Abstract
Context: Mutually exclusive mutations of RET, RAS, or BRAF are present in about 70% of papillary thyroid carcinomas, whereas only the latter two are seen in poorly differentiated and anaplastic cancers. Although the signal output common to these oncoproteins is ERK, a recent report showed that only BRAF mutations consistently predicted responsiveness to MAPK kinase (MEK) inhibitors.
Objectives: Here we investigated whether sensitivity to MEK inhibition was determined by oncogene status in 13 human thyroid cancer cell lines: four with BRAF mutations, four RAS, one RET/PTC1, and four wild type.
Results: Growth of BRAF (+) cells was inhibited by the MEK antagonist PD0325901 with an IC50 of less than 5 nm. By contrast, RAS, RET/PTC1, or wild-type cells had IC50 of 4 nm to greater than 1000nm. Sensitivity was not predicted by coexisting mutations in PIK3CA or by PTEN status. Similar effects were obtained with the MEK inhibitor AZD6244. PD0325901 induced a sustained G1/S arrest in BRAF (+) but not BRAF (−) lines. PD0325901 was equipotent at inhibiting pERK1/2 after 2 h, regardless of genetic background, but pERK rebounded at 24 h in most lines. MEK inhibitor resistance was associated with partial refractoriness of pERK to further inhibition by the compounds. AZD6244 was more potent at inhibiting growth of NPA (BRAF +) than Cal62 (KRAS +) xenografts.
Conclusion: Thyroid cancers with BRAF mutation are preferentially sensitive to MEK inhibitors, whereas tumors with other MEK-ERK effector pathway gene mutations have variable responses, either because they are only partially dependent on ERK and/or because feedback responses elicit partial refractoriness to MEK inhibition.
Thyroid cancer cell lines with BRAF mutations are consistently sensitive to growth inhibition by MEK inhibitors whereas cell lines with RAS or RET mutations have variable responses.
Papillary thyroid carcinomas (PTC) are the most frequent type of thyroid malignancy. These tumors are associated with characteristic genetic alterations, which are believed to be involved in tumor initiation. These include rearrangements of the tyrosine kinase receptor oncogenes RET (1) or NTRK1(2), leading to illegitimate expression of the chimeric proteins RET/PTC (of which there are multiple variants) or TRK, respectively, and constitutive activation of their tyrosine kinase activities. Activating RAS mutations, particularly NRAS and HRAS, are seen in follicular thyroid carcinoma as well as follicular variant of PTC (3,4). Activating mutations of BRAF are the most common genetic alterations in PTC (5,6,7). Moreover, BRAF mutations are also found in poorly differentiated or anaplastic thyroid carcinoma (8). The BRAF mutation is almost exclusively a thymine-to-adenine transversion at position 1799, leading to a valine-to-glutamate substitution at residue 600 (V600E) (9). Altogether, approximately 70% of PTCs harbor a mutation in either RET, NTRK1, NRAS, HRAS, or BRAF. These mutations are nonoverlapping, suggesting that no selective advantage is derived by acquiring more than one of these abnormalities during tumor development (5,7,10). All these oncoproteins have the common property of activating MAPK kinase (MEK) and ERK pathway, leading to the hypothesis that inappropriate signaling through this pathway is critical to tumor initiation and transformation, and presumably for tumor maintenance.
The premise that MEK-ERK activity is required for viability of cancer cells in which the pathway is activated by upstream receptor mutations has been tested in thyroid cells. RET/PTC-induced activation of MEK-ERK in rat thyroid cells is blocked by small interfering RNA-mediated knockdown of BRAF but not CRAF (11). Knockdown of BRAF or pharmacological MEK inhibition disrupts RET-induced expression of a large set of genes, including functional clusters likely required for cell cycle progression and tumor invasiveness (12,13). This in turn suggests that RAF proteins, and in particular BRAF, may represent legitimate therapeutic targets for patients with papillary thyroid cancer. Accordingly, treatment of human thyroid cancer cell lines with the small-molecule RAF kinase inhibitors AAL881 or LBT617, isoquinolines with submicromolar IC50 activity on wild-type RAF proteins and mutant BRAF, was effective at inhibiting growth of human thyroid cancer cells with endogenous RET/PTC or BRAF mutations (14). Although these compounds inhibit RAF, they also have inhibitory activity on other kinases (i.e. abl and kinase insert domain-containing receptor), and it is therefore not possible, based on this study alone, to conclude that RAF is a valid therapeutic target for thyroid cancers, regardless of the oncogenic event responsible for activating MEK-ERK (14,15).
Recently Solit et al. (16) demonstrated that BRAF mutation predicted sensitivity to MEK inhibition in a panel of human cancer cell lines of different lineages. They showed that pharmacological MEK inhibition potently impaired tumor growth in BRAF mutant xenografts, whereas RAS mutant tumors were only partially inhibited. This study used the NCI60 cell line panel, which does not include thyroid cancer cell lines. After this observation and while this study was in progress, two groups examined the effect of MEK inhibition on a small panel of human thyroid cancer cell lines (17,18) and found that sensitivity to MEK inhibitors was confined to cells with BRAF mutation. It remains unclear whether these findings can be generalized and whether thyroid cancer cell lines with RAS mutations in particular are insensitive to MEK inhibition. Here we examined these questions in a larger panel of thyroid cancer cell lines to determine the genetic determinants of MEK dependency for growth, using two highly selective MEK inhibitors.
