Abstract
Background: Gender disparities were found in reports of early pediatric recombinant human GH (rhGH) use in the United States. With rhGH entering its third decade, we sought to examine U.S. gender-based treatment patterns and how these patterns compare with that of other countries.
Methods: All children entered in the Pfizer International Growth Study, a database designed to document long-term outcomes and safety of Genotropin (Pfizer, New York, NY), were categorized by gender, location, date and age of therapy initiation, and diagnosis. Measures of national health status, health care expenditure, general economic indices, and mean adult heights were also compared.
Results: Throughout the past 20 yr, the United States had an almost 2:1 male to female ratio overall. The gender ratio depended on the specific indication and age. There was no consistent relation to geographical region, pediatric population size, or density of pediatric endocrinologists. Male predominance was seen in Asia (mostly Japan), the United States, and Europe/Australia/New Zealand (65, 64, and 55%, respectively), but not the rest of the world (47%), where rhGH was prescribed less frequently. In the countries with the greatest rhGH use, the gender ratios depended on the specific indications but did not correlate with mean adult height, national health care measures, or general economic indices.
Conclusions: Male predominance among U.S. pediatric rhGH recipients persists, especially for indications without a clear organic etiology. Global differences in gender ratios suggest that factors other than biology are at play. We speculate that social and cultural pressures and the health care systems’ permissiveness toward paying for rhGH therapy contribute to these international differences.
A database review finds that nearly twice as many boys than girls were treated with recombinant human GH in the U.S. and Asia (mostly Japan), but that the gender differences were smaller in other countries.
The advent of recombinant human GH (rhGH) in 1985 revolutionized the treatment of pediatric growth failure. Before 1985, the limited supply of human pituitary (cadaveric) GH restricted treatment to children with severe GH deficiency (GHD). Because rhGH is manufactured, production can match demand, making its availability theoretically boundless, and its use limited only by cost and what is deemed medically appropriate. Thus, global rhGH use expanded, with both increasing doses and greater numbers of approved indications that include non-GH deficient causes of growth failure. As the first recombinant biopharmaceutical product, rhGH has been continuously monitored by several post-marketing surveillance studies.
In the first description of U.S. children receiving rhGH from one such surveillance study, the National Cooperative Growth Study, 2331 children from 112 centers were started on treatment for GHD and other causes of short stature between October 1985 and October 1987. Seventy percent were male, which the authors suggested reflected an ascertainment bias; septooptic dysplasia was the only category in which boys did not significantly outnumber girls, and among children with idiopathic GHD, the most disparate category (male to female ratio of 2.5:1), girls had greater height deficit at diagnosis (1).
This issue was included at a conference of mostly American pediatric endocrinologists, medical ethicists, medical economists, and psychologists convened in 1991 to explore the allocation of rhGH (2). With another roughly 10,000 children from 350 endocrine centers enrolled in National Cooperative Growth Study at that time, idiopathic GHD again showed the greatest gender discordance (male to female ratio of 2.9:1), and female height deficits again exceeded that of males at diagnosis of idiopathic GHD and other causes of short stature, but not organic GHD or septooptic dysplasia. The Pfizer International Growth Study (KIGS) 10-yr data similarly found that boys received rhGH by ratios of about 2:1, depending on the diagnosis, with the highest ratio for idiopathic short stature (ISS) (3).
In 2003 the U.S. Food and Drug Administration (FDA) approved the use of rhGH for the treatment of ISS. Because ISS historically has been the most gender-disparate indication (3), the growing number of children treated for this indication will likely further the gender disparities. Now that rhGH has entered its third decade of clinical use, we sought to examine the gender-based patterns of rhGH use in the United States and how it compares with that of other countries.
Subjects and Methods
KIGS data collection
KIGS, created in 1987 and introduced in the United States in 1996, is the world’s largest post-marketing surveillance database of pediatric rhGH. The KIGS registry is an international database developed with the objective of documenting long-term outcomes and safety of Genotropin (Pfizer, New York, NY). Pediatric patients and their legal guardians are informed and consent/assent to participate in the survey, which permits anonymous use of the data in compliance with privacy guidelines. Institutional review board approval was obtained per standards of the place and time.
