Abstract
Context: Although the inner fetal zone (FZ) of the midgestation human fetal adrenal (HFA) produces dehydroepiandrosterone sulfate, the function of the outer definitive zone (DZ) remains less clear. We have proposed that the DZ phenotype is that of a pool of progenitor cells, many of which are mitotically active. Recently, we studied HFA expression of a family of vascular endothelial cell-specific angiogenic factors, the angiopoietins (Angs), and demonstrated that Ang2 was localized predominantly in the periphery of the gland. Ang1 stabilizes, whereas Ang2 destabilizes, vessels, increasing responsiveness to angiogenic stimuli such as vascular endothelial growth factor (VEGF)-A and fibroblast growth factor (FGF)-2.
Objective: Our objective was to test the hypothesis that the periphery of the HFA is a site of angiogenesis.
Design: Studies were conducted involving RNA, frozen sections, and primary cell cultures from midgestation HFAs.
Main Outcome Measures: Immunofluorescence, laser capture microdissection, and real-time quantitative RT-PCR were used.
Results: Double immunostaining demonstrated that proliferating endothelial cells were limited to the DZ and DZ/FZ border. Ang2 mRNA was primarily expressed in the DZ, whereas Ang1 mRNA was primarily in the FZ. VEGF-A and FGF-2 mRNA levels were higher in the DZ. FGF-2 (10 ng/ml) induced Ang2 mRNA by 4-fold in both zones of cells (P < 0.01, at 24 h), but not Ang1 or VEGF-A mRNA.
Conclusion: Data suggest that angiogenesis occurs at the periphery of the HFA. The DZ-predominant expression of Ang2 may be explained, in part, by the parallel pattern of FGF-2 expression.
This study suggests that angiogenesis occurs at the periphery of the human fetal adrenal gland, as high levels of Ang2, FGF-2, and VEGF-A were found in this region. Coordinated organ and vascular growth can be seen by the parallel expression of Ang2 in endothelial cells of the outer definitive zone and FGF-2 in the adrenal cortical cells.
The human fetal adrenal (HFA) gland plays an essential role in intrauterine homeostasis, fetal organ maturation, preparation for extrauterine life, and parturition in several species (1,2). The HFA is morphologically and functionally different from the adult adrenal. For most of gestation, the HFA has two morphologically recognizable zones: the outer, narrow definitive zone (DZ); and the inner, large fetal zone (FZ). The FZ produces large quantities of dehydroepiandrosterone and its sulfate, precursors of placental estrogen synthesis, whereas the DZ does not have the capacity to produce steroids before the third trimester. The DZ consists of small, tightly packed cells exhibiting ultrastructural characteristics typical of cellular proliferation (3). We confirmed this difference in proliferative activity between the DZ and FZ by immunostaining for proliferating cell nuclear antigen (4). Thus, we proposed that proliferating cells in the DZ migrate centripetally, differentiate, and finally undergo senescence in the central part of the HFA (1). After birth, the DZ likely differentiates into the zona glomerulosa, zona fasciculata, and zona reticularis, whereas the FZ regresses.
The HFA undergoes a phase of rapid growth in midgestation. Although the central drive for the HFA growth appears to be provided by ACTH, the growth-stimulatory actions of ACTH may be mediated, at least in part, by locally produced growth factors such as fibroblast growth factor (FGF)-2 (basic FGF) and IGF-II, acting in an autocrine and/or paracrine fashion (5,6,7). Angiogenesis, the process of formation of new capillaries from preexisting blood vessels, likely is essential for the rapid growth of the HFA. In addition, the HFA requires the development of an extensive vasculature for delivery of steroid hormone precursors to the gland and secretion of hormone products into the peripheral circulation. A variety of factors are implicated in the regulation of angiogenesis. We have studied expression and regulation of the vascular endothelial cell-specific angiogenic factors, vascular endothelial growth factor (VEGF)-A (8), angiopoietins (Angs) 1 and 2 (9) in the midgestation HFA. We showed that these factors are expressed in the HFA and that ACTH up-regulates them in isolated HFA cortical cells, suggesting that these factors may be key local regulators of HFA angiogenesis. Thus, they may mediate the tropic action of ACTH, exerting parallel control over the vasculature. Of particular note, ACTH induces an altered Ang balance in which Ang2 predominates over Ang1. Furthermore, Ang2 protein is predominantly localized in the periphery of the HFA (i.e. the DZ and an outer region of the FZ). Ang2 expression has been restricted to sites of vascular remodeling, and it has been proposed that Ang2 renders endothelial cells responsive to angiogenic stimuli, such as VEGF-A and FGF-2 (10,11,12,13). Viewed in this light, the outer zone-predominant Ang2 localization in the HFA may reflect the primary site of angiogenesis in the organ.