Materials and Methods
Cell lines
The human thyroid carcinoma cell lines NPA, ARO, 8505c, WRO, C643, Cal62, Hth74, Hth83, and Kat 18 were maintained in RPMI 1640 supplemented with 10% fetal calf serum. The human papillary thyroid cancer cell line TPC-1 was maintained in DMEM with 10% fetal calf serum. The human thyroid carcinoma cell lines ACT1, TTA1, and OCUT1 were maintained in DMEM supplemented with 5% fetal calf serum. All experiments were performed with the cells grown in their respective media unless specified otherwise. The cell lines were genotyped by Sequenom mass spectrometry for mutations of BRAF, all RAS genes, and for the 31 most common PIK3CA mutations. Presence of RET/PTC rearrangements was examined by RT-PCR as described (19). The entire coding region of PTEN was sequenced in the following cell lines: NPA, ARO, 8505c, WRO, OCUT-1, ACT1, and TPC-1. No mutations were found.
Reagents
The MEK inhibitors PD0325901 and AZD6244 (ARRY-142886) are allosteric ATP noncompetitive inhibitors of MEK and were provided by Judith Leopold (Pfizer, Groton, CT) and Paul Smith (AstraZeneca, Cheshire, UK), respectively. The IC50 of PD0325901 for isolated MEK is 1 nm. The IC50 of AZD6244 is 12 nm against isolated MEK, and inhibits ERK phosphorylation in a range of cultured tumor cells with an IC50 of approximately 10 nm. We performed additional experiments using AZD6244 because while this study was underway, clinical development of PD0325901 was discontinued, making it desirable to explore the action of a compound with a similar mechanism of action that was still in clinical trials. The following antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA): ERK1 rabbit polyclonal (sc-94), pERK mouse monoclonal (sc-7383). The Rb (4H1) mouse monoclonal antibody (no. 9309), the pMEK1/2 rabbit polyclonal antibody (no. 9121), and the pRb (ser780) rabbit polyclonal antibody (no. 9307) were from Cell Signaling Technology (Beverly, MA). The antiphosphatidylinositol 3-kinase (PI3K; p85) rabbit polyclonal antibody (no. 06–195) was from Upstate Cell Signaling Solutions (Charlottesville, VA). Fetal bovine serum and penicillin-streptomycin-l-glutamine were purchased from Life Technologies, Inc. (Gaithersburg, MD). Propidium iodide (PI) was purchased from Sigma (St. Louis, MO).
Growth curves
Cells were plated in triplicate into 6-well plates at 6 × 104 cells/well, and treated with or without the indicated concentrations of PD0325901 or AZD6244, with media changes every 2 d. Cells were collected by trypsinization and counted in a Vi-Cell series cell viability analyzer (Beckman Coulter, Inc., Fullerton, CA).
Cell cycle analyses
Cal62, WRO, OCUT1, ARO, and NPA were plated in triplicate into 60-ml dishes at 2 × 105 cells/well. The following day cells were incubated with fresh medium with or without 10 nm PD0325901. Cells were collected at 24 and 48 h and fixed in 70% ethanol at −20 C overnight. Fixed cells were centrifuged and washed once with PBS. Five hundred microliters of DNA staining solution (distilled water, Triton X-100, and sodium citrate) were added followed by PI staining solution (final concentration of 30 μg/ml) and ribonuclease A (final concentration of 20 μg/ml) and the proportion of cells in S, G2/M, and G1/G0 determined by fluorescence-activated cell sorting analysis using a Cell Lab Quanta flow cytometer (Beckman Coulter), at an excitation range of 488 nm (argon laser) and 620 BP for PI.
Western blotting
Cells were harvested by trypsinization, washed once with cold PBS, and lysed in a buffer containing 20 mm Tris (pH 7.4), 135 mmol/liter NaCl, 2 mmol/liter EDTA, 1% Triton X-100, 25 mmol/liter β-glycerophosphate, 1 mmol/liter sodium orthovanadate, 1 mmol/liter sodium fluoride, 1 mmol/liter phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin, 10 μg/ml aprotinin, and 10 μg/ml EA64 for 20 min. Lysates were repeatedly passed through a G27 needle, centrifuged, and protein concentration determined using the Micro BCA kit (Pierce, Rockford, IL). For xenografted tumors, about 50–100 mg fresh frozen tissue were homogenized in lysis buffer in a power homogenizer (PT3000; Polytron, Duluth, GA). The tissue lysates were centrifuged and supernatants collected. Western blots were performed on 35 μg protein separated by SDS-10% PAGE using the indicated antibodies, except for Rb and pRb where the Western blots were performed on 10 μg protein samples separated by SDS-7.5% PAGE.
Tumor xenografts
Female nudeν/ν athymic mice (Charles River Laboratory, Inc., Wilmington, MA) of approximately 4–6 wk of age were injected sc in the right flank with either 107 NPA or 107 Cal62 cells suspended in 20% fetal bovine serum. Treatment was initiated when tumor volume approached about 500 mm3 as estimated by measuring length and width with calipers (width2 × length/0.52). Tumor-bearing mice were randomized into two groups consisting of a control group (vehicle only) and a treatment group. Mice were weighed at the start of the treatment and every 3 d during the course of therapy. AZD6244 was dissolved in a mixture of 0.5% hydroxypropylmethyl cellulose (Sigma-Aldrich) and 0.1% polysorbate (Tween-80, Sigma-Aldrich) to a concentration of 20 mg/ml. Treatments were administered by oral gavage in a volume of approximately 100 μl using a sterile animal feeding needle. A dose of AZD6244 was 100 mg/kg twice daily. Animals were killed by CO2 anesthesia 4 h after the last dose of AZD6244. Tumors were measured every 3 d with calipers. At the time the animals were killed, the tumors were dissected free of vessels, fibrous tissue, and surrounding dermis. Tumors were then weighed, cut longitudinally to provide a representative fragment for immunohistochemistry, and the remainder flash frozen in liquid N2 for subsequent protein or RNA isolation. All animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center.
Statistical analysis
All data are presented as the mean ± sd. Statistical significance of differences observed in tumor volumes of treated and untreated animals was determined using the Mann-Whitney test. P < 0.05 was considered statistically significant. All analyses were performed using SPSS software for Windows version 14 (SPSS, Inc., Chicago, IL).
Results
The genotype of the lines used in this study is shown in Table 1. Of 13 thyroid cancer cell lines, four had BRAF mutation, four had RAS mutations (one NRAS, two HRAS, and one KRAS), one had a RET/PTC1 rearrangement, and the remaining four were wild type for all genes tested (Table 1). Besides harboring a heterozygous BRAFT1799A substitution, the OCUT1 line was homozygous for the activating PIK3CAH1047R mutation.
Table 1.
Genotype of thyroid cancer cell lines used in this study
| Cell line | BRAFV600E | NRASQ61K | HRASG12A/Q61R | KRASG12R | PIK3CAH1047R | RET/PTC1 |
|---|---|---|---|---|---|---|
| WRO | wt | wt | wt | wt | wt | wt |
| 8505c | +/+ | wt | wt | wt | wt | wt |
| ACT1 | wt | +/− | wt | wt | wt | wt |
| ARO | +/− | wt | wt | wt | wt | wt |
| C643 | wt | wt | G12A+/− | wt | wt | wt |
| Cal62 | wt | wt | wt | +/+ | wt | wt |
| Hth-74 | wt | wt | wt | wt | wt | wt |
| Hth-83 | wt | wt | Q61R+/− | wt | wt | wt |
| Kat18 | wt | wt | wt | wt | wt | wt |
| NPA | +/+ | wt | wt | wt | wt | wt |
| OCUT-1 | +/− | wt | wt | wt | +/+ | wt |
| TPC1 | wt | wt | wt | wt | wt | yes |
| TTA1 | wt | wt | wt | wt | wt | wt |
Preferential inhibition of growth of BRAF (+) cell lines by PD0325901 and AZD6244
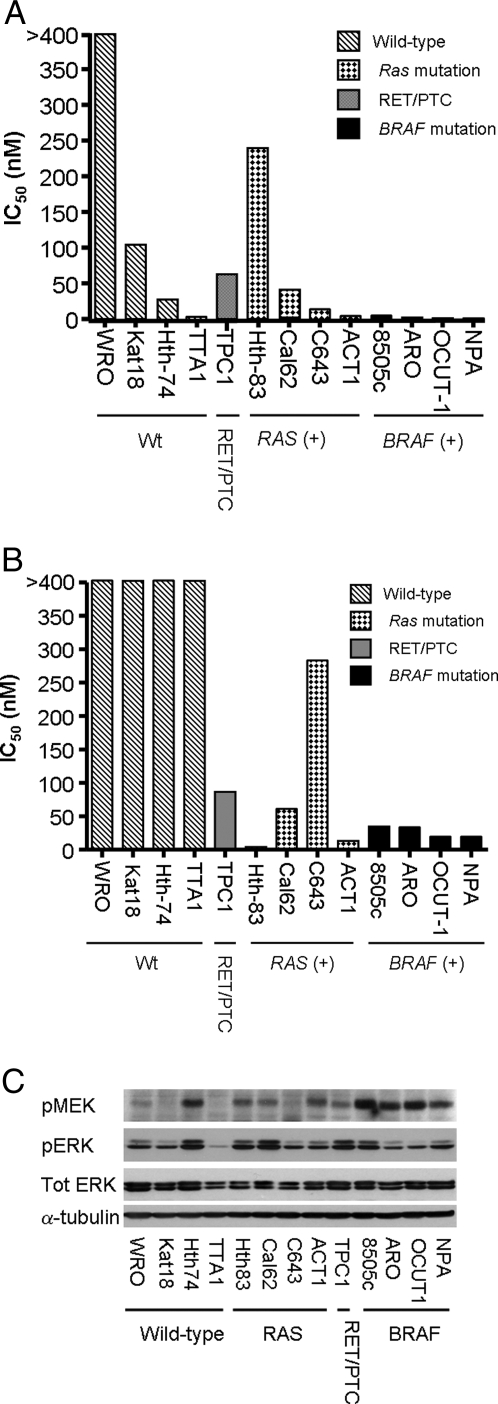