Data from all patients enrolled in the KIGS registry were included in this analysis. Patients were categorized into four geopolitical regions: 1) the United States; 2) Europe/Australia/New Zealand (Australia, Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Luxemburg, Malta, The Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, and Yugoslavia); 3) Asia (Hong Kong, India, Israel, Japan, Korea, Russia, Taiwan, and Thailand); and 4) rest of the world (ROW) (Argentina, Brazil, Colombia, Egypt, El Salvador, Guatemala, Mexico, South Africa, and Venezuela). The U.S. portion of the database, updated in 2006, was further divided into 10 geographical regions, according to the U.S. Postal Service zip code.
Key variables of interest were gender, city and state, year of therapy initiation, age, and diagnosis. Data for the individual variables were missing in fewer than 1% of U.S. subjects. The past two decades of U.S. rhGH clinical use were divided into three periods, based on the sequence of FDA-approved indications: 1992 and before comprised the classical GHD era (GHD remained the only indication); 1993–2000 the non-GHD pathophysiology era [FDA-approved rhGH for specific causes of growth failure such as chronic renal insufficiency, Turner syndrome, and Prader-Willi syndrome (PWS), or for nongrowth effects of GH as in AIDS wasting syndrome and adult GHD]; and 2001 onwards, the height-based era [FDA-approved rhGH for size-based, rather than pathophysiology based, indications like small for gestational age (SGA) and ISS].
Diagnoses were made by individual KIGS investigators according to the KIGS Etiology Classification List. For purposes of this analysis, the more than 100 KIGS diagnoses were collapsed into eight categories: 1) congenital GHD (includes known genetic causes of GHD and resistance to GH or IGF-I, congenital forms of GHD and central malformations, genetic syndromes that involve congenital GHD, and prenatal infections); 2) organic acquired GHD (includes intracranial and other solid tumors, leukemias/lymphomas, head trauma, central nervous system infection and inflammatory conditions, hydrocephalus and vascular anomalies); 3) renal insufficiency (all causes); 4) Turner syndrome; 5) PWS; 6) SGA, intrauterine growth retardation (IUGR); 7) familial short stature (FSS)/constitutional growth delay (CGD)/ISS; and 8) idiopathic, neurosecretory, and transient GHDs (idiopathic GHD [IGHD]). Such broad categories were intended to minimize the potential diagnostic inconsistencies across investigators, geographical regions, and time.
Non-KIGS data
The number of children in each U.S. geographical region was extracted from the U.S. Census of 2000 (4), and the number of pediatric endocrinologists in each area who prescribe rhGH was obtained from Verispan, LLC (Yardley, PA). To enhance the robustness of the data, we focused on individual countries with over 1000 children in the registry. Mean adult heights for each country, age trends (percentage of the population under age 15 yr), measures of national health outcomes (life expectancy at birth, and infant deaths per 1,000 live births), health care environment (number of physicians per 100,000 population, and total health expenditure as percentage of gross national product), and general economic indices (Gini index of income inequality) (5) were all collected from “Highlights on Health” from the World Health Organization (6).
Statistical analyses
Standard descriptive statistics were used to evaluate the frequencies of patient gender, location, year of therapy initiation, and diagnosis. Statistical models were developed using the binomial test procedure looking at gender against a test proportion of 50% and splitting the sample by year of initiation of therapy and diagnosis (SPSS version 11.0.1; SPSS, Inc., Chicago, IL). Age of rhGH initiation, by gender, was compared by the Wilcoxon rank sum test. To measure the linear association between pairs of socioeconomic variables, sample Pearson correlation coefficients were computed. To evaluate statistical significance, for each variable pair a two-sided t test (via Fisher’s transformation) was conducted (without any multiplicity adjustment) using the Minitab software program (Release 14; Minitab, Inc., State College, PA). All analyses were conducted using two-sided tests of hypotheses and a type I (α) error rate of 0.05.
