Abstract
Background: Although weight loss and exercise ameliorates frailty and improves cardiac risk factors in obese older adults, the long-term effect of lifestyle intervention on bone metabolism and mass is unknown.
Objective: The objective was to evaluate the effects of diet-induced weight loss in conjunction with exercise on bone metabolism and mass in obese older adults.
Design and Setting: We conducted a one-year randomized, controlled clinical trial in a university-based research center.
Participants: Twenty-seven frail, obese (body mass index = 39 ± 5 kg/m2), older (age 70 ± 5 yr) adults participated in the study.
Intervention: Participants were randomly assigned to diet and exercise (treatment group; n = 17) or no therapy (control group; n = 10).
Outcome Measures: Body weight decreased in the treatment group but not in the control group (−10 ± 2 vs. +1 ± 1%, P < 0.001). Compared with the control group, the treatment group had greater changes in bone mass, bone markers, and hormones, including 1) bone mineral density (BMD) in total hip (0.1 ± 2.1 vs. −2.4 ± 2.5%), trochanter (0.2 ± 3.3 vs. −3.3 ± 3.1%), and intertrochanter (0.3 ± 2.7 vs. −2.7 ± .3.0%); 2) C-terminal telopeptide (12 ± 35 vs. 101 ± 79%) and osteocalcin (−5 ± 15 vs. 66 ± 61%); and 3) leptin (2 ± 12 vs. −30 ± 25%) and estradiol (0.1 ± 14% vs. −14 ± 21%) (all P < 0.05). Changes in weight (r = 0.55), bone markers (r = −0.54), and leptin (r = 0.61) correlated with changes in hip BMD (all P < 0.05).
Conclusion: Weight loss, even when combined with exercise, decreases hip BMD in obese older adults. It is not known whether the beneficial effects of weight loss and exercise on physical function lower the overall risk of falls and fractures, despite the decline in hip BMD.
A 1-year randomized controlled trial in frail, obese, older adults finds that weight loss combined with a multi-component exercise is associated with bone loss at the hip and bone turnover increase. It is not known whether the beneficial effects of weight loss and exercise on physical function lower the overall risk of falls and fractures, despite the decline in hip bone mineral density.
Obesity exacerbates the age-related decline in physical function in older adults, which causes frailty, impairs quality of life, and increases nursing home admissions (1,2,3). Therefore, obesity in the elderly population has considerable public health implications in the United States, because both the number of older adults and the prevalence of obesity among older adults are increasing (4).
We have recently demonstrated that lifestyle intervention ameliorates frailty (5) and improves metabolic coronary heart disease risk factors in obese older adults (6). In contrast, data from prospective interventional studies suggest that diet-induced weight loss could have deleterious effects in older adults by causing bone loss (7,8,9,10,11,12). Adding exercise training (ET) to a dietary weight loss program might be particularly important in older adults because ET is used to prevent and treat osteoporosis (13) and reduces the risk of injurious falls (14,15). However, the long-term effects of weight loss and ET on bone mass and bone metabolism in obese older persons have not been studied.
The purpose of the present study was to conduct a randomized controlled trial to determine the effects of a diet-induced weight loss program conducted in conjunction with ET on bone metabolism and mass in obese older adults. We hypothesized that a strength and endurance ET program would not be adequate to prevent increased bone turnover and bone loss induced by dietary weight loss.
Subjects and Methods
Subjects
This study was conducted at Washington University School of Medicine and was approved by the Institutional Review Board. Written informed consent was obtained from each participant. Volunteers were recruited by using local advertisements.
All potential subjects completed a comprehensive screening procedure, which included a medical history, physical examination, standard blood and urine chemistries, and a treadmill exercise stress test. To be eligible for this study, volunteers had to meet the following criteria: 1) older age (≥65 yr), 2) obese [body mass index (BMI) ≥ 30 kg/m2], 3) sedentary (did not participate in regular exercise more than twice a week), 4) stable body weight (±2 kg) over the past year, and 5) treatment with medications was unchanged for at least 6 months before enrollment. Moreover, all subjects had to have mild to moderate frailty, based on meeting at least two of the three following criteria (1,5): 1) physical performance test score of 18–32, 2) peak O2 consumption of 11–18 ml/kg·min, and 3) difficulty or need for assistance in two instrumental activities of daily living or one basic activity of daily living. Subjects who had severe cardiopulmonary disease, neuromuscular impairments that preclude ET, visual, hearing, or cognitive impairments, history of malignant neoplasm, and treatment with bone-acting drugs (e.g. bisphosphonates, glucocorticoids, sex-steroid compounds) during the previous year were excluded from participation. The effects of diet and exercise on physical function and cardiac risk factors in these subjects were reported previously (5,6).