The effects of the MEK inhibitor PD0325901 and AZD6244 on growth of the 13 cell lines was determined by incubating them with a range of concentrations over a 6-d period. The four cell lines harboring a BRAF mutation (8505c, ARO, OCUT-1, and NPA) had IC50 less than 5 nm, whereas cells with RAS or RET/PTC mutations had more variable responses, with IC50 ranging from 5 to 250 nm, and WT cells had IC50 of 3–1000 nm when treated with PD0325901 (Fig. 1A). We also determined the IC50 of a different allosteric noncompetitive inhibitor of MEK, AZD6244, on the same panel of lines. As shown in Fig. 1B, all cell lines with BRAF mutation were highly sensitive to the compound, and the overall spectrum of activity closely resembled that of PD0325901. Some cell lines, such as Hth83, TTA1, and C643, exhibit slightly different responses. It is possible that these compounds may be metabolized differently, which may account for some of these changes. As shown in Fig. 1C, the basal levels of activation of MEK in serum-free conditions was highest in cell lines with BRAF mutation, whereas RAS and RET/PTC mutant lines tended to have an intermediate level of pMEK abundance. Overall, pMEK did not predict sensitivity to MEK inhibition. For example, TTA1 cells are very sensitive to MEK inhibition yet had undetectable pMEK, whereas Hth74 cells had high pMEK and were refractory to PD0325901. Basal pERK levels were highly variable, possible reflecting the fact that the MEK-ERK pathway is subject to multiple feedback regulatory controls.
Figure 1.
A, Growth-inhibitory effects of PD0325901 on human thyroid cancer cell lines. The indicated thyroid cancer cell lines were treated for 6 d with PD0325901 (0.2–1000 nm), and the IC50 determined by nonlinear regression using GraphPad Prism version 4 (GraphPad Software Inc., San Diego, CA). B, Growth-inhibitory effects of AZD6244. The indicated cell lines were assessed for response to the MEK inhibitor AZD6244 as described in A, with concentrations ranging from 1 to 5000 nm. C, Basal level of pMEK and pERK in human thyroid cancer cell lines. Western blot of lysates of the indicated thyroid cancer cell lines extracted 72 h after incubation in serum-free media with 0.1% BSA. Membranes were incubated with the indicated antibodies.
PD0325901 induces a block in G1 in BRAF (+) human thyroid cancer cells
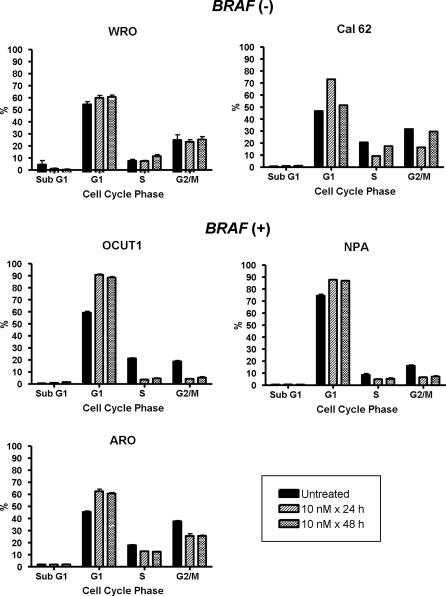
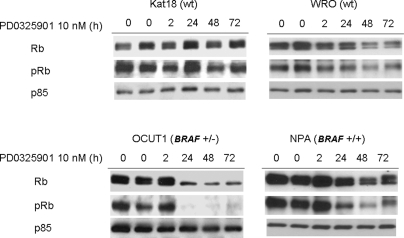
Treatment of BRAF (+) cell lines (NPA, OCUT1, and ARO) with PD0325901 resulted in a cell cycle block in G1 that was sustained through 48 h. There was no detectable sub-G1 peak, indicative of no induction of apoptosis. By contrast, the compound had no effect on cell cycle progression of WRO cells, which are wild type for these thyroid oncogenes. Cal62 cells, which harbor a homozygous KRAS mutation, exhibited a transient delay in G1/S at 24 h, with a subsequent escape and progression to S and G2/M after 48 h (Fig. 2). Treatment of the BRAF (+) cell lines NPA and OCUT1 with PD0325901 was associated with Rb hypophosphorylation, which was also accompanied by lower levels of total Rb. By contrast, Rb was largely unchanged or only slightly diminished in the BRAF (−) lines Kat 18 and WRO, respectively (Fig. 3).
Figure 2.
Effect of PD0325901 on cell cycle progression of thyroid cancer cell lines with or without BRAF mutation. Fluorescence-activated cell sorting analysis of the indicated cell lines 24 and 48 h after treatment with 10 nm PD0325901. Bars indicate the percent of cells at the indicated stage of the cell cycle.
Figure 3.
Effect of PD0325901 on Rb phosphorylation in thyroid cancer cell lines with or without BRAF mutation. Western blots of cell lysates harvested from the indicated cell lines at various time points after addition of 10 nm PD0325901. Membranes were incubated with the indicated antibodies. Hybridization with p85 served as a loading control.
Effect of PD0325901 on ERK phosphorylation in human thyroid cancer cell lines
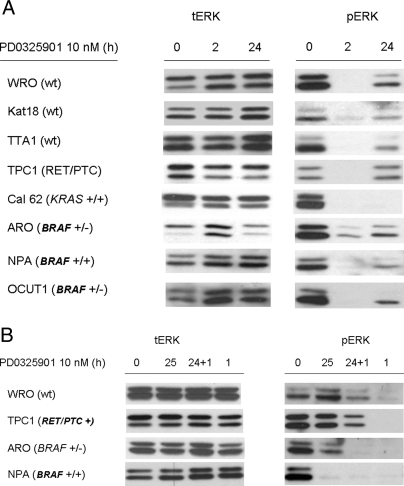
pERK levels were markedly inhibited in all cell lines tested 2 h after treatment with PD0325901, regardless of genotype (Fig. 4A). There was a rebound in pERK after 24 h, which was more pronounced in cells that were comparatively less sensitive to the growth-inhibitory effects of the compound (i.e. WRO, Kat18, and TPC1), although there was also some recovery of ERK phosphorylation in ARO and TTA1 cells, which were exquisitely sensitive to MEK inhibitors (Fig. 4A). This property is distinct from effects of MEK inhibitors on other cancer cell lines, such as melanomas, in which PD0325901 induces a sustained inhibition of ERK phosphorylation through at least 72 h (not shown). We explored whether the rebound in ERK phosphorylation was associated with refractoriness to a rechallenge with MEK inhibitor. For this we examined three cell lines showing the greatest increase in pERK 24 h after exposure to PD0325901, two of which were relatively resistant to the growth-inhibitory effects of the compound (WRO and TPC1) and one that was highly sensitive (ARO). In addition, we also tested NPA cells, which had no significant rebound in pERK. Cells were treated with PD0325901 for 24 h, and then refed with fresh compound for 1 h before analysis (Fig. 4B). TPC1 and WRO cells showed a near complete recovery of pERK after 25 h, and retreatment for 1 h failed to completely inhibit ERK phosphorylation, compared with the acute response seen after initial exposure to the compound. By contrast, pERK levels in ARO cells, which also recovered partially at 25 h, became undetectable after a rechallenge with the compound. NPA cells did not show any rebound in pERK after 25 h of exposure to the MEK inhibitor.
Figure 4.
A, Time course of ERK phosphorylation after treatment of thyroid cancer cell lines with the MEK inhibitor PD0325901. Cells were harvested at the indicated times after treatment with 10 nm PD0325901 and lysates Western blotted with antibody to total (left panel) or phosphorylated ERK (right panel). B, ERK phosphorylation rebound after 24 h of treatment with MEK inhibitor. Cells were harvested at the indicated times after treatment with 10 nm PD0325901 for 25 h by changing the media and the drug was readded or added for the first time 1 h before harvesting the cells. Lysates were Western blotted with antibody to total (left panel) or phosphorylated ERK (right panel).
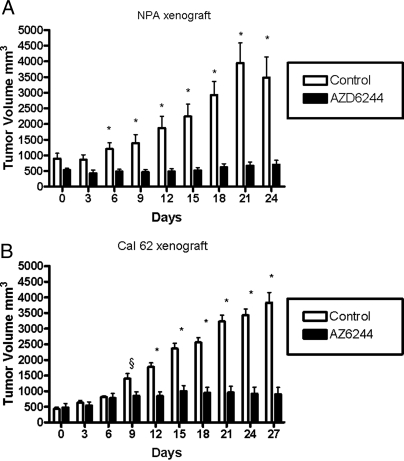
Effect of AZD6244 on NPA and Cal62 tumor xenografts
We compared the effects of MEK inhibition on xenografts of cells with high (NPA, BRAF+/+) and intermediate (Cal62, KRAS+/+) sensitivity to MEK inhibitors in vitro. It was not possible to examine the in vivo effects of AZD6244 on the more refractory lines (i.e. WRO, Kat18) because they did not grow as xenografts. NPA tumor xenografts grew by about 4-fold in vehicle-treated mice after 24 d. Growth of NPA was completely inhibited by AZD6244 (Fig. 5A). Growth suppression was associated with a decline in proliferation index in AZD6244, compared with vehicle-treated mice (8.8 ± 3.4 vs. 20.3 ± 5.6%, respectively; P = 0.02). Cal62 (KRAS+/+) tumor xenografts grew by about 7-fold in the vehicle-treated mice after 24 d, and growth was blunted but not completely prevented by AZD6244 (Fig. 5B). Although the proliferation index was also lower in Cal62 xenografts in AZD6244-treated mice, compared with vehicle controls, the difference was not statistically significant (40 ± 11.5 vs. 20.3 ± 18.3%, vehicle vs. AZD6244, respectively; P = 0.2). There was an overall decrease in the level of pERK staining by immunohistochemistry in drug- vs. vehicle-treated controls in both tumor xenografts (data not shown).
Figure 5.
Effect of treatment with AZD6244 on growth of thyroid cancer cell line xenografts in nude mice. A, NPA cells were implanted into the flanks of athymic ν/ν mice, and allowed to reach a volume of about 500 mm3. Mice (n = 9 per group) were then treated with AZD6244 (100 mg/kg, twice a day) by gavage or vehicle. Data represent the mean ± sd of tumor volumes as measured with calipers in the two groups. *, P < 0.02 AZD6244 vs. vehicle-treated mice. At the time the animals were killed, tumor weights were 0.48 ± 0.25 vs. 0.09 ± 0.05, in AZD6244, respectively (P= 0.04). B, Cal62 xenografts were allowed to grow to about 500 mm3 before treatment of mice (n = 4 per group) with vehicle or AZD6244 (100 mg/kg, twice a day) as described in Fig. 6A. Data represent the mean ± sd of tumor volumes as measured with calipers in the two groups. §, P = 0.04, *, P < 0.02 AZD6244 vs. vehicle-treated mice. At the time the animals were killed, tumor weight was 0.4 ± 0.09 vs. 0.09 ± 0.02 g, control vs. treatment, respectively (P = 0.02).
Discussion
Activating mutations of RET/PTC, RAS, and BRAF are highly prevalent and do not overlap in papillary thyroid cancer specimens. RET/PTC and BRAF are involved in the early stages of thyroid tumorigenesis. The fact that they are mutually exclusive suggests that they share a common mechanism for transformation, thus implicating unregulated MEK-ERK signaling in thyroid cancer development (5,7,10). Alternatively, if any of these oncoproteins is activated by mutation, there may be no selective advantage for additional effector mutations in the pathway, which does not necessarily imply that ERK is the key driver of the process. A key question is whether thyroid cancers require continued MEK-ERK activity for their viability and whether tumor genotype determines this requirement. This information is of great consequence because MEK inhibitors are presently in clinical trials for several malignancies, including thyroid cancers. Solit et al. (16) reported that exquisite MEK dependency is a uniform property of cells with BRAF mutation, independent of cell lineage. Their data were particularly enriched with melanoma lines, and no thyroid cancer cells were studied. While this study was in progress, two reports showed that the MEK inhibitors AZD6244 (17) and CI-1040 (18) preferentially inhibited thyroid cancer cell lines with BRAF mutation. The data shown here reaffirm these observations, using a larger panel of lines that were verified to be genetically distinct from each other.
The predictive value of preclinical studies performed in human cancer cell lines is limited, in part because their growth requirements may change during adaptation to in vitro conditions. Findings are more likely to be generalizable if they are corroborated on multiple, independently derived lines, which was one of the objectives of this study. To this end we performed genomic fingerprinting on 28 human thyroid cancer cell lines obtained from different primary sources and found that many of them were not unique. The problem of cell line cross-contamination is well recognized in the literature. Estimates of the fraction of research papers whose conclusions may be compromised by the use of misidentified and cross-contaminated cell cultures approximate 15–20% (20). Based on our analysis, it is likely that most publications exploring the role of kinase inhibitors in thyroid cancer suffer from this problem. All but two of the 13 lines used in this study (WRO and Kat18) were examined by single-nucleotide polymorphism-comparative genomic hybridization or other single-nucleotide polymorphism-comparative genomic hybridization (SNP-CGH)-based genotyping approaches and verified to be distinct from each other.
Liu et al. (18) tested the response of a single cell line (C643) with a RAS mutation to the MEK inhibitor CI-1040 and found an IC50 comparable with that of cell lines with BRAF mutation. The four thyroid cancer cell lines with RAS mutations we examined varied markedly in their sensitivity to MEK inhibitors, consistent with the findings on the NCI60 lines harboring RAS mutations (16). None of the thyroid lines with RAS mutation had coexisting point mutations of PIK3CA or AKT1 that could account for a primary dependence on this alternative pathway for growth. However, it is clear from recent cancer genome resequencing studies of colorectal and breast cancers that there are numerous other somatic mutations of genes encoding effectors that signal via PI3K (21). The basal level of pMEK in serum-free conditions was not predictive of the response of cell lines to MEK inhibitors.
Treatment of thyroid cell lines with BRAF mutations with MEK inhibitors was associated with Rb hypophosphorylation and impairment of progression into S and G2/M. However, there was no accumulation of cells in a sub-G1 fraction, indicating no induction of apoptosis. Accordingly, the growth of NPA xenografts was completely inhibited by AZD6244, but there was no tumor regression or apoptosis. These data are consistent with the mode of action of MEK inhibitors in most melanoma cell lines with BRAF mutations, and on the effects of the pan-RAF inhibitors AAL881 and LBT613 in thyroid cancer cells (14). PD0325901 only induced a transient delay in G1 in Cal62 cells, which harbor a homozygous KRAS mutation, and have intermediate sensitivity to the growth-inhibitory effects of the compound. This is consistent with a partial dependence on MEK signaling for growth, which was also apparent in the xenograft experiment. By contrast to these observations in cell lines and xenografts, pharmacological inhibition of MEK blocked lung tumor growth in mice with doxycycline-inducible expression of oncogenic BRAF or KRAS in alveolar epithelial cells (22). Moreover, CI-1040 induced apoptosis in these tumors, indicating that in this context MEK activity is required for BRAF or RAS-induced tumor cell viability in transgenic mice.
MEK inhibitors potently abrogated ERK phosphorylation at early time points in all cell lines tested. However, there was a significant recovery of pERK in most lines, irrespective of genotype or sensitivity to MEK inhibitors. This is quite distinct from melanoma cells with BRAF mutation, in which the inhibitory effects of PD0325901 on pERK are sustained. Readdition of the MEK inhibitor after 24 h in BRAF (+) ARO cells fully inhibited ERK phosphorylation, suggesting that the rebound might be attributable to efflux or rapid metabolism of the drug. The ATP-cassette binding transporters ABCB1 (Mdr-1; P-glycoprotein) and/or ABCG2 are expressed in thyroid cancer tissues and ARO cells (23), are known to interact with kinase inhibitors, and can mediate resistance to their action (24). Moreover, the Raf-MEK-ERK pathway has been implicated in the regulation of Mdr-1 (25). By contrast, readdition of compound to WRO and TPC1 cells did not inhibit ERK phosphorylation to the same degree observed after initial exposure. The Ras/Raf/MEK/ERK pathway is subject to an extensive and complex set of feedback regulatory controls (reviewed in Ref. 26). Activated ERK phosphorylates Raf1 at multiple sites and appears to mediate its inactivation after mitogen stimulation (27), although the precise role of individual phosphorylation sites is controversial (28). ERK also phosphorylates BRaf at T753, which destabilizes Raf1-BRaf heterodimers (29). In addition to restoration of the stimulatory input into MEK, inhibition of Raf-MEK-ERK signaling results in a potent and durable down-regulation of expression of the dual-specificity MAPK phosphatases DUSP5 and DUSP6, which could also contribute to partial restoration of ERK activity (14). Constitutive activation of the PI3K pathway could also result in resistance to MEK-ERK pathway inhibitors at several levels. Thus, p21-activated kinase 1, whose activity is controlled in part by PI3K, phosphorylates and activates Raf1 as well as MEK (30). Moreover, the PI3K pathway plays a role in regulation of DUSP6 gene expression (31). Given the complexity of these interactions, we have not yet determined their possible role in the development of partial refractoriness of ERK to MEK inhibition in some of the cell lines with resistance to the growth-inhibitory effects of the compound. However, there is a sound conceptual basis that establishes the importance of duration of ERK activity on cell proliferation (32), emphasizing the potential significance of this issue in determining the efficacy of therapies targeting this pathway.
Thus, the BRAFV600E mutation predicts sensitivity to MEK inhibition in thyroid cancer cell lines. Some thyroid cell lines with RAS mutations are also highly sensitive, yet the factors that determine dependence on MEK activity are not understood. These findings provide rationale for testing MEK inhibitors in patients with radioiodine refractory and poorly differentiated thyroid cancers, particularly those with BRAF or RAS mutations. Clinical trials with these or similar compounds, presently ongoing, should determine whether tumor genotype will serve as a predictor of response to therapy. It is critical that potential mechanisms of failure to respond to MEK inhibitors in clinical trials be rigorously explored because the cumulative information derived from human thyroid cancer genetics, mouse models, and human cell lines strongly implicates this pathway in tumor initiation and maintenance.
Note Added in Proof
After this paper was accepted for publication, the original derivation of NPA and ARO cells has been questioned, with indications that they may not be of thyroid origin.
Acknowledgments
We thank Ronald A. Ghossein for his help in the interpretation of the IHC stains of xenograft tumors and Julio Ricarte Filho for his help with genotyping cell lines.
Footnotes
This work was supported by National Institutes of Health Grant CA72597, a Byrne award from Memorial Sloan-Kettering Cancer Center, and the Marcus and Ann Rosenberg Foundation.
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 1, 2008
Abbreviations: MEK, MAPK kinase; PI, propidium iodide; PI3K, phosphatidylinositol 3-kinase; PTC, papillary thyroid carcinoma.
References
- Santoro M, Melillo RM, Carlomagno F, Fusco A, Vecchio G 2002 Molecular mechanisms of RET activation in human cancer. Ann NY Acad Sci 963:116–121 [DOI] [PubMed] [Google Scholar]
- Greco A, Pierotti MA, Bongarzone I, Pagliardini S, Lanzi C, Della Porta G 1992 TRK-T1 is a novel oncogene formed by the fusion of TPR and TRK genes in human papillary thyroid carcinomas. Oncogene 7:237–242 [PubMed] [Google Scholar]
- Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE 1999 Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol (Oxf) 50:529–535 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE 2003 Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 120:71–77 [DOI] [PubMed] [Google Scholar]
- Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA 2003 High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454–1457 [PubMed] [Google Scholar]
- Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D 2003 BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 95:625–627 [DOI] [PubMed] [Google Scholar]
- Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, Maximo V, Botelho T, Seruca R, Sobrinho-Simoes M 2003 BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 22:4578–4580 [DOI] [PubMed] [Google Scholar]
- Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE 2003 BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88:5399–5404 [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA 2002 Mutations of the BRAF gene in human cancer. Nature 417:949–954 [DOI] [PubMed] [Google Scholar]
- Frattini M, Ferrario C, Bressan P, Balestra D, De Cecco L, Mondellini P, Bongarzone I, Collini P, Gariboldi M, Pilotti S, Pierotti MA, Greco A 2004 Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene 23:7436–7440 [DOI] [PubMed] [Google Scholar]
- Mitsutake N, Miyagishi M, Mitsutake S, Akeno N, Mesa Jr C, Knauf JA, Zhang L, Taira K, Fagin JA 2006 BRAF mediates RET/PTC-induced mitogen-activated protein kinase activation in thyroid cells: functional support for requirement of the RET/PTC-RAS-BRAF pathway in papillary thyroid carcinogenesis. Endocrinology 147:1014–1019 [DOI] [PubMed] [Google Scholar]
- Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R, Kruhoffer M, Orntoft T, Fusco A, Santoro M 2005 The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest 115:1068–1081 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mesa Jr C, Mirza M, Mitsutake N, Sartor M, Medvedovic M, Tomlinson C, Knauf JA, Weber GF, Fagin JA 2006 Conditional activation of RET/PTC3 and BRAFV600E in thyroid cells is associated with gene expression profiles that predict a preferential role of BRAF in extracellular matrix remodeling. Cancer Res 66:6521–6529 [DOI] [PubMed] [Google Scholar]
- Ouyang B, Knauf JA, Smith EP, Zhang L, Ramsey T, Yusuff N, Batt D, Fagin JA 2006 Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clin Cancer Res 12:1785–1793 [DOI] [PubMed] [Google Scholar]
- Chiloeches A, Marais R 2006 Is BRAF the Achilles’ heel of thyroid cancer? Clin Cancer Res 12:1661–1664 [DOI] [PubMed] [Google Scholar]
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N 2006 BRAF mutation predicts sensitivity to MEK inhibition. Nature 439:358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball DW, Jin N, Rosen DM, Dackiw A, Sidransky D, Xing M, Nelkin BD 2007 Selective growth inhibition in BRAF mutant thyroid cancer by the MEK 1/2 inhibitor AZD6244 (ARRY-142886). J Clin Endocrinol Metab 92:4712–4718 [DOI] [PubMed] [Google Scholar]
- Liu D, Liu Z, Jiang D, Dackiw AP, Xing M 2007 Inhibitory effects of the MEK inhibitor CI-1040 on the proliferation and tumor growth of thyroid cancer cells with BRAF or RAS mutations. J Clin Endocrinol Metab 92:4686–4695 [DOI] [PubMed] [Google Scholar]
- Nikiforov YE, Rowland JM, Bove KE, Monforte-Munoz H, Fagin JA 1997 Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res 57:1690–1694 [PubMed] [Google Scholar]
- Nardone RM 2007 Eradication of cross-contaminated cell lines: a call for action. Cell Biol Toxicol 23:367–372 [DOI] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B 2007 The genomic landscapes of human breast and colorectal cancers. Science 318:1108–1113 [DOI] [PubMed] [Google Scholar]
- Ji H, Wang Z, Perera SA, Li D, Liang MC, Zaghlul S, McNamara K, Chen L, Albert M, Sun Y, Al-Hashem R, Chirieac LR, Padera R, Bronson RT, Thomas RK, Garraway LA, Janne PA, Johnson BE, Chin L, Wong KK 2007 Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res 67:4933–4939 [DOI] [PubMed] [Google Scholar]
- Lopez JP, Wang-Rodriguez J, Chang C, Chen JS, Pardo FS, Aguilera J, Ongkeko WM 2007 Gefitinib inhibition of drug resistance to doxorubicin by inactivating ABCG2 in thyroid cancer cell lines. Arch Otolaryngol Head Neck Surg 133:1022–1027 [DOI] [PubMed] [Google Scholar]
- Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A, Boudriot U, Neubauer A 2007 Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia 21:1267–1275 [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M 2006 Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 46:249–279 [DOI] [PubMed] [Google Scholar]
- Dhillon AS, von Kriegsheim A, Grindlay J, Kolch W 2007 Phosphatase and feedback regulation of Raf-1 signaling. Cell Cycle 6:3–7 [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrads TP, Veenstra TD, Lu KP, Morrison DK 2005 Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell 17:215–224 [DOI] [PubMed] [Google Scholar]
- Balan V, Leicht DT, Zhu J, Balan K, Kaplun A, Singh-Gupta V, Qin J, Ruan H, Comb MJ, Tzivion G 2006 Identification of novel in vivo Raf-1 phosphorylation sites mediating positive feedback Raf-1 regulation by extracellular signal-regulated kinase. Mol Biol Cell 17:1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth LK, Hindley AD, O'Neill E, Kolch W 2006 Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol 26:2262–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ 2006 p21-activated kinases in cancer. Nature Rev 6:459–471 [DOI] [PubMed] [Google Scholar]
- Owens DM, Keyse SM 2007 Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26:3203–3213 [DOI] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J 2002 Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol 4:556–564 [DOI] [PubMed] [Google Scholar]