Results
U.S. patterns
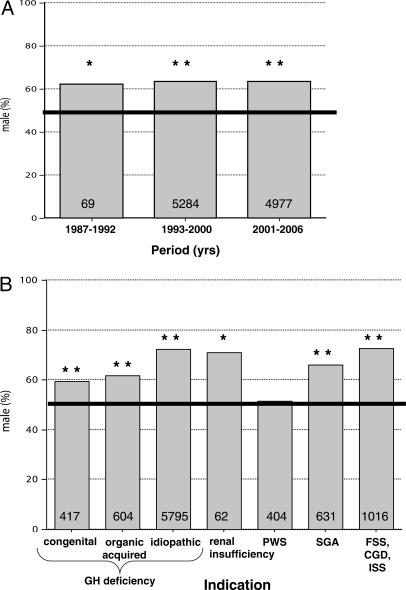
Historical trends revealed a consistent overall male predominance among U.S. pediatric rhGH recipients, at almost 2:1. The gender ratio did not change significantly across the three time periods defined by the sequence of FDA-approved indications (Fig. 1A). Off-label rhGH use was evident because a substantial number of children were enrolled under diagnoses before their FDA approval for Pfizer or its predecessor (data not shown).
Figure 1.
Gender distribution (male percentage) of U.S. pediatric rhGH recipients. A, All indications combined by time period as defined by the sequence of FDA-approved indications. B, All time periods combined by diagnostic indication. For both panels, the total number of children is included within each column. Fifty percent is highlighted by the horizontal bar. Difference from 50%, by χ2 testing, is indicated. *P < 0.05. **P ≤ 0.0001.
However, the degree of male predominance depended on the diagnostic indication (Fig. 1B). All indications except PWS significantly exceeded 50% males. Turner syndrome had no males, as expected, and, therefore, served as negative control for the data (data not shown). The male predominance for all nonorganic indications combined (72%) exceeded that for the organic indications, with or without Turner syndrome (38 and 59% male, respectively; P < 0.0001 for both).
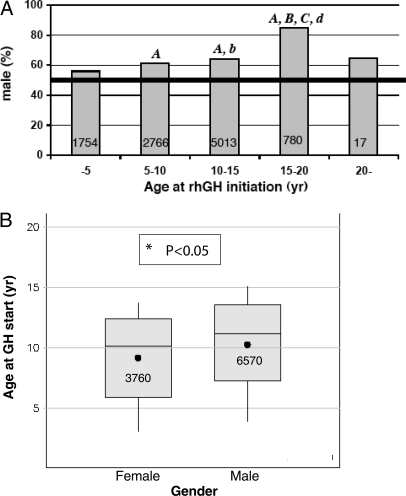
Males outnumbered females at all ages, but especially during the second decade (Fig. 2A). The mean age of initiating rhGH therapy was about 11 yr for boys and 10 for girls (Fig. 2B).
Figure 2.
Age at rhGH initiation by gender. A, Gender distribution (male percentage) of U.S. pediatric rhGH recipients per 5-yr strata of age. B, Box plots of the mean, median, and range of age at initiation of rhGH therapy for girls and boys. For both panels, the total number of children is included within each column or box. In panel A, significant differences are indicated with lowercase letters for P < 0.05 and uppercase letters for P < 0.0005: a, compared with less than 5 yr; b, compared with 5–10 yr; c, compared with 10–15 yr; and d, compared with 20+ yr. After applying the Bonferroni adjustment to account for multiplicity of testing, comparisons indicated by capital letters remained statistically significant at P < 0.05.