Design
Eligible volunteers were randomized to receive either 52 wk of diet and exercise therapy (treatment group) or no treatment (control group), in an approximately 1.5:1 sequence, by using a computer-generated block random permutation procedure stratified for sex.
Treatment group intervention. The treatment group intervention involved a combination of an energy-deficit diet, behavior therapy, and a multicomponent exercise therapy. Subjects met weekly as a group with a study dietitian, who was experienced in group behavioral therapy. Standard behavioral techniques were used to change eating habits (16). Participants were prescribed a balanced diet to provide an energy deficit of 500–750 kcal/d, which contained about 30% of energy as fat, 50% as carbohydrate, and 20% as protein. In addition, subjects were given a daily multivitamin supplement and were counseled to consume adequate dietary calcium and vitamin D (1200–1500 mg Ca/d and 1000 IU vitamin D/d) (17). Total calorie intake was adjusted to prevent more than a 1.5% loss of body weight per week. The goal was to achieve a 10% weight loss at 6 months, followed by weight maintenance for an additional 6 months.
The exercise program focused on improving endurance, strength, and balance. ET sessions were conducted as a group on three nonconsecutive days each week at our exercise facility. Each session lasted about 90 min: 15 min of flexibility exercises, 30 min of endurance exercise, 30 min of strength training, and 15 min of balance exercises. Endurance exercises included walking on a treadmill, step-ups, stair climbing, stationary cycling, and Stairmaster exercise. Subjects exercised at moderate intensity (∼75% of peak heart rate), and the intensity of exercise was gradually increased over several weeks to between 80 and 90% of peak heart rate. Resistance exercises were performed by using weight-lifting machines and free weights. One-repetition maximums (1-RMs), which is the maximal amount of weight subjects lifted one time, were used to adjust resistance exercises. Weight-lifting sessions consisted of one to two sets performed at a resistance of about 65% of 1-RM, which allowed the completion of eight to 12 repetitions. The volume of exercise was gradually increased to two to three sets at a resistance of about 80% of 1-RM, which allowed the completion of six to eight repetitions. These sessions were supervised by a physical therapist.
Control group intervention. Participants randomized to the control group were instructed to maintain their usual diet and activities during the study period and were asked not to participate in any weight-loss or exercise programs.
Outcome assessments
Body weight and bone mass
Body weight was measured at baseline, 6 months, and 12 months in the morning after subjects had fasted for 12 h. Bone mineral density (BMD) and bone mineral content (BMC) of the lumbar spine, proximal femur, and total body were measured at baseline, 6 months, and 12 months by using dual-energy x-ray absorptiometry (Delphi 4500-W; Hologic Corp., Waltham, MA). BMD and BMC of the lumbar spine were calculated as the mean of vertebrae L1–L4. The coefficient of variation (CV) for this technique at our center is 1.1% for the lumbar spine and 1.2% for the proximal femur (18).
Serum markers of bone metabolism
Venous blood samples were obtained in the morning after subjects fasted for at least 12 h at baseline, 6 months, and 12 months. ELISA kits were used to measure C-terminal telopeptide of type I collagen (CTX) (Crosslaps; Nordic Bioscience Diagnostics, Herlev, Denmark; CV, 2.1%) as a marker of bone resorption and osteocalcin (Metra OC; Quidel Corp., San Diego, CA; CV, 4.4%) and bone-specific alkaline phosphatase (Metra BAP; Quidel; CV, 4.9%) as markers of bone formation. RIA kits were used to measure serum estradiol (Ultra-sensitive estradiol DSL-4800; Diagnostic Systems Laboratories Inc., Webster, TX), leptin (Leptin HL-81K; Linco Research Inc., St. Charles, MO), IGF-I (Diagnostic Products Group, Los Angeles, CA), cortisol (Diagnostic Systems Laboratories), 25-hydroxyvitamin D [25(OH)D] (DiaSorin, Stillwater, MN), and 1,25-dihydroxyvitamin D [1,25(OH)2D] (DiaSorin) concentrations. Serum PTH concentration was measured by using chemiluminescence immunoassay (ADVIA Centaur intact PTH). The CV for these hormone measurements were less than 10%. Blood tests at 6 and 12 months were obtained about 40 h after the last bout of exercise.