In this study we localized proliferating endothelial cells in the midgestation HFA, and investigated the zonal differential expression of Ang1, Ang2, VEGF-A, and FGF-2. In addition, we examined regulation of the vascular endothelial cell-specific angiogenic factors in isolated HFA cortical cells.
Subjects and Methods
Tissue preparation and reagents
HFA glands (15–24 wk gestation, n = 18) were obtained from women who opted for elective termination of pregnancy for social indications (i.e. no known fetal pathology) by dilatation and evacuation. Gestational age was estimated by foot length. The study protocol was approved by the Committee on Human Research, University of California, San Francisco. Adrenal glands were collected and placed in ice-cold medium for primary cell culture, in RNA Later (Ambion, Inc., Austin, TX) for RNA extraction, or in 4% paraformaldehyde in PBS for histological examinations, or snap frozen for laser capture microdissection (LCM) studies. Recombinant human FGF-2 was purchased from R&D Systems, Inc. (Minneapolis, MN). Recombinant human IGF-II was from Upstate Biotechnology, Inc. (Lake Placid, NY).
LCM
LCM was performed as described previously (14,15). Captured cells from the DZ or FZ were immediately processed for RNA extraction.
RNA isolation and real-time quantitative RT (qRT)-PCR
Total RNA from primary culture cells and cells captured by LCM was isolated and purified as described previously (9). RT reactions with random primers were performed with Omniscript reverse transcriptase (QIAGEN, Inc., Valencia, CA) under conditions described by the supplier. Expression of Ang1, Ang2, FGF-2, VEGF-A, and 17α -hydroxylase/17, 20 lyase (P450c17) were analyzed using real-time TaqMan RT-PCR as we have described previously (9,16,17). The levels of expression of each gene were normalized using β-glucuronidase (GUS) levels after the comparative threshold cycle method (9,16,18). Sequences for the PCR primers and TaqMan fluorogenic probe for FGF-2 were: forward primer, ACCCCGACGGCCGA; reverse primer, TCTTCTGCTTGAAGTTGTAGCTTGA; and TaqMan probe, FAM (6-carboxy-fluorescein)-TCCGGGAGAAGAGCGACCCTCACATAMRA (6-carboxytetramethyl-rhodamine) (Integrated DNA Technologies, Coralville, IA) (19).
Immunofluorescence studies
Frozen sections were prepared from HFAs, and processed for immunofluorescence and subsequent imaging analysis as previously described (15). Primary antibody incubation was performed for 1 h at room temperature with the following antibodies: a 1:20 dilution of rabbit antihuman VEGF-A polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); a 1:15 dilution of mouse antihuman FGF-2 monoclonal antibody (BD Biosciences, San Jose, CA); a combination of a 1:10 dilution of rabbit antihuman Ki67 antibody (Zymed Laboratories, Inc., South San Francisco, CA) and a 1:10 dilution of sheep antihuman CD31 polyclonal antibody (R&D Systems); a combination of a 1:10 dilution of the Ki67 antibody and a 1:10 dilution of mouse antihuman CD31 monoclonal antibody (Dako Corp., Carpinteria, CA); or a combination of the anti-Ki67 antibody and a 1:20 dilution of rhodamine-labeled ulex europaeus agglutinin (UEA) I (Vector Laboratories, Burlingame, CA). After washing, incubation with Cy3-conjugated goat antirabbit or antimouse antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was performed at room temperature for 30 min for VEGF-A or FGF-2 localization, respectively. For double-color immunohistochemical studies, Cy3- or FITC-conjugated, appropriate secondary antibodies (Jackson ImmunoResearch Laboratories) were used. After washing, slides were mounted with VectorShield mounting medium with or without 4, 6-diamidino-2-phenylindole (Vector Laboratories). Sections were visualized, and images were captured using a Nikon inverted fluorescence microscope (Nikon Corp., Tokyo, Japan) with MetaMorph software (Universal Imaging, Downingtown, PA). Confocal microscopy was performed using a Carl Zeiss (Jena, Germany) 510 META Laser Scanning Microscope. Negative controls were HFA sections incubated with unconjugated mouse IgG, or with rabbit or sheep serum. Background staining using these controls under the conditions described was minimal. The endothelium of vessels in the capsule of the HFA served as a positive control for CD31 and UEA I staining. The human fetal lung and kidney served as positive controls for VEGF-A and FGF-2 staining.