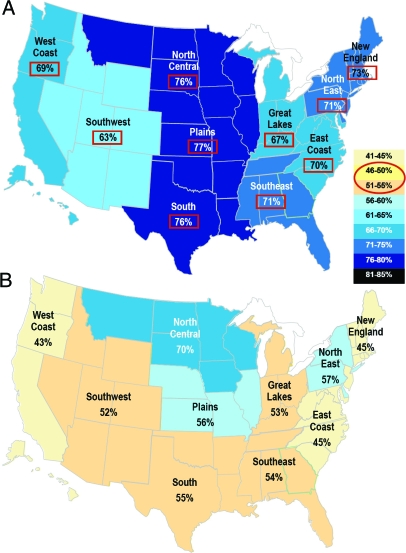
U.S. regional patterns were explored for possible correlates of the male predominance. When the male percentage of pediatric rhGH recipients was geocoded by zip code region, the areas with maximal and minimal percentages differed for each indication. For example, two indications with particularly high and low percentages of treated males are shown in Fig. 3. Likewise, the percentage of males did not correlate with either the number of children in each geographical area or the ratio of pediatric endocrinologists to children in each area (data not shown).
Figure 3.
Gender distribution (male percentage) of U.S. pediatric rhGH recipients geocoded by zip code region. A, FSS, CGD, and ISS indications. B, PWS indication. Red squares highlight percentages that significantly differ from 50%.
Global patterns
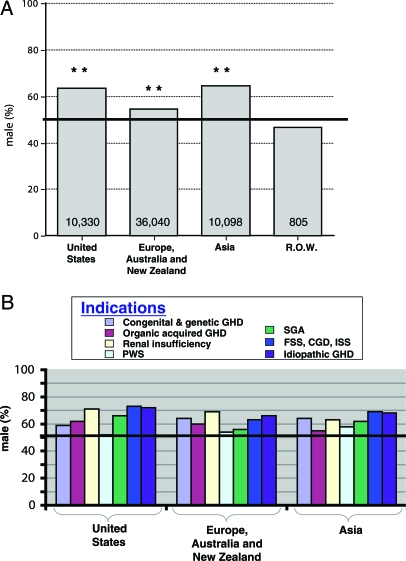
Comparing the U.S. experience with global patterns revealed the United States to have the second greatest male predominance, exceeded by Asia (mostly Japan) but greater than Europe/Australia/New Zealand (Fig. 4A). All three world regions significantly exceeded 50% males, whereas rhGH recipients in the ROW region were 47% male. Because the number of children receiving rhGH was so much smaller in the ROW, they were excluded from further analyses. Similar to the United States, global gender distributions of rhGH recipients depended on the specific indication (Fig. 4B).
Figure 4.
Gender distribution (male percentage) of global pediatric rhGH recipients. All indications combined by geopolitical region (A) and diagnostic indication for each of the regions with over 10,000 patients enrolled in KIGS (B). Total number of children enrolled is included within each column. **, Significant difference from 50% (highlighted by the horizontal bar) on χ2 testing (P ≤ 0.0001).
The top 10 countries with over 1000 patients enrolled in KIGS were compared to determine factors that may contribute to the gender disparities in rhGH use (Table 1). Neither adult height nor markers of population health, health care, or financial heterogeneity correlated with the percentage of male rhGH recipients.
Table 1.
Correlation of health and economic measures with the male percentage of patients enrolled in the KIGS registry, in decreasing order, for the 10 most represented countries
| Country
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Japan | United States | Italy | Turkey | Sweden | Australia | Germany | Spain | France | UK | P value | |
| rhGH (% males) | 65 | 64 | 59 | 59 | 58 | 57 | 57 | 54 | 54 | 53 | |
| Mean adult height (cm) | |||||||||||
| Male | 169 | 177 | 176 | 180 | 179 | 178 | 177 | 176 | 177 | 0.13 | |
| Female | 157 | 163 | 162 | 167 | 164 | 165 | 164 | 163 | 163 | 0.17 | |
| δ | 12 | 14 | 14 | 13 | 15 | 13 | 13 | 13 | 14 | 0.25 | |
| Age trends | |||||||||||
| Percentage of population age 0–14 yr | 14 | 20 | 14 | 37 | 18 | 20 | 15 | 15 | 19 | 18 | 0.93 |
| Health outcomes | |||||||||||
| Life expectancy at birth (yr) | 81 | 78 | 80 | 69 | 80 | 81 | 79 | 81 | 79 | 79 | 0.85 |
| Infant deaths per 1,000 live births | 3 | 7 | 5 | 28 | 3 | 5 | 4 | 4 | 4 | 5 | 0.76 |
| Health care environment | |||||||||||
| Physicians per 100,000 | 196 | 256 | 386 | 139 | 325 | 247 | 340 | 322 | 340 | 213 | 0.39 |
| Total health expenditure (% of GDP) | 8 | 15 | 9 | 8 | 10 | 10 | 11 | 8 | 10 | 8 | 0.28 |
| General economic indices | |||||||||||
| Gini indexa | 38 | 45 | 36 | 42 | 25 | 35 | 28 | 33 | 33 | 37 | 0.17 |
GDP, Gross domestic product.
The higher the Gini index, the greater the country’s income inequality.
Discussion
Male predominance among U.S. pediatric rhGH recipients, described at the introduction of rhGH, persisted into this third decade of use. The factor that most consistently affected the gender distribution was the diagnostic indication, with the greatest gender disparity appearing in indications without clear organic etiologies, like ISS. Interestingly, PWS was the only indication with gender equality in the United States. Although the FDA specifically approved rhGH to ameliorate the growth failure frequently associated with PWS, many clinicians and patient families primarily seek rhGH for its metabolic benefits; rhGH can improve the hypotonia and abnormal body composition (decreased lean body mass and increased fat mass) that far outweigh height in producing PWS-associated morbidity (7,8,9).
The gender distribution was also related to age at rhGH initiation. The gender disparity increased into the second decade, and boys on average were older than girls at the initiation of treatment. Because boys typically start puberty later than girls and hit their growth spurt later in the sequence of pubertal development, boys who lag behind their peers in sexual development are more likely to be perceived as having a growth problem. Likewise, boys may still be growing at ages when most girls have achieved epiphyseal fusion and, thus, may still benefit from starting rhGH at older ages. However, differences in pubertal maturation cannot account for the male predominance seen at the younger ages.
Analysis of global pediatric rhGH recipients showed that the United States closely followed Japan, with a roughly 2:1 male to female ratio overall; Europe/Australia/New Zealand also exceeded an equal gender distribution, with an average of 55% males. The absence of male predominance in the ROW region raises the question of cultural influences on rhGH use. Furthermore, the fact that children from the ROW numbered an order of magnitude fewer than the regions with male predominance suggests other possible contributing factors. Perhaps countries that cannot financially support extensive rhGH use are less likely to have physicians promote pharmacological male height enhancement. Alternatively, perhaps physicians in countries that have not experienced extensive rhGH use have not yet developed sufficient comfort with this agent to favor its use when considering treatment for male height enhancement.
Biological differences between the genders, leading to an unequal incidence of growth disorders, are another potential explanation. For example, chronic renal insufficiency occurs more often in males (10). GHD can be inherited in an autosomal dominant, recessive or X-linked manner, but the majority of cases are idiopathic (11). Reports from the United States (12) and Denmark (13) found more childhood-onset GHD among males than females. However, each study may have an ascertainment bias. The U.S. study based its prevalence calculation on the provocative testing of subjects who completed diagnostic evaluation. However, not all children identified by the screening program as having a worrisome growth pattern underwent diagnostic evaluation due to the lack of parental concern or refusal to allow follow-up. The history of specialist use by the study population suggests that this limitation may have skewed the gender ratio results; of the children diagnosed with GHD (mostly male), half had been previously referred to and 25% had seen an endocrinologist, whereas 17% of the girls with Turner syndrome had been referred, and none had seen an endocrinologist (12). The Danish study based its incidence calculations on cases of GHD recorded in three national registries: patients hospitalized with a cancer diagnosis, all inpatients, and all deceased patients. Outpatient hospital visits were included in one of the registries only during the last 4 yr of the 19-yr study period (13). Thus, the results were dependent on the hospital referral patterns of the general practitioners and may have overrepresented organic GHD relative to idiopathic isolated GHD. Even if the two studies were not affected by ascertainment bias, the reported male predominance in GHD is insufficient to explain the gender disparities in the KIGS registry. The prevalence of GHD was calculated at 1:3,500 children in the U.S. study (12), and its average incidence rate 1:38,800 boys and 1:58,800 girls less than 18 yr old in the Danish study (13). The prevalence of Turner syndrome, 1:2000 to 1:3125 live-born girls depending on cytogenetic screening (14,15) vs. postnatal diagnosis (16), would be expected to offset the male excess of GHD in tallying up total rhGH use. Finally, the wide discordance between the male proportions of rhGH recipients in the KIGS registry from these two countries (64% in the United States vs. 54% in Denmark) suggests that more than biology was at play.
The relatively high cost of rhGH treatment (17) makes financial considerations a reasonable factor to contemplate. We examined proxy variables for funds available for medical care across countries. The percentage of male rhGH recipients did not correlate with markers of health outcomes, health care environment, or general economic indices in the 10 countries most represented in the KIGS registry.
Countries differ in their financing of rhGH therapy. Of the10 greatest Genotropin users, the United States is the only country with a commercial third-party payor health system; the remaining countries essentially have a single (government) payor, even when decentralized with both national and local government programs. In some countries, such as Spain, private insurance companies can sell supplemental health care coverage to supplement the national plan (6). The United States is also the only country in which ISS became a government-approved indication for pediatric rhGH therapy. The FDA approved rhGH for children with ISS with height z-scores below −2.25 sd, i.e. shortest 1.2% of children (18), whereas −2.0 sd (shortest 2.3%) is generally accepted as the cutoff between normal and abnormal height (19,20). In Japan, the only country to exceed the male predominance of the United States, −2.0 sd of the national reference data are also the accepted height threshold for treating GHD, with 70% coverage from the national health insurance system. However, for Japanese children with heights −2.5 sd or below (shortest 0.7%), supplemental support from the local government increases rhGH coverage to almost 90% (21). In contrast, the United Kingdom, which had the lowest male predominance of the 10 greatest Genotropin users, recommends a threshold of −2.65 sd (the shortest 0.4%) on height screening for initiating referral and evaluation (22,23). Using this cutoff, the National Institute for Clinical Excellence concluded that rhGH treatment had a worthwhile cost-effectiveness analysis for the United Kingdom (24).
A limitation of our report is that the rhGH recipient data were drawn from a post-marketing surveillance database. Clinicians voluntarily participate in KIGS and provide data on the children in their practices who receive Genotropin. Thus, the study does not capture every child on this product because clinicians can prescribe the drug without contributing to the database. KIGS captures approximately 22 ± 3% of the pediatric patients started on Genotropin therapy; this figure varies by major market and year. The study also misses all the children who receive another brand of rhGH. Genotropin’s market share varies from country to country, region to region, and by time, but Genotropin is currently one of the three leading brands of rhGH in each of the areas analyzed, with no less than a 20% share in any of the major markets. Therefore, conclusions based on Genotropin use are largely representative of the market as a whole.
There is no reason to suspect that the reporting mechanism, albeit nonexhaustive, would preferentially include children of one gender over the other and, thereby, introduce a systematic ascertainment bias based on gender. Likewise, although the various rhGH manufacturers received FDA approval for different indications at different times, a significant number of children were enrolled in KIGS under diagnoses years before Pfizer (or its predecessor) specifically, and in some cases, any manufacturer, received FDA approval for that indication. Such off-label prescription practices minimize the potential impact of systematic manufacturer/diagnosis-based bias on our results.
Where could the gender disparity in U.S. rhGH use originate? Pediatric endocrinologists are the principal prescribers of rhGH for children in the United States. In a national survey published in 1996, North American pediatric endocrinologists were 1.3 times more likely to prescribe rhGH for identical case scenarios that described male rather than female patients (25). More recently, a referral bias was documented in a U.S. academic pediatric endocrinology center. Girls were referred for short stature evaluations half as often as boys, with greater height deficits relative to both the general population and their midparental target heights, and were more likely to have an identifiable underlying disease (26). It is unclear to what extent the primary care physicians, patient families, or combination contributed to this referral bias. Correcting the referral bias will be important to avoid delayed or missed diagnoses in girls of growth stunting conditions that extend beyond rhGH treatment.
In conclusion, male predominance among U.S. pediatric rhGH recipients persists into its third decade. The gender ratio depends on the diagnostic indication and age at initiating treatment, and varies among different countries. Social pressures for tallness affect boys more than girls (27), and the extent of such pressures is influenced by cultural mores. These data suggest that social and cultural differences, in conjunction with perceived acceptable rhGH expenditures, foster greater gender disparities in pediatric rhGH use in Japan and the United States than in other countries. Medical care providers need to be aware of this pattern of practice bias, and carefully consider girls with growth failure to ensure timely diagnosis and treatment of underlying health problems. Conversely, the potential overtreatment of short boys should also be considered.
Acknowledgments
We thank all the clinicians who enrolled their patients in the Pfizer International Growth Study registry, without whom such research would not be possible. We also thank Dr. Virginia Stallings for her thoughtful review of the manuscript.
Footnotes
The Pfizer International Growth Study is supported by Pfizer Global Pharmaceuticals. E.S. and M.P.W. are employees of Pfizer. A.G. is funded by National Institutes of Health Grant 5K08 DK64352 from the National Institute of Diabetes and Digestive and Kidney Diseases and by a Foerderer/Murray Award from the Children’s Hospital of Philadelphia. Pfizer granted permission for data extraction from the Pfizer International Growth Study but did not influence the analyses and/or conclusions of the study. A.G. was primarily responsible for the study design, collection, interpretation of data, writing of the manuscript, and the decision to submit for publication.
Disclosure Statement: E.S. and M.P.W. are employees of Pfizer, Inc. A.G. received an honorarium and travel expenses from Pfizer, Inc., to present the preliminary data at the 2006 Pfizer International Growth Study United States Investigators’ Meeting, San Francisco, California.
First Published Online March 11, 2008
Abbreviations: CGD, Constitutional growth delay; FDA, U.S. Food and Drug Administration; FSS, familial short stature; GHD, GH deficiency; ISS, idiopathic short stature; KIGS, Pfizer International Growth Study; PWS, Prader-Willi syndrome; rhGH, recombinant human GH; ROW, rest of the world; SGA, small for gestational age.
References
- August GP, Lippe BM, Blethen SL, Rosenfeld RG, Seelig SA, Johanson AJ, Compton PG, Frane JW, McClellan BH, Sherman BM 1990 Growth hormone treatment in the United States: demographic and diagnostic features of 2331 children. J Pediatr 116:899–903 [DOI] [PubMed] [Google Scholar]
- Lippe BM 1992 Growth hormone treatment: does ascertainment bias determine treatment practices? Growth Genet Horm 8(Suppl 1):31–34 [Google Scholar]
- Chatelain P 1999 Trends in the diagnosis and treatment of short stature as revealed by KIGS. In: Ranke MB, Wilton P, eds. Growth hormone therapy in KIGS: 10 years’ experience. Heidelberg: Barth; 1–20 [Google Scholar]
- US Census Bureau 2000 United States Census 2000. http://www.census.gov/main/www/cen2000.html [Google Scholar]
- Lindstrom C, Lindstrom M 2006 “Social capital,” GNP per capita, relative income, and health: an ecological study of 23 countries. Int J Health Serv 36:679–696 [DOI] [PubMed] [Google Scholar]
- World Health Organization Highlights on health. http://www.who.int/countries/en (accessed 2007) [Google Scholar]
- Myers SE, Whitman BY, Carrel AL, Moerchen V, Bekx MT, Allen DB 2007 Two years of growth hormone therapy in young children with Prader-Willi syndrome: physical and neurodevelopmental benefits. Am J Med Genet A 143:443–448 [DOI] [PubMed] [Google Scholar]
- Carrel AL, Moerchen V, Myers SE, Bekx MT, Whitman BY, Allen DB 2004 Growth hormone improves mobility and body composition in infants and toddlers with Prader-Willi syndrome. J Pediatr 145:744–749 [DOI] [PubMed] [Google Scholar]
- l'Allemand D, Eiholzer U, Schlumpf M, Steinert H, Riesen W 2000 Cardiovascular risk factors improve during 3 years of growth hormone therapy in Prader-Willi syndrome. Eur J Pediatr 159:835–842 [DOI] [PubMed] [Google Scholar]
- Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR 2002 Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol 17:450–455 [DOI] [PubMed] [Google Scholar]
- Grimberg A, De Leon D 2005 Disorders of growth. In: Moshang Jr T, ed. Pediatric endocrinology: the requisites in pediatrics. St. Louis: Elsevier Mosby; 127–167 [Google Scholar]
- Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M 1994 Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr 125:29–35 [DOI] [PubMed] [Google Scholar]
- Stochholm K, Gravholt CH, Laursen T, Jørgensen JO, Laurberg P, Andersen M, Kristensen LØ, Feldt-Rasmussen U, Christiansen JS, Frydenberg M, Green A 2006 Incidence of GH deficiency–a nationwide study. Eur J Endocrinol 155:61–71 [DOI] [PubMed] [Google Scholar]
- Gravholt CH 2005 Clinical practice in Turner syndrome. Nat Clin Pract Endocrinol Metab 1:41–52 [DOI] [PubMed] [Google Scholar]
- Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH 2006 Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab 91:3897–3902 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Juul S, Naeraa RW, Hansen J 1996 Prenatal and postnatal prevalence of Turner’s syndrome: a registry study. BMJ 312:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Davis MM, Clark SJ, Hofer TP, Kemper AR 2006 Estimated cost-effectiveness of growth hormone therapy for idiopathic short stature. Arch Pediatr Adolesc Med 160:263–269 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration 2003 FDA approves Humatrope for short stature. www.fda.gov/bbs/topics/ANSWERS/2003/ANS01242.html [Google Scholar]
- Growth Hormone Research Society 2000 Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab 85:3990–3993 [DOI] [PubMed] [Google Scholar]
- Gharib H, Cook DM, Saenger PH, Bengtsson BA, Feld S, Nippoldt TB, Rodbard HW, Seibel JA, Vance ML, Zimmerman D, Palumbo PJ, Bergman DA, Garber JR, Hamilton Jr CR, Petak SM, Rettinger HI, Service FJ, Shankar TP, Stoffer SS, Tourletot JB, American Association of Clinical Endocrinologists Growth Hormone Task Force 2003 American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in adults and children–2003 update. Endocr Pract 9:65–75 [DOI] [PubMed] [Google Scholar]
- Chihara K 2005 Diagnosis guideline for pediatric growth hormone deficiency. In: Study Report for Pituitary Dysfunction. Tokyo, Japan: Japan Ministry of Health, Labour and Welfare. 118–120 [Google Scholar]
- Hall DM 2000 Growth monitoring. Arch Dis Child 82:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Screening Committee 2004 Child health sub-group report–growth disorders. Leeds: National Screening Committee. http://www.library.nhs.uk/screening/ViewResource.aspx?resID=61017 [Google Scholar]
- National Institute for Clinical Excellence 2005 Guidance on the use of human growth hormone (somatropin) in children with growth failure. Technology Appraisal Guidance No. 42, 1–19 [Google Scholar]
- Cuttler L, Silvers JB, Singh J, Marrero U, Finkelstein B, Tannin G, Neuhauser D 1996 Short stature and growth hormone therapy. A national study of physician recommendation patterns. JAMA 276:531–537 [PubMed] [Google Scholar]
- Grimberg A, Kutikov JK, Cucchiara AJ 2005 Sex differences in patients referred for evaluation of poor growth. J Pediatr 146:212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS 2006 Size matters: how height affects the health, happiness, and success of boys–and the men they become. Boston: Houghton Mifflin Co. [Google Scholar]