Statistical analysis
The primary outcome in this study was changes in total hip BMD. It was estimated that 10 control and 15 treatment subjects would be needed to detect a clinically meaningful 2.5 ± 3.3% greater decrease in total hip BMD in the treatment compared with control groups, with a power of 0.9 and an α-level of 0.05. Secondary outcomes included changes in bone-related hormones. When follow-up data were not available, the last observation was carried forward. Differences in baseline characteristics between groups were evaluated by using independent t tests (continuous variables) and χ2 tests (categorical variables). Repeated-measures ANOVA was used to compare treatment effects, with a group factor (treatment) and a trial factor (time). Baseline values and sex were included as covariates in the ANOVA. When a significant treatment-by-time interaction was detected, changes from baseline to 6 months and from baseline to 12 months were evaluated by the ANOVA model using linear contrasts, controlling for baseline values and sex. Pearson’s correlations were performed to assess associations between changes in selected variables. A P value of <0.05 was considered statistically significant. Results are reported as mean ± sd, except in the figures, which report data as mean ± se.
Results
Of 40 obese older volunteers who were screened, 27 were eligible and were randomized to the treatment group (n = 17) or control group (n = 10). Twenty-four participants successfully completed the study; two participants in the treatment group dropped out because of difficulty with compliance, and one participant in the control group dropped out because of relocation to another state.
Baseline characteristics, including age, sex, BMI, physical function, BMD, BMC, and T-scores, in treatment and control groups were similar (Table 1). Based on T-scores, no subject had osteoporosis; 40% had osteopenia. All participants had above average Z-scores (>0), which were not statistically different between groups: lumbar spine (2.1 ± 1.8 vs. 2.2 ± 1.2) and total hip (0.9 ± 0.8 vs. 1.3 ± 1.1) in treatment and control groups, respectively. Baseline serum markers of bone turnover and hormones did not differ between groups (Tables 1 and 2).
Table 1.
Baseline characteristics
| Variable | Control group (n = 10) | Treatment group (n = 17) | P value |
|---|---|---|---|
| Age (yr) | 71.1 ± 5.1 | 69.4 ± 4.6 | 0.37 |
| Female sex [n (%)] | 6 (60) | 12 (71) | 0.57 |
| Weight (kg) | 103.2 ± 19.8 | 99.7 ± 13.6 | 0.60 |
| BMI (kg/m2) | 39.0 ± 5.0 | 38.5 ± 5.3 | 0.81 |
| BMD (g/cm2) | |||
| Lumbar spine | 1.127 ± 0.132 | 1.107 ± 0.127 | 0.70 |
| Total hip | 0.993 ± 0.141 | 0.947 ± 0.115 | 0.37 |
| Femoral neck | 0.804 ± 0.104 | 0.810 ± 0.120 | 0.88 |
| Trochanter | 0.747 ± 0.152 | 0.716 ± 0.107 | 0.54 |
| Intertrochanter | 1.189 ± 0.137 | 1.129 ± 0.129 | 0.27 |
| Whole body | 1.197 ± 0.138 | 1.151 ± 0.127 | 0.39 |
| BMC (g) | |||
| Lumbar spine | 67.7 ± 17.1 | 65.5 ± 11.6 | 0.70 |
| Total hip | 38.1 ± 11.1 | 35.3 ± 9.6 | 0.50 |
| Femoral neck | 4.3 ± 0.9 | 4.1 ± 0.7 | 0.60 |
| Trochanter | 8.9 ± 2.5 | 8.8 ± 2.6 | 0.90 |
| Intertrochanter | 24.8 ± 7.8 | 22.4 ± 7.0 | 0.39 |
| Whole body | 2606 ± 669 | 2423 ± 474 | 0.42 |
| T-score | |||
| Lumbar spine | 0.4 ± 1.2 | 0.3 ± 1.0 | 0.69 |
| Total hip | 0.1 ± 0.9 | −0.2 ± 0.7 | 0.42 |
| Femoral neck | −0.8 ± 0.7 | −0.7 ± 0.9 | 0.77 |
| Bone turnover markers | |||
| Osteocalcin (ng/ml) | 8.3 ± 2.6 | 6.3 ± 2.9 | 0.08 |
| BAP (U/liter) | 25.7 ± 25.7 | 24.9 ± 3.7 | 0.72 |
| CTX (ng/ml) | 0.353 ± 0.090 | 0.282 ± 0.098 | 0.08 |
Values are means ± sd. BAP, Bone alkaline phosphatase; CTX, C-terminal telopeptide. To convert osteocalcin and CTX to nanomoles per liter, multiply by 0.17 and 7.8, respectively.
Table 2.
Serum concentrations of bone-related hormones
| Control group | Treatment group | |
|---|---|---|
| 25(OH)D (ng/ml) | ||
| Baseline | 17.2 ± 8.8 | 20.2 ± 7.7 |
| 6 months | 17.2 ± 6.6 | 24.0 ± 8.9b,d |
| 1 yr | 20.4 ± 11.0 | 25.8 ± 8.0b |
| 1,25(OH)2D (pg/ml) | ||
| Baseline | 38.7 ± 20.3 | 32.8 ± 16.6 |
| 6 months | 32.4 ± 19.2 | 27.6 ± 14.8 |
| 1yr | 31.3 ± 19.7 | 26.6 ± 11.5 |
| PTH (pg/ml) | ||
| Baseline | 41.4 ± 21.3 | 48.8 ± 25.2 |
| 6 months | 49.4 ± 23.7 | 63.3 ± 22.6 |
| 1 yr | 55.7 ± 19.2 | 62.7 ± 28.2 |
| Leptin (U/liter) | ||
| Baseline | 33.2 ± 12.9 | 33.5 ± 11.3 |
| 6 months | 33.4 ± 12.7 | 27.2 ± 14.2b,d |
| 1 yr | 34.3 ± 16.7 | 24.7 ± 12.7a,e |
| Estradiol (pg/ml) | ||
| Baseline | 20.3 ± 4.4 | 25.4 ± 9.8 |
| 6 months | 19.2 ± 2.8 | 20.8 ± 6.1b,d |
| 1 yr | 19.9 ± 2.3 | 20.6 ± 5.3c,d |
| IGF-I (ng/ml) | ||
| Baseline | 156.0 ± 40.4 | 154.2 ± 85.7 |
| 6 months | 159.8 ± 35.7 | 156.0 ± 76.0 |
| 1 yr | 148.8 ± 36.5 | 156.5 ± 85.7 |
| Cortisol (μ g/dl) | ||
| Baseline | 9.4 ± 3.3 | 11.5 ± 2.7 |
| 6 months | 10.4 ± 4.6 | 11.2 ± 3.7 |
| 1 yr | 9.4 ± 3.5 | 10.7 ± 3.1 |
Values are mean ± sd. To convert 25(OH)D to nanomoles per liter, 1,25(OH)2D to nanomoles per liter, estradiol to picomoles per liter, and cortisol to nanomoles per liter, multiply by 27.6, 2.5, 2.6, 3.7, and 27.6, respectively.
a–c Value significantly different from baseline value:
P < 0.001;
P < 0.01;
P < 0.05.
d,e Value significantly different from control group value:
P < 0.05;
P < 0.01.
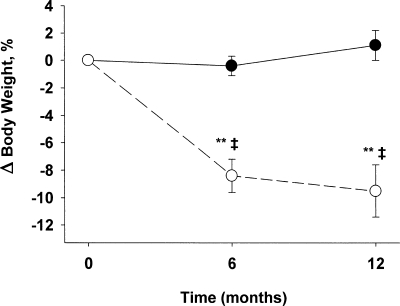
Mean attendance at the weekly group behavioral and nutrition education sessions was 81.1 ± 12.7%. Mean attendance at the exercise sessions was 83 ± 8.7%, performed at a frequency of 2.5 ± 0.3 d/wk. At 12 months, the treatment group lost 10.1 ± 2.0% body weight, whereas weight did not change significantly (+1.2 ± 1.3%) in the control group (Fig. 1). Relative improvements in strength, assessed by 1-RM, were detected for both upper body (bench curl, 50 ± 55%; bench press, 33 ± 48%; seated row, 20 ± 20%) and lower body (knee flexion, 32 ± 32%; knee extension, 63 ± 56%; leg press, 55 ± 40%) muscle groups (all P < 0.05).
Figure 1.
Changes in body weight in the treatment (○) and control (•) groups.
Decreases in BMD were greater in the treatment than control group at total hip (−2.4 ± 2.5 vs. 0.1 ± 2.1%; P = 0.02), trochanter (−3.3 ± 3.1 vs. 0.2 ± 3.3%; P = 0.04), and intertrochanter (−2.7 ± 3.0 vs. 0.3 ± 2.7%; P = 0.02) sites (Fig. 2). The treatment group also had greater decreases in BMC than the control group at total hip (−2.4 ± 4.7 vs. 0.9 ± 2.0%; P = 0.02), trochanter (−4.1 ± 7.0 vs. 1.4 ± 6.1%; P = 0.048), and intertrochanter (−2.4 ± 5.7 vs. 0.6 ± 2.0%; P = 0.04). No differences between groups were detected in changes in spine BMD (0.9 ± 3.1 vs. 1.3 ± 5.8%), spine BMC (2.1 ± 6.1 vs. 2.1 ± 4.9%), and whole-body BMD (−0.9 ± 1.7 vs. 0.3 ± 2.1%) and BMC (−1.4 ± 2.5 vs. −1.7 ± 2.4%) (all P > 0.05). Final T-scores were 0.4 ± 1.0, −0.5 ± 0.8, and −0.9 ± 0.9 in the treatment group and 0.6 ± 1.7, 0.1 ± 1.1, and −0.8 ± 0.7 in the control group at spine, total hip, and femoral neck, respectively.
Figure 2.
Changes from baseline in total hip BMD (A), femoral neck BMD (B), trochanter BMD (C), and intertrochanter BMD (D) in obese older adults randomized to treatment group (○) or control group (•). Values are mean ± se. Value significantly different from baseline value: **, P < 0.01; *, P < 0.05. Value significantly different from control value: ‡, P < 0.01; †, P < 0.05.
At 6 months, serum CTX (101 ± 79 vs. 12 ± 35%; P = 0.02) and serum osteocalcin (66 ± 61 vs. −5 ± 15%; P = 0.02) concentrations increased in the treatment group but not in the control group (Fig. 3). At 12 months, changes in serum CTX (86 ± 91 vs. 5 ± 44%; P = 0.12) and osteocalcin (47 ± 65 vs. 3 ± 37%; P = 0.08) tended to be greater in the treatment group than in the control group, but the differences were no longer statistically significant. However, both serum CTX and osteocalcin were greater at 12 months compared with baseline values in the treatment group (within-group P < 0.05). There were no significant changes in serum bone alkaline phosphatase concentrations.
Figure 3.
Changes from baseline in serum markers of bone turnover: CTX (A), osteocalcin (B), and bone alkaline phosphatase (C) in obese older adults randomized to treatment group (○) or control group (•). Values are mean ± se. Value significantly different from baseline value: *, P < 0.05. Value significantly different from control value: †, P < 0.05.
Serum 25(OH)D (24 ± 30 vs. 15 ± 56%; P = 0.02) concentrations increased at 6 months, whereas serum leptin (−30 ± 25 vs. 2 ± 12%; P < 0.001) and estradiol (−14 ± 21 vs. 0.1 ± 14%; P = 0.04) decreased at 6 and 12 months in the treatment compared with the control group (Table 2). There were no significant changes in serum 1,25-dihydroxyvitamin D, PTH, IGF-I, or cortisol concentrations.
Changes in body weight correlated directly with changes in BMD at the total hip (r = 0.55; P = 0.004), trochanter (r = 0.40; P = 0.05) and intertrochanter (r = 0.45; P = 0.02) sites.
Several markers associated with bone metabolism also correlated with changes in BMD: 1) changes in serum CTX concentrations correlated negatively with changes in total hip BMD (r = −0.54; P = 0.007), trochanter BMD (r = −0.56; P = 0.006), and intertrochanter BMD (−0.40; P = 0.04); 2) changes in osteocalcin concentration correlated negatively with the changes in trochanter BMD (r = −0.50; P = 0.01), and 3) changes in leptin concentration were directly correlated with changes in BMD at the total hip (r = 0.61; P < 0.001), trochanter (r = 0.56; P = 0.004), and intertrochanter (r = 0.56; P = 0.003) sites. Changes in other serum hormone concentrations that were affected by weight loss, such as estradiol and 25(OH)D, did not correlate with changes in BMD (all P > 0.05).
Discussion
Obesity in older adults exacerbates the age-related decline in physical function (1,2,19,20), which can lead to a loss of independence and admission to a chronic care facility (3). Although a reduction in body weight can improve physical function in obese older adults (5,21), weight loss is also associated with bone loss and decreased BMD (7,8,9,10,11,12), which could increase fracture risk. Therefore, we conducted a 1-yr randomized controlled trial in obese older adults to evaluate whether ET, which is often recommended to prevent or treat osteoporosis, can be used to prevent loss of bone that occurs with diet-induced weight loss. The results of the present study demonstrate that treatment with an energy-deficit diet, increased bone turnover, and decreased hip bone mass in obese older adults, despite concomitant regular ET. These findings have important implications for weight-loss therapy in obese older adults and underscore the need to monitor BMD and to consider adjunctive therapeutic interventions to reduce the risk of bone loss in this patient population.
The decrease in hip BMD observed in our study subjects was directly correlated with their decrease in body weight. Data from previous weight-loss studies that were conducted in young and middle-aged adults also found that bone loss was proportional to the amount of weight loss (7,8,9,10,11,12). The 2–3% decrease in hip BMD in our obese older adults is within the range reported in other studies of obese subjects who lost a similar amount (∼10%) of body weight (7,8,9,10,11,12). Therefore, the effect of weight loss on BMD in older adults is probably similar to that in younger adults. The clinical significance of the decrease in hip BMD induced by weight loss in obese older adults is not clear. All our subjects had high baseline BMD Z-scores, and none had evidence of osteoporosis after weight loss. In addition, the increased fracture risk caused by decreases in hip BMD and the protective cushioning of body fat might be offset by improved physical function and balance (15), which can decrease the risk of falls and bone injury. Moreover, BMD decreased in the hip but not in the spine, suggesting that our exercise intervention was more effective in preventing bone loss in the spine, which is a load-bearing region rich in trabecular bone (22).
The marked increase in serum CTX (∼100-fold) and osteocalcin (∼60-fold) concentrations in response to weight loss in our participants indicate that bone resorption and formation, respectively, were stimulated. Moreover, the increases in both CTX and osteocalcin concentrations correlated with decreases in hip BMD, suggesting that weight-loss-induced bone loss is due to increased bone turnover, with greater stimulation of bone resorption than bone formation. These results support data from previous studies conducted in younger obese adults, which found weight loss was associated with a disproportionate increase in bone resorption (12,23). Although physical activity itself can stimulate bone turnover, it is unlikely that exercise training contributed to bone turnover in our subjects, because samples were collected ∼40 h after the last bout of exercise (24).
The precise mechanisms responsible for weight-loss-induced bone loss are not known. One hypothesis is that weight loss decreases the mechanical stress on the weight-bearing skeleton (11,25) mediated by changes in local bone factors (e.g. prostaglandins) and by changes in the mechanostat (26) that result in a decrease in bone mass. Accordingly, obesity, which increases weight-bearing stress on the skeleton, is associated with high bone mass (27), and exercise-induced mechanical strain on the skeleton is osteogenic and helps maintain BMD (13). Therefore, we included exercises that stimulated major muscles attached to bone to our weight-loss program in an attempt to prevent bone loss. However, our ET did not prevent a decrease in BMD or BMC in our study subjects. Our ET program was specifically designed to improve physical function and ameliorate frailty in obese older adults (1,5) and therefore not necessarily the most bone-loading exercises (13). Although our results are consistent with previous studies showing that increased physical activity does not prevent weight-loss-induced bone loss (10,28), one study showed that a weight-bearing endurance exercise was able to maintain hip BMD (29).
Alterations in bone-acting hormones has also been proposed as a mechanism for the weight-loss-induced decrease in bone, particularly bone loss in non-weight-bearing sites (30,31). Leptin, which is produced by adipose tissue, has important effects on bone metabolism (32). Leptin has a direct positive effect on osteoblastic differentiation (33) and inhibits the expression of receptor activator of nuclear factor-κB ligand levels (34). Serum leptin concentration correlates directly with bone mass (35) and percent body fat and BMI (36) and decreases with weight loss (12). In our subjects, weight loss was associated with a 25% reduction in serum leptin concentrations. Moreover, the decrease in leptin was strongly correlated with a decrease in hip BMD. A decrease in estrogen production also has been suggested to mediate weight-loss-induced bone loss (30). Although weight loss caused a decrease in serum estradiol concentrations in our study subjects, we did not detect a correlation between changes in serum estradiol and changes in BMD. Other bone-active hormones, such as IGF-I and cortisol, which are anabolic and catabolic to bone, respectively, are affected by weight loss. Data from studies conducted in obese young adults have found that serum IGF-I concentrations decrease (37), whereas serum cortisol concentrations increase, with weight loss (23). In contrast, we did not detect any changes in these hormone concentrations in our subjects, suggesting that growth factors and endogenous steroids were not involved in mediating the weight-loss-induced decrease in bone mass observed in our obese older adults. Weight loss can also increase serum PTH concentrations (38), which stimulates bone resorption, but we did not detect significant changes in PTH concentrations in either of our study groups. Serum 25(OH)D levels increased in the treatment group, presumably because subjects were instructed to take a daily multivitamin that contained vitamin D. However, serum 25(OH)D concentrations did not reach the optimal range, raising the possibility that greater vitamin D supplementation could reduce bone loss.
To our knowledge, this is the first study to determine the effect of a 1-yr weight loss and ET program on bone mass in obese older adults within a randomized, controlled trial. Because an important goal of weight-loss therapy in obese older adults is to improve physical function (19), we added a multicomponent ET program to improve balance, endurance, and strength, in conjunction with a low-calorie weight-loss diet. The adherence by our participants to the intervention program (∼80% attendance at behavior education and supervised exercise sessions) was as good or better than that observed in previous studies conducted in young and middle-aged adults (39). This compliance was responsible for the successful weight management experienced by our subjects (∼10% at 6 months, which was maintained for another 6 months) and contradicts the notion that older adults will be unlikely to lose weight because of the difficulty in changing longstanding lifestyle behaviors (40).
Our study also has several limitations. First, we did not measure bone quality (e.g. bone architecture and geometry), which is an additional determinant of bone fracture (41). Even though ET did not maintain BMD, it is possible that ET had a beneficial effect on bone quality (42). Second, the small number of subjects and short duration of this study precluded an assessment of the intervention on falls and fracture. It is possible that the aggregate beneficial effects of weight loss and ET on muscle strength, balance, and potentially bone quality could lower the risk of falls and fractures, despite the decline in BMD (14). Third, we provided a multivitamin supplement as part of a standard regimen for weight-loss therapy and counseled participants about adequate Ca and vitamin D in their diet but were unable to monitor the dietary intake of calcium and vitamin D in our participants. Fourth, we could not examine sex differences in BMD because of the small sample size but controlled for the effect of sex by including it as a covariate in the repeated-measures ANOVA. Finally, changes in BMD after weight loss could have been exaggerated because of technical limitations of dual-energy x-ray absorptiometry (43). However, the decrease in BMD observed in our subjects was corroborated by similar decreases in BMC and correlation with changes in markers of bone turnover.
In conclusion, the results of the present study demonstrate that diet-induced weight loss in frail, obese older adults increases bone turnover and causes a decline in hip BMD, despite participation in a concomitant exercise program. However, it is not known whether the beneficial effects of weight loss and ET on muscle strength, balance, and physical function lower the overall risk of falls and fractures, despite the decline in BMD. Further studies are needed to determine the clinical significance of such bone loss and whether additional therapeutic interventions can prevent the weight-loss-induced decline in hip BMD.
Acknowledgments
We are grateful to Joan Heins, R.D., M.S., for weight loss therapy, Ellen Frye, P.T., for exercise training, and the participants for their cooperation in this study.
Footnotes
This study was supported by Grants AG025501, AG2116401, AG00078, DK37948, RR00036, and DK56341 from the National Institutes of Health and by a grant from the Barnes Jewish Hospital Foundation.
Disclosure information: D.T.V., K.S., M.R.B., D.R.S., and S.K. have nothing to declare.
First Published Online March 25, 2008
Abbreviations: BMC, Bone mineral content; BMD, bone mineral density; BMI, body mass index; CTX, C-terminal telopeptide of type I collagen; CV, coefficient of variation; ET, exercise training; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
References
- Villareal DT, Banks M, Siener C, Sinacore DR, Klein S 2004 Physical frailty and body composition in obese elderly men and women. Obes Res 12:913–920 [DOI] [PubMed] [Google Scholar]
- Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP 2005 The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc 53:927–934 [DOI] [PubMed] [Google Scholar]
- Zizza CA, Herring A, Stevens J, Popkin BM 2002 Obesity affects nursing-care facility admission among whites but not blacks. Obes Res 10:816–823 [DOI] [PubMed] [Google Scholar]
- Arterburn DE, Crane PK, Sullivan SD 2004 The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc 52:1907–1912 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Banks M, Sinacore DR, Siener C, Klein S 2006 Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 166:860–866 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Miller III BV, Banks M, Fontana L, Sinacore DR, Klein S 2006 Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr 84:1317–1323 [DOI] [PubMed] [Google Scholar]
- Avenell A, Richmond PR, Lean ME, Reid DM 1994 Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr 48:561–566 [PubMed] [Google Scholar]
- Jensen LB, Quaade F, Sørensen OH 1994 Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res 9:459–463 [DOI] [PubMed] [Google Scholar]
- Pritchard JE, Nowson CA, Wark JD 1996 Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord 20:513–520 [PubMed] [Google Scholar]
- Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, Applegate WB, Ettinger WHJ 2000 Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc 48:753–759 [DOI] [PubMed] [Google Scholar]
- Jensen LB, Kollerup G, Quaade F, Sorensen OH 2001 Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res 16:141–147 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein, S, Holloszy JO 2006 Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 166:2502–2510 [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR 2004 American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc 36:1985–1996 [DOI] [PubMed] [Google Scholar]
- Robertson MC, Campbell AJ, Gardner MM, Devlin N 2002 Preventing injuries in older people by preventing falls: a meta-analysis of individual-level data. J Am Geriatr Soc 50:905–911 [DOI] [PubMed] [Google Scholar]
- Weatherall M 2004 Prevention of falls and fall-related fractures in community-dwelling older adults: a meta-analysis of estimates of effectiveness based on recent guidelines. Intern Med J 34:102–108 [DOI] [PubMed] [Google Scholar]
- Klein S, Wadden T, Sugerman HJ 2002 AGA technical review on obesity. Gastroenterology 123:882–932 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services 2005 Dietary Guidelines for Americans 2005. http://www.healthierus.gov/dietaryguidelines/ [Google Scholar]
- Napoli N, Villareal DT, Mumm S, Halstead L, Sheikh S, Cagaanan M, Rini GB, Armamento-Villareal R 2005 Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res 20:232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Apovian CM, Kushner RF, Klein S 2005 Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 82:923–934 (also published in: Obes Res 13:1849–1863) [DOI] [PubMed] [Google Scholar]
- Al SS, Ottenbacher KJ, Markides KS, Kuo YF, Eschbach K, Goodwin JS 2007 The effect of obesity on disability vs mortality in older Americans. Arch Intern Med 167:774–780 [DOI] [PubMed] [Google Scholar]
- Jensen GL, Roy MA, Buchanan AE, Berg MB 2004 Weight loss intervention for obese older women: improvements in performance and function. Obes Res 12:1814–1820 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Binder EF, Yarasheski KE, Williams DB, Brown M, Sinacore DR Kohrt WM 2003 Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. J Am Geriatr Soc 51:985–990 [DOI] [PubMed] [Google Scholar]
- Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA 2005 Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res 20:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J 2000 The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 11(Suppl 6):S2–S17 [DOI] [PubMed] [Google Scholar]
- Keen RW 1999 Effects of lifestyle interventions on bone health. Lancet 354:1923–1924 [DOI] [PubMed] [Google Scholar]
- Frost HM, Ferretti JL, Jee WS 1998 Perspectives: some roles of mechanical usage, muscle strength, and the mechanostat in skeletal physiology, disease, and research. Calcif Tissue Int 62:1–7 [DOI] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, Anderson JJ 1993 Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8:567–573 [DOI] [PubMed] [Google Scholar]
- Svendsen OL, Hassager C, Christiansen C 1993 Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med 95:131–140 [DOI] [PubMed] [Google Scholar]
- Ryan AS, Nicklas BJ, Dennis KE 1998 Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol 84:1305–1310 [DOI] [PubMed] [Google Scholar]
- Reid IR 2002 Relationships among body mass, its components, and bone. Bone 31:547–555 [DOI] [PubMed] [Google Scholar]
- Reid IR, Cornish J, Baldock PA 2006 Nutrition-related peptides and bone homeostasis. J Bone Miner Res 21:495–500 [DOI] [PubMed] [Google Scholar]
- Thomas T, Burguera B 2002 Is leptin the link between fat and bone mass? J Bone Miner Res 17:1563–1569 [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR 2002 Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175:405–415 [DOI] [PubMed] [Google Scholar]
- Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT 2001 Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 142:3546–3553 [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, Nicholson GC 2001 Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab 86:1884–1887 [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL 1996 Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- Ammann P, Bourrin S, Bonjour JP, Meyer JM, Rizzoli R 2000 Protein undernutrition-induced bone loss is associated with decreased IGF-I levels and estrogen deficiency. J Bone Miner Res 15:683–690 [DOI] [PubMed] [Google Scholar]
- Ricci TA, Heymsfield SB, Pierson Jr RN, Stahl T, Chowdhury HA, Shapses SA 2001 Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr 73:347–352 [DOI] [PubMed] [Google Scholar]
- McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, Lohr KN 2003 Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 139:933–949 [DOI] [PubMed] [Google Scholar]
- Elia M 2001 Obesity in the elderly. Obes Res 9(Suppl 4):244S–248S [DOI] [PubMed] [Google Scholar]
- Seeman E, Delmas PD 2006 Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261 [DOI] [PubMed] [Google Scholar]
- Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, Kohn DH 2007 Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone 40:1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothill P, Hannan WJ 2000 Comparisons between Hologic QDR 1000W, QDR 4500A, and Lunar Expert dual-energy x-ray absorptiometry scanners used for measuring total body bone and soft tissue. Ann NY Acad Sci 904:63–71 [DOI] [PubMed] [Google Scholar]