||||
|---|---|---|---|---|---|---|
| BMI | Waist | BMI | Waist | BMI | Waist | |
| yr 2 | ||||||
| Total T | 0.04 | 0.03 | 0.06 | 0.14 | 0.03 | 0.03 |
| Free T | 0.21 | 0.19 | 0.11 | 0.18 | 0.00 | −0.02 |
| SHBG | −.33 | −0.33 | −0.06 | −0.08 | 0.01 | 0.04 |
| Change, yr 2–10 | ||||||
| Total T | 0.09 | 0.11 | 0.03 | −0.03 | 0.00 | −0.04 |
| Free T | −0.02 | 0.00 | 0.10 | 0.04 | −0.03 | −0.07 |
| SHBG | 0.07 | 0.05 | −0.33 | −0.27 | 0.05 | 0.07 |
| Change, yr 10–16 | ||||||
| Total T | −0.12 | −0.13 | −0.06 | −0.09 | −0.02 | −0.01 |
| Free T | −0.11 | −0.13 | −0.09 | −0.12 | 0.06 | 0.04 |
| SHBG | 0.07 | 0.08 | 0.03 | 0.01 | −0.27 | −0.17 |
Bolded correlations are significantly different from zero, P < 0.05.
Excludes women who were on OCs or pregnant at time of hormone measurement or breastfeeding at yr 2 or 10.
Excludes women who were on OCs or pregnant at either one or both of the times of hormone measurement or breastfeeding at yr 2 and/or yr 10.
In general, yr-2 BMI and waist circumference were not correlated with androgen changes between either yr 2 and 10 or yr 10 and 16, although there were weak, inverse associations with change in total T and free T between yr 10 and 16. Similarly, yr-2 androgens were generally not correlated with changes in BMI or waist circumference, although both total T and free T showed a weak direct association with change in waist circumference between yr 2 and 10.
The only other correlations of notable magnitude (i.e. r > 0.20) were between concurrent change in SHBG and changes in BMI and waist circumference; concurrent changes in BMI and waist circumference were not associated with either change in total T or free T. Furthermore, yr-2 to -10 change in BMI or waist circumference was not associated with change between yr 10 and 16 in any of the androgen markers, nor did earlier changes in any of the androgens relate substantially to later changes in either BMI or waist circumference. These findings were generally similar in both Blacks and Whites (data not shown).
Because neither baseline BMI or waist circumference or change in BMI or waist circumference were consistently related to changes in total T or free T, multivariable analyses of these two hormones were not pursued.
The results presented in Table 4 demonstrate further the influence that concurrent changes in BMI had on changes in SHBG. For both Black and White women, annual increases in BMI over the 14-yr follow-up period were significantly associated with annual decreases in SHBG. Stratification by race showed that the starting level of BMI (yr 2) was marginally associated with change in SHBG in Blacks but not in Whites, whereas the baseline level of SHBG was associated with SHBG change in Whites but not Blacks. However, interaction terms were not significant (data not shown). Similar results were obtained for waist circumference (data not shown).
Table 4.
Associations of annualized change in SHBG from yr 2–10 and yr 10–16 with concurrent annualized change in BMI, baseline BMI, and other factors, stratified by race
| Blacks
|
Whites
|
|||||
|---|---|---|---|---|---|---|
| β | se | P value | β | se | P value | |
| BMI, yr 2 (kg/m2) | −0.029 | 0.016 | 0.07 | 0.02 | 0.029 | 0.46 |
| Annualized change in BMI, yr 2–10 and yr 10–15 (kg/m2)a | −0.768 | 0.205 | 0.0002 | −1.2 | 0.29 | <0.0001 |
| SHBG, yr 2 (ng/dl) | −0.001 | 0.007 | 0.87 | −0.023 | 0.009 | 0.009 |
| Age, yr 2 (yr) | 0.046 | 0.029 | 0.12 | 0.03 | 0.043 | 0.49 |
| OC use, yr 2 | ||||||
| No | Ref. | Ref. | ||||
| Yes | −0.827 | 0.506 | 0.104 | −0.026 | 0.469 | 0.96 |
| Change in OC use, yr 2–10 and yr 10–16a | ||||||
| Stop | −4.4 | 0.648 | <0.0001 | −1.21 | 0.7 | 0.08 |
| Start | 2.250 | 0.422 | <0.0001 | 2.95 | 0.46 | <0.0001 |
| No change | Ref. | Ref. | ||||
Time-dependent variable in repeated-measures linear regression model.
In all models, stopping OC use was independently associated with declines in SHBG, whereas starting OCs was associated with increases in SHBG. Further adjustment for education, smoking, parity, and physical activity did not alter these relations (data not shown), and none of these variables were included in any of the final models.
In contrast to the strong associations between concurrent changes in BMI and SHBG, annualized change in BMI between yr 2 and 10 was not related to annualized change in SHBG between yr 10 and 16 (Table 5). Models that included waist circumference rather than BMI showed similar results (data not shown).
Table 5.
Associations of annualized change in SHBG from yr 10–16 with previous annualized change in BMI, baseline BMI, and other factorsa, stratified by race
| Blacks
|
Whites
|
|||||
|---|---|---|---|---|---|---|
| β | se | P value | β | se | P value | |
| BMI, yr 2 (kg/(m)2) | −0.006 | 0.015 | 0.70 | −0.012 | 0.034 | 0.73 |
| Annualized change in BMI, yr 2–10 (kg/m2) | −0.008 | 0.222 | 0.97 | 0.587 | 0.386 | 0.13 |
| SHBG, yr 2 (ng/dl) | 0.013 | 0.011 | 0.24 | −0.022 | 0.015 | 0.16 |
| Age, yr 2 (yr) | 0.065 | 0.030 | 0.03 | 0.009 | 0.050 | 0.87 |
Excludes women who were OC users at any of the three points in time.
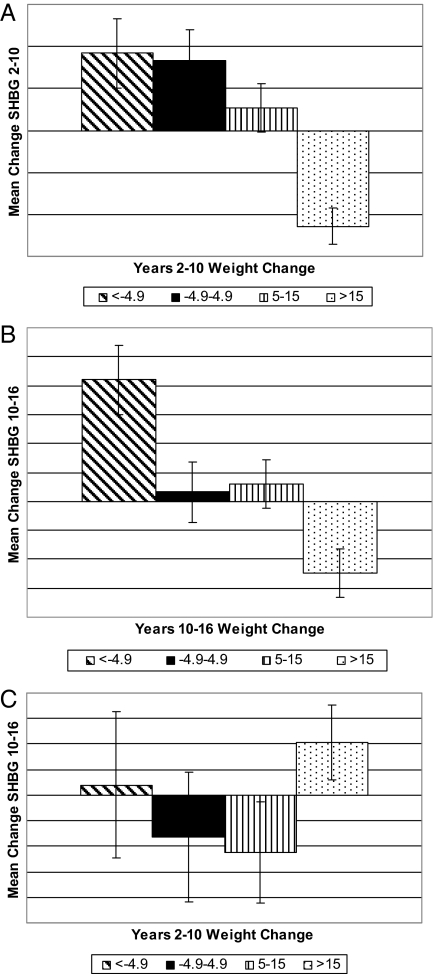
The differences between how concurrent changes and previous changes in BMI relate to change in SHBG is further illustrated in Fig. 1, A–C. Figure 1A shows that SHBG increased between yr 2 and 10 in women who either lost weight or stayed essentially weight stable between those same years, remained relatively unchanged in women who gained a moderate amount of weight, and decreased significantly in those with a large weight gain. Figure 1B shows a generally similar pattern for the mean change in SHBG between yr 10 and 16 by weight change category between those same years. In contrast, Fig. 1C demonstrates that previous changes in weight (between yr 2 and 10) had no clear relation to later change in SHBG (between yr 10 and 16).
Figure 1.
A, Mean change in SHBG (and se) between yr 2 and 10 by change in weight (pounds) between yr 2 and 10, adjusted for yr-2 age, yr-2 BMI, and race; B, mean change in SHBG (and se) between yr 10 and 16 by change in weight (pounds) between yr 10 and 16, adjusted for yr-2 age, yr-2 BMI, and race; C, mean change in SHBG (and se) between yr 10 and 16 by change in weight (pounds) between yr 2 and 10, adjusted for yr-2 age, yr-2 BMI, and race.
A series of sensitivity analyses were conducted to examine whether these findings were unduly influenced by the inclusion of various subgroups of participants (specifically, 44 women who self-reported ever having a diagnosis of polycystic ovary syndrome, 93 women with more than 35 d between the last menstrual period and the yr-2 exam, and 49 women who reported taking medications for diabetes). None of these exclusions substantially altered the results (data not shown).
Discussion
In this previously unpublished, longitudinal analysis of the relation of 14-yr changes in BMI and waist circumference to changes in androgen markers, higher BMI and larger waist circumference were inversely associated, cross-sectionally, with SHBG and directly associated with free T. Increases in both BMI and waist circumference were associated with decreases, over the same time period, in SHBG but were not associated with increases in total or free T. In addition, earlier changes in BMI and waist circumference were not associated with change in any of the androgens over a later time. Finally, the inverse associations between concurrent changes in BMI or waist circumference and SHBG were independent of initial levels of BMI or waist circumference and SHBG. For the sample as a whole, there was only a minimal decline in SHBG over time, suggesting little effect of aging on this androgen marker in reproductive-age women. These findings were generally similar in both Black and White women.
The moderately strong cross-sectional relations between BMI and androgenicity observed in this study are consistent with a number of other studies (12,20) and relatively well understood in terms of physiological mechanisms (21). Less well known is whether change in body fat relates to change in androgenicity. The current study suggests that change in BMI has little relation to changes in testosterone but is closely associated with changes in SHBG and that as both total adiposity, represented by BMI, and central adiposity, represented by waist circumference, increase, SHBG decreases. However, given the lack of association between earlier change in BMI and later change in SHBG, this study also suggests that long-term changes in body fat and SHBG occur in parallel, rather than one clearly preceding the other. Some experimental studies suggest that an increase in SHBG results from weight loss (22,23,24), but none of these studies have made the frequent and simultaneous measures of weight and SHBG that may be necessary to establish the precise timing of how changes in these factors occur relative to each other.
At baseline, Black women in CWS had lower SHBG than White women, due, presumably, to higher BMI and greater waist circumference. Also, baseline BMI was inversely related to change in SHBG, independently of change in BMI, in the Blacks but not in the Whites, even though a formal test for interaction was not significant. Previous cross-sectional studies have also found racial differences in SHBG concentration in relation to body fat. For instance, in a study of nonobese premenopausal women, Blacks had lower SHBG than Whites (25), whereas in a study of obese premenopausal women, the opposite was true (i.e. SHBG was higher in Blacks) (26). On the other hand, comparable levels of SHBG were observed in overweight and obese postmenopausal Black and White women, despite greater central adiposity in Blacks (27).
These somewhat inconsistent findings suggest the possibility that genetic variation may influence the generally tight coupling of body fat and SHBG. However, both the HERITAGE study and the San Antonio Family Heart study estimated the heritability of SHBG concentration at only about 30% (28,29) and concluded that most of the correlation between SHBG and body fat is not genetically based (28,30). Thus, the finding of racial differences in the relation between baseline BMI and change in SHBG in the present study may be due to chance or incomplete control of confounding.
The lack of any relation between change in BMI and change in free T was somewhat unexpected, particularly given the direct cross-sectional associations between the variables, and may also be due to chance. On the other hand, the fact that change in BMI was strongly related to concurrent change in SHBG but not change in free T may suggest that the changes in SHBG are not as indicative of increases in androgenicity as they are of increases in insulin.
Several considerations may limit the inferences that can be drawn from our findings. First, we did not measure estrogens. Although SHBG is an indirect index of the relative balance of estrogens to androgens (31), it is only an indirect measure. Second, the CWS relied on surrogate measures of overall body fat and central adiposity (BMI and waist circumference, respectively). Although both measures are highly correlated with more sophisticated measures of body fat and central adiposity (32,33), small changes in visceral adiposity may not be accurately reflected in changes in waist circumference. Also, waist circumference may overestimate the extent of visceral adiposity in Black women (34,35) and, therefore, may not be as equally valid in both Whites and African-Americans. In addition, the measurement of waist circumference is subject to error, particularly when repeated measures are made by different personnel over long periods of time. However, standardized protocols and rigorous quality control procedures were implemented, and the high correlations between waist circumference measures over time (r ranging from 0.74–0.90) suggest that this measurement error was minimal. Similarly, the lack of standardization with regard to day of the menstrual cycle for the yr-2 and -10 blood draws may have introduced variability in the testosterone measures that could limit our ability to detect meaningful associations with change in BMI. However, total and free T remain relatively stable throughout the menstrual cycle, except for a slight increase a few days before the LH surge (36). Finally, the tight coupling of body fat with markers of androgenicity and the long period of time between observations poses a problem with time-dependent confounding that limits the ability of this analysis to establish the direction of causality.
This study also has two notable strengths. First, the biracial, population-based sample allows for broad generalizability of the findings. And second, the availability of repeated measures of BMI, waist circumference, hormones, and other covariates is essentially unique. Without the repeated measures of the variables of interest, it would be impossible to address the issue of how changes in BMI related to changes in androgenicity, the central issue of this analysis.
In conclusion, this study showed that increasing androgenicity in women over time, as indicated by decreases in SHBG, is tightly connected to increases in both BMI and waist circumference. Although the debate over androgenicity as an independent risk factor for diabetes and cardiovascular disease continues, from a public health perspective, the current study supports the importance of lifestyle interventions, such as increased physical activity and healthy eating behavior, known to limit increases in body fat in reproductive-age women, as an effective strategy for limiting the development of a more androgenic hormonal profile and the adverse health outcomes that accompany that profile.
Footnotes
This research was supported by a grant from the National Heart, Lung, and Blood Institute (R01-HL065611) and contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, and N01-HC-48050.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 11, 2008
Abbreviations: BMI, Body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CWS, CARDIA Women’s Study; OC, oral contraceptive; T, testosterone.
References
- Lindstedt G, Lundberg PA, Lapidus L, Lundgren H, Bengtsson C, Bjorntorp P 1991 Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM: 12-yr follow-up of population study of women in Gothenburg, Sweden. Diabetes 40:123–128 [DOI] [PubMed] [Google Scholar]
- Haffner SM, Valdez RA, Morales PA, Hazuda HP, Stern MP 1993 Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J Clin Endocrinol Metab 77:56–60 [DOI] [PubMed] [Google Scholar]
- Haffner SM 2000 Sex hormones, obesity, fat distribution, type 2 diabetes and insulin resistance: epidemiological and clinical correlation. Int J Obes Relat Metab Disord 24(Suppl 2):S56–S58 [DOI] [PubMed] [Google Scholar]
- Kumagai S, Kai Y, Sasaki H 2001 Relationship between insulin resistance, sex hormones and sex hormone-binding globulin in the serum lipid and lipoprotein profiles of Japanese postmenopausal women. J Atheroscler Thromb 8:14–20 [DOI] [PubMed] [Google Scholar]
- Falkner B, Sherif K, Sumner A, Kushner H 1999 Hyperinsulinism and sex hormones in young adult African Americans. Metabolism 48:107–112 [DOI] [PubMed] [Google Scholar]
- Tchernof A, Toth MJ, Poehlman ET 1999 Sex hormone-binding globulin levels in middle-aged premenopausal women. Associations with visceral obesity and metabolic profile. Diabetes Care 22:1875–1881 [DOI] [PubMed] [Google Scholar]
- Ivandic A, Prpic-Krizevac I, Sucic M, Juric M 1998 Hyperinsulinemia and sex hormones in healthy premenopausal women: relative contribution of obesity, obesity type, and duration of obesity. Metabolism 47:13–19 [DOI] [PubMed] [Google Scholar]
- Goodman-Gruen D, Barrett-Connor E 1997 Sex hormone-binding globulin and glucose tolerance in postmenopausal women. The Rancho Bernardo Study. Diabetes Care 20:645–649 [DOI] [PubMed] [Google Scholar]
- Lee CC, Kasa-Vubu JZ, Supiano MA 2004 Androgenicity and obesity are independently associated with insulin sensitivity in postmenopausal women. Metabolism 53:507–512 [DOI] [PubMed] [Google Scholar]
- Plymate SR, Matej LA, Jones RE, Friedl KE 1988 Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67:460–464 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P 1991 Metabolic implications of body fat distribution. Diabetes Care 14:1132–1143 [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Bertazzo D, Casimirri F, Pascal G, Tortelli O, Labate AM 1997 Determinants of sex hormone-binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Virgilio-Menopause-Health Group. Metabolism 46:5–9 [DOI] [PubMed] [Google Scholar]
- Haffner SM, Katz MS, Dunn JF 1991 Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int J Obes 15:471–478 [PubMed] [Google Scholar]
- Raison J, Bonithon-Kopp C, Egloff M, Ducimetiere P, Guy-Grand B 1990 Hormonal influences on the relationships between body fatness, body fat distribution, lipids, lipoproteins, glucose and blood pressure in French working women. Atherosclerosis 85:185–192 [DOI] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs Jr DR, Liu K, Savage PJ 1988 CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41:1105–1116 [DOI] [PubMed] [Google Scholar]
- Pearlman WH, Crepy O, Murphy M 1967 Testosterone-binding levels in the serum of women during the normal menstrual cycle, pregnancy, and the post-partum period. J Clin Endocrinol Metab 27:1012–1018 [DOI] [PubMed] [Google Scholar]
- Folsom AR, Burke GL, Ballew C, Jacobs Jr DR, Haskell WL, Donahue RP, Liu KA, Hilner JE 1989 Relation of body fatness and its distribution to cardiovascular risk factors in young blacks and whites. The role of insulin. Am J Epidemiol 130:911–924 [DOI] [PubMed] [Google Scholar]
- Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs Jr DR 1996 Seven-year trends in body weight and associations of weight change with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health 87:635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs Jr DR, Hahn LP, Haskell WL, Pirie P, Sidney S 1989 Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil 9:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, Gilliland F, Stanczyk FZ, Yasui Y, Ballard-Barbash R 2003 Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol 21:1961–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG 1991 A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72:83–89 [DOI] [PubMed] [Google Scholar]
- Tolino A, Gambardella V, Caccavale C, D'Ettore A, Giannotti F, D'Anto V, De Falco CL 2005 Evaluation of ovarian functionality after a dietary treatment in obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 119:87–93 [DOI] [PubMed] [Google Scholar]
- Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Franks S 1992 Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 36:105–111 [DOI] [PubMed] [Google Scholar]
- Kopp HP, Krzyzanowska K, Schernthaner GH, Kriwanek S, Schernthaner G 2006 Relationship of androgens to insulin resistance and chronic inflammation in morbidly obese premenopausal women: studies before and after vertical banded gastroplasty. Obes Surg 16:1214–1220 [DOI] [PubMed] [Google Scholar]
- Hughes GS, Mathur RS, Margolius HS 1989 Sex steroid hormones are altered in essential hypertension. J Hypertens 7:181–187 [PubMed] [Google Scholar]
- Dowling HJ, Pi-Sunyer FX 1993 Race-dependent health risks of upper body obesity. Diabetes 42:537–543 [PubMed] [Google Scholar]
- Berman DM, Rodrigues LM, Nicklas BJ, Ryan AS, Dennis KE, Goldberg AP 2001 Racial disparities in metabolism, central obesity, and sex hormone-binding globulin in postmenopausal women. J Clin Endocrinol Metab 86:97–103 [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Blangero J, Mahaney MC, Haffner SM, Mitchell BD, Stern MP, MacCluer JW 1996 Genetic and environmental correlations among hormone levels and measures of body fat accumulation and topography. J Clin Endocrinol Metab 81:597–600 [DOI] [PubMed] [Google Scholar]
- An P, Rice T, Gagnon J, Hong Y, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC 2000 A genetic study of sex hormone-binding globulin measured before and after a 20-week endurance exercise training program: the HERITAGE Family Study. Metabolism 49:1014–1020 [DOI] [PubMed] [Google Scholar]
- Feitosa MF, Borecki IB, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC 2003 Lack of pleiotropic genetic effects between adiposity and sex hormone-binding globulin concentrations before and after 20 weeks of exercise training: the HERITAGE family study. Metabolism 52:35–41 [DOI] [PubMed] [Google Scholar]
- Anderson DC 1974 Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 3:69–96 [DOI] [PubMed] [Google Scholar]
- Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C 1999 The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord 23:801–809 [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R 2002 Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr 75:683–688 [DOI] [PubMed] [Google Scholar]
- Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm E 1999 Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) Study. Am J Clin Nutr 69:381–387 [DOI] [PubMed] [Google Scholar]
- Conway JM, Yanovski SZ, Avila NA, Hubbard VS 1995 Visceral adipose tissue differences in black and white women. Am J Clin Nutr 61:765–771 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S 1998 The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab 83:1312–1318 [DOI] [PubMed] [Google Scholar]