Calculating the proportion of Ki67-positive endothelial cells in all the Ki67-positive proliferating cells in the DZ was assessed in three sections per adrenal. The demonstration of a Ki67-positive endothelial cell depended on the identification of a Ki67-positive nucleus surrounded by a CD31-positive cell membrane.
Fetal adrenal cortical cell culture
The capsule with the adherent DZ was carefully peeled away from the HFA to separate the DZ from the FZ as described previously (20,21). Briefly, cells in the separated zones were dispersed by enzymatic digestion and plated on plastic culture dishes at a density of approximately 25,000 cells/cm2. Culture medium consisted of a 1:1 (vol/vol) mixture of DMEM H-16 and Ham’s F-12 with 10% fetal calf serum, 2 mm glutamine, and antibiotics. Cells were incubated at 37 C in a humidified environment consisting of 5% CO2 in air. After 48 h in culture, the medium was changed to one containing 2% fetal calf serum, and nonadherent cells were removed. At the initiation of experiments (usually 96 h after plating), the medium was renewed, and IGF-II (100 ng/ml) or FGF-2 (10 ng/ml) was added to the cells. These concentrations of IGF-II and FGF-2 were chosen because they represent the concentrations used in previous studies of human adrenal cortical cells and are effective in inducing various biological activities (6,21,22,23). All experiments were replicated on adrenal cortical cells obtained from at least three different fetuses.
Statistical analysis
Data are presented as means ± se, and were analyzed by ANOVA, followed by the Dunnett’s test for multiple comparisons. Differences were considered significant at P < 0.05.
Results
Zonal expression of Ang1, Ang2, VEGF-A, and FGF-2 mRNA
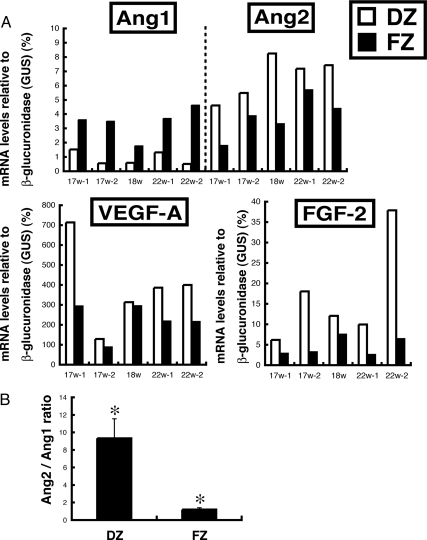
Zonal differential mRNA expression of Ang1, Ang2, VEGF-A, and FGF-2 was investigated by LCM and qRT-PCR. Ang2 mRNA was primarily expressed in the DZ, whereas Ang1 mRNA was primarily in the FZ (Fig. 1A). Because Ang1 and Ang2 can have opposing effects, the arbitrary ratio of Ang2 to Ang1 mRNA was calculated. The Ang2/Ang1 mRNA ratio was 9.3 ± 2.3 and 1.2 ± 0.2, in the DZ and FZ, respectively (P < 0.05) (Fig. 1B). Levels of VEGF-A and FGF-2 mRNA were 1.4- and 2.5-fold higher, respectively, in the DZ than in the FZ of the HFA at midgestation (Fig. 1A).
Figure 1.
A, Zonal expression of mRNAs encoding Ang1, Ang2, VEGF-A, and FGF-2. Outer DZ and inner FZ cells in the midgestation HFA (17–22 wk) were collected using LCM. Total RNA was extracted from cells of the respective zones and analyzed by qRT-PCR as described in Materials and Methods. GUS-normalized data are shown. Each bar represents the mean value of three determinations from a single fetal adrenal. Ang2, VEGF-A, and FGF-2 mRNA levels were higher in the DZ (white bars) than the FZ (black bars). B, The arbitrary ratio of Ang2/Ang1 mRNA levels in DZ and FZ cells. *, P < 0.05.
Proliferating endothelial cells
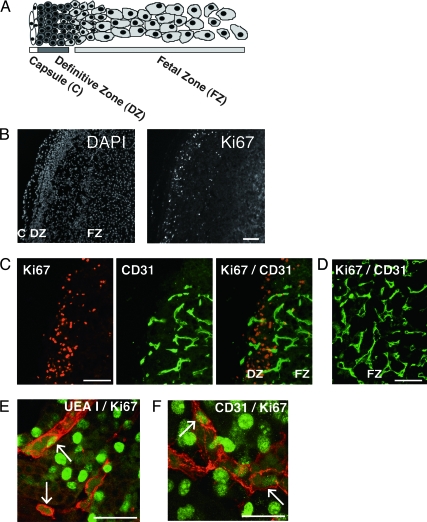
We evaluated zonal differential mitotic activity in the midgestation HFA by staining tissue sections with an antibody against the proliferation marker, Ki67. Ki67 immunoreaction was almost exclusively restricted to cell nuclei at the periphery of the HFA. Proliferating endothelial cells were detected by double immunofluorescence with Ki67 and the endothelial cell markers, CD31 or UEA I. Double immunostaining demonstrated that proliferating endothelial cells were limited to the DZ and DZ/FZ border (Fig. 2C) but that the endothelium located in the more central portion of the gland was quiescent (Fig. 2D). Similar results were obtained from HFAs studied (i.e. 17–24 wk). In the DZ, the mean percentage of proliferating endothelial cells was 4.7 ± 0.4% (n = 5) of total Ki67-positive cells. The percentage of proliferating endothelial cells does not appear to change over the age range studied (i.e. 17–24 wk).
Figure 2.
Proliferating endothelial cells in the HFA gland. A, Schematic illustration of the morphology of the HFA. B, Low-power views spanning the DZ and FZ of a 15-wk HFA are shown. Nuclear staining with 4, 6-diamidino-2-phenylindole (DAPI) or immunostaining with antibody against the proliferation marker, Ki67. Ki67-immunoreactive nuclei were almost exclusively restricted to cells at the periphery of the gland. C and D, Immunostaining of a 18-wk HFA gland with anti-Ki67 (red) and anti-CD31 (green) antibodies. Low-power views spanning the periphery of the gland (i.e. the outer DZ and DZ/FZ border; C) and the inner FZ (D). E and F, Confocal microscopy images illustrating labeling for Ki67, and UEA I (UEA I) or CD31 in the DZ. Green indicates Ki67-positive nuclei. Red indicates UEA I-positive (E) or CD31-positive cells (F). Note costaining for Ki67 in the nuclei, and UEA I or CD31 on the cell membrane, indicating proliferating endothelial cells (arrows). Images are from a 17-wk adrenal (E and F). Magnification bar, 100 μm (B, C, and D) and 50 μm (E and F). Original magnification, ×100 (B), ×200 (C and D), and ×630 (E and F).
FGF-2 and VEGF-A protein localization
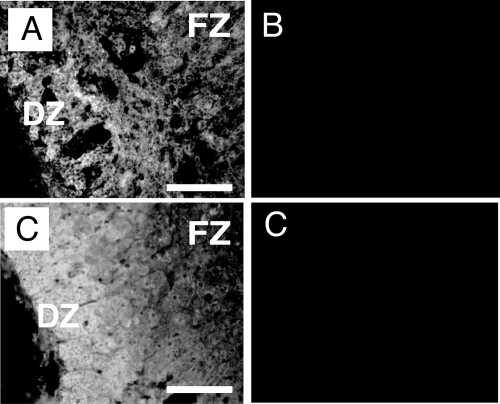
We further localized VEGF-A and FGF-2 proteins in the midgestation HFA. Immunoreactive VEGF-A and FGF-2 had a zonal expression pattern similar to those of VEGF-A and FGF-2 mRNAs (Fig. 3). Predominant staining for FGF-2 was evident in the periphery of the gland. Immunoreactive VEGF-A was detected throughout the gland. There was no apparent effect of gestational age on the pattern of staining for FGF-2 and VEGF-A over the age range studied (i.e. 17–24 wk).
Figure 3.
VEGF-A and FGF-2 protein expression in the HFA gland. A–D, Immunofluorescence of 17-wk (A and B) and 18-wk (C and D) gestation HFAs showing localization of VEGF-A and FGF-2, respectively. VEGF-A protein (A) localized throughout the gland. FGF-2 protein (C) localized principally in the outer region of the gland. B, Control slide incubated with normal rabbit serum. D, Control slide incubated with normal mouse IgG. Magnification bar, 100 μm. Original magnification, ×200.
Regulation of Ang1, Ang2, and VEGF-A mRNA
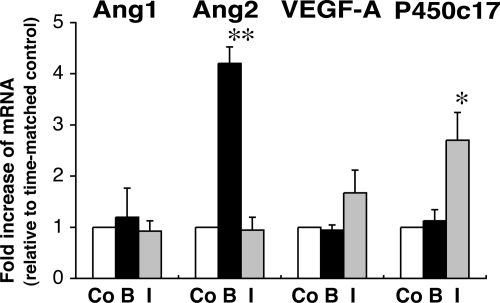
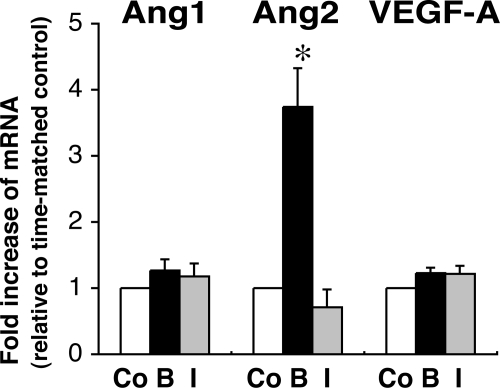
Because FGF-2 and IGF-II appear to be locally produced growth factors implicated in HFA development (5,6), and both have regulated expression of vascular endothelial cell-specific angiogenic factors in certain cell types (24,25), we examined whether FGF-2 or IGF-II can regulate mRNA expression of VEGF-A, Ang1, and Ang2 in cultured HFA cells. Treatment of isolated FZ cells with FGF-2 (10 ng/ml) for 24 h increased Ang2 mRNA by 4-fold, but not Ang1 or VEGF mRNA (Fig. 4). In contrast, IGF-II (100 ng/ml) did not significantly change mRNA levels of Ang1, Ang2, or VEGF, whereas the action of IGF-II on FZ cells was confirmed by assessing its effect on the abundance of mRNA encoding P450c17, which is up-regulated by IGF-II (22,26) (Fig. 4). Similarly, treatment of cultured DZ cells with FGF-2, but not IGF-II, selectively increased Ang2 mRNA, as shown in Fig. 5.
Figure 4.
Effects of FGF-2 or IGF-II on Ang1, Ang2, VEGF-A, and P450c17 mRNA levels in FZ cells. Isolated FZ cells were exposed to FGF-2 (basic FGF) (B) (10 ng/ml) or IGF-II (I) (100 ng/ml) for 24 h. Total RNA was extracted and analyzed by qRT-PCR as described in Materials and Methods. For comparison, GUS-normalized values are presented as fold increase relative to time-matched, unstimulated controls (Co) (arbitrarily presented as one). Data shown are from four experiments using different adrenals. *, P < 0.05, **, P < 0.01, vs. time-matched, unstimulated control.
Figure 5.
Effects of FGF-2 or IGF-II on Ang1, Ang2, and VEGF-A mRNA levels in DZ cells. Isolated DZ cells were exposed to FGF-2 (basic FGF) (B) (10 ng/ml) or IGF-II (I) (100 ng/ml) for 24 h. For comparison, GUS-normalized values are presented as fold increase relative to time-matched, unstimulated controls (Co) (arbitrarily presented as one). Data shown are from three experiments using different adrenals. *, P < 0.01, vs. time-matched, unstimulated control.
Discussion
The significance of angiogenesis in HFA development and function may reflect the gland being one of the most highly vascularized organs in the human fetus (27). However, angiogenesis in the HFA has not been extensively studied. Here, we provide evidence that identifies the periphery of the HFA as a primary site of angiogenesis, and propose that locally produced angiogenic factors such as Ang2, VEGF-A, and FGF-2 likely play a key role in HFA angiogenesis.
Angiogenesis is considered an integral process for organ growth that is mediated in part by pro-angiogenic factors, including FGF-2 and VEGF-A, both of which are potent mitogens for endothelial cells (13,28). Ang1 and Ang2 belong to a more recently identified family of endothelial cell-specific growth factors that also play a key role in angiogenesis (12,13,29). Ang1 is expressed in a wide variety of tissues, whereas Ang2 expression is found primarily at sites of vascular remodeling, including the reproductive tract and placenta (10,16,30,31). Both Ang1 and Ang2 bind their Tie2 receptor with high affinity. The currently accepted hypothesis is that Ang1 signals via Tie2 and promotes vessel stabilization and maturation, whereas Ang2 antagonizes Ang1/Tie2 signaling and destabilizes vessels, leading to either angiogenesis or vessel regression, depending on the presence of angiogenic stimuli such as VEGF-A and FGF-2 (11,12,13). In the present study, we sought to investigate the zonal pattern of mRNA expression of the angiogenic factors in the midgestation HFA. To address this in a sensitive, quantitative, and spatially accurate fashion, we used LCM and qRT-PCR, which demonstrated that Ang2 mRNA levels in vivo were higher in the DZ than in the FZ, consistent with our previous immunohistochemical findings of Ang2 localization predominantly in the outer zone (9). In contrast, Ang1 mRNA levels were lower in the DZ than in the FZ. VEGF-A and FGF-2 mRNA levels were higher in the DZ than in the FZ. Immunoreactive VEGF-A and FGF-2 exhibited a zonal pattern similar to that of their mRNA. Therefore, given the presumed roles of the angiogenic factors discussed previously, the outer-zone predominant expression of Ang2, along with abundant VEGF-A and FGF-2, likely indicates that angiogenesis in the midgestation HFA occurs primarily in the periphery of the gland. The higher Ang2 to Ang1 mRNA ratios in the DZ further support this observation. Conversely, increased Ang1 expression in the FZ implies greater vessel maturity. We speculate that a stable vasculature is necessary for the FZ because of the early initiation of hormone synthesis and sustained production of dehydroepiandrosterone and its sulfate in the zone. Further studies are required to explore this tenet.
The localization of VEGF protein throughout the midgestation HFA indicates its roles not only in the DZ but the FZ. Vittet et al. (32) reported that VEGF-A was strongly expressed in both glomerulosa and fasciculata cells of the adult bovine adrenal cortex where the endothelium is quiescent, suggesting a role for VEGF-A in the maintenance of the dense fenestrated vascular bed. Similarly, we have data that VEGF-A mRNA levels in human adult adrenals are comparable to those in midgestation HFAs (data not shown). Vittet et al. (32) also demonstrated that mRNA expression of the signaling VEGF receptors VEGFR-1 and VEGFR-2 was restricted to endothelial cells of the adult bovine adrenal cortex. In our hands, qRT-PCR analysis revealed that VEGFR-2 mRNA was expressed in both the DZ and FZ, and that VEGFR-2 mRNA expression relative to CD31 (an index of abundance of endothelial cells) was higher in the inner FZ than the outer DZ (data not shown). Because VEGF-A is known to exert antiapoptotic effects on endothelial cells and induce endothelial fenestration (28), very likely via VEGFR-2 on the fetal adrenal endothelium, it may play an important role in maintaining vascular homeostasis in the midgestation HFA, an active endocrine organ.
We demonstrate, for the first time, that proliferating endothelial cells were limited to the periphery of the HFA (i.e. the DZ and DZ/FZ border) during midgestation. The DZ may benefit from a more plastic vascular state to accommodate its proliferative phenotype. In a previous study using microcorrosion casts and scanning electron microscopy, a dense network of irregular capillaries at the periphery of the HFA was described (33). The dense vascularization enables proximity between adrenocytes and endothelial cells. Thus, we hypothesize that interactions between adrenal cortical and endothelial cells ensure a coordinate expansion of the vascular network as DZ cells proliferate and the organ grows. A similar coordination of growth of the vasculature and organ has been demonstrated in murine embryonic lung morphogenesis (34).
Because Ang2 often plays a pivotal role in vessel destabilization, the initial step in angiogenesis (12,13), understanding the mechanisms that regulate Ang2 expression, is of significant importance. In most tissues, the primary source of Ang2 is endothelial cells (10,35). Ang2 expression is almost absent in the quiescent vasculature and is related to endothelial activation (29). Environmental cues such as hypoxia and several different endotheliotropic cytokines, including FGF-2 and VEGF-A, can up-regulate expression of Ang2 mRNA in endothelial cells (24,36). Therefore, Ang2 may act in an autocrine manner to control endothelial responsiveness. On the other hand, several investigators have described nonendothelial expression of Ang2 in certain tissues (9,16,37,38). For example, in the human corpus luteum, Ang2 mRNA was detected in luteal cells as well as endothelial cells (37). Recently, we showed that Ang2 is expressed in the periphery of the midgestation HFA, whereas the Tie2 receptor is exclusively in endothelial cells throughout the gland (9). We also demonstrated that isolated HFA cortical cells produce Ang2 and VEGF-A, particularly in response to ACTH stimulation, indicating that fetal adrenal cortical cells can be a paracrine source to trigger angiogenesis (8,9). In the current investigation, FGF-2 up-regulated mRNA encoding Ang2, but not Ang1, in isolated DZ and FZ cells. Of particular interest, FGF-2 expression exhibited a DZ-predominant pattern similar to that of Ang2. Thus, Ang2 may be partly under the control of FGF-2 in vivo. Because FGF-2 is one of the most potent mitogens for HFA cells as well as for endothelial cells (5,28), this suggests an efficient mechanism by which FGF-2 can promote angiogenesis directly and indirectly through inducing Ang2 to support the vascular supply proportionate to organ growth. Furthermore, because both FGF-2 and Ang2 are regulated by ACTH (5,9), these results are consistent with a concept, proposed by us and other investigators, that ACTH coordinates adrenal organ growth and angiogenesis (9,39,40).
In summary, the present study shows that endothelial cell proliferation occurs at the outer region of the HFA gland. This outer-zone predominance is parallel to localization of the key angiogenic factors, Ang2, FGF-2, and VEGF-A. Moreover, the parallelism observed in the in vivo expression patterns of Ang2 and FGF-2 may in part be explained by the up-regulation of Ang2 mRNA by FGF-2 in HFA cortical cells. This study highlights the importance of coordinated organ and vasculature growth by interactions between adrenal cortical cells and endothelial cells in which FGF-2 and the vascular endothelial-specific growth factors, Ang2 and VEGF-A, are likely involved.
Acknowledgments
We thank Mikiye Nakanishi for technical assistance and Michiyo Ishimoto for assistance with manuscript preparation.
Footnotes
This work was supported in part by National Institutes of Health Grant HD08478 (to R.B.J.), and Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science No. 19591288 (to H.I.) and No.18659313 (to Y.Y).
Results from this work were presented in part at the 85th Annual Meeting of The Endocrine Society, Philadelphia, Pennsylvania, June 19–22, 2003, and the 54th Annual Scientific Meeting of The Society for Gynecologic Investigation, Reno, Nevada, March 14–17, 2007.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 25, 2008
Abbreviations: Ang, Angiopoietin; DZ, definitive zone; FGF, fibroblast growth factor; FZ, fetal zone; GUS, β-glucuronidase; HFA, human fetal adrenal; P450c17, 17α-hydroxylase/17, 20 lyase; LCM, laser capture microdissection; qRT, real-time quantitative RT; UEA, ulex europaeus agglutinin; VEGF, vascular endothelial growth factor.
References
- Mesiano S, Jaffe RB 1997 Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18:378–403 [DOI] [PubMed] [Google Scholar]
- Jaffe RB 2001 Role of the human fetal adrenal gland in the initiation of parturition. In: Smith R, ed. The endocrinology of parturition: basic science and clinical application. Basel: Karger; 75–85 [DOI] [PubMed] [Google Scholar]
- Johannisson E 1968 The foetal adrenal cortex in the human. Its ultrastructure at different stages of development and in different functional states. Acta Endocrinol (Copenh) 58(Suppl 130):1–107 [PubMed] [Google Scholar]
- Spencer SJ, Mesiano S, Lee JY, Jaffe RB 1999 Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodeling. J Clin Endocrinol Metab 84:1110–1115 [DOI] [PubMed] [Google Scholar]
- Mesiano S, Mellon SH, Gospodarowicz D, Di Blasio AM, Jaffe RB 1991 Basic fibroblast growth factor expression is regulated by corticotropin in the human fetal adrenal: a model for adrenal growth regulation. Proc Natl Acad Sci USA 88:5428–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Mellon SH, Jaffe RB 1993 Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J Clin Endocrinol Metab 76:968–976 [DOI] [PubMed] [Google Scholar]
- Coulter CL, Goldsmith PC, Mesiano S, Voytek CC, Martin MC, Han VK, Jaffe RB 1996 Functional maturation of the primate fetal adrenal in vivo: I. Role of insulin-like growth factors (IGFs), IGF-I receptor, and IGF binding proteins in growth regulation. Endocrinology 137:4487–4498 [DOI] [PubMed] [Google Scholar]
- Shifren JL, Mesiano S, Taylor RN, Ferrara N, Jaffe RB 1998 Corticotropin regulates vascular endothelial growth factor expression in human fetal adrenal cortical cells. J Clin Endocrinol Metab 83:1342–1347 [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Ginzinger DG, Jaffe RB 2006 Adrenocorticotropin preferentially up-regulates angiopoietin 2 in the human fetal adrenal gland: implications for coordinated adrenal organ growth and angiogenesis. J Clin Endocrinol Metab 91:1909–1915 [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD 1997 Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55–60 [DOI] [PubMed] [Google Scholar]
- Ramsauer M, D'Amore PA 2002 Getting Tie(2)d up in angiogenesis. J Clin Invest 110:1615–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J 2000 Vascular-specific growth factors and blood vessel formation. Nature 407:242–248 [DOI] [PubMed] [Google Scholar]
- Bouis D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA 2006 A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol Res 53:89–103 [DOI] [PubMed] [Google Scholar]
- Ratcliffe J, Nakanishi M, Jaffe RB 2003 Identification of definitive and fetal zone markers in the human fetal adrenal gland reveals putative developmental genes. J Clin Endocrinol Metab 88:3272–3277 [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Ginzinger DG, Matsumoto T, Hattori Y, Furuya M, Minegishi K, Tanaka M, Yoshimura Y, Jaffe RB 2006 Differential zonal expression and adrenocorticotropin regulation of secreted protein acidic and rich in cysteine (SPARC), a matricellular protein, in the midgestation human fetal adrenal gland: implications for adrenal development. J Clin Endocrinol Metab 91:3208–3214 [DOI] [PubMed] [Google Scholar]
- Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB 2002 Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab 87:4213–4224 [DOI] [PubMed] [Google Scholar]
- Muench MO, Ratcliffe JV, Nakanishi M, Ishimoto H, Jaffe RB 2003 Isolation of definitive zone and chromaffin cells based upon expression of CD56 (neural cell adhesion molecule) in the human fetal adrenal gland. J Clin Endocrinol Metab 88:3921–3930 [DOI] [PubMed] [Google Scholar]
- Ginzinger DG, Godfrey TE, Nigro J, Moore 2nd DH, Suzuki S, Pallavicini MG, Gray JW, Jensen RH 2000 Measurement of DNA copy number at microsatellite loci using quantitative PCR analysis. Cancer Res 60:5405–5409 [PubMed] [Google Scholar]
- Van Trappen PO, Ryan A, Carroll M, Lecoeur C, Goff L, Gyselman VG, Young BD, Lowe DG, Pepper MS, Shepherd JH, Jacobs IJ 2002 A model for co-expression pattern analysis of genes implicated in angiogenesis and tumour cell invasion in cervical cancer. Br J Cancer 87:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Fujimoto VY, Nelson LR, Lee JY, Voytek CC, Jaffe RB 1996 Localization and regulation of corticotropin receptor expression in the midgestation human fetal adrenal cortex: implications for in utero homeostasis. J Clin Endocrinol Metab 81:340–345 [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Muench MO, Higuchi T, Minegishi K, Tanaka M, Yoshimura Y, Jaffe RB 2006 Midkine, a heparin-binding growth factor, selectively stimulates proliferation of definitive zone cells of the human fetal adrenal gland. J Clin Endocrinol Metab 91:4050–4056 [DOI] [PubMed] [Google Scholar]
- Mesiano S, Jaffe RB 1993 Interaction of insulin-like growth factor-II and estradiol directs steroidogenesis in the human fetal adrenal toward dehydroepiandrosterone sulfate production. J Clin Endocrinol Metab 77:754–758 [DOI] [PubMed] [Google Scholar]
- Lafont J, Laurent M, Thibout H, Lallemand F, Le Bouc Y, Atfi A, Martinerie C 2002 The expression of novH in adrenocortical cells is down-regulated by TGFbeta 1 through c-Jun in a Smad-independent manner. J Biol Chem 277:41220–41229 [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Pepper MS 1998 Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 83:852–859 [DOI] [PubMed] [Google Scholar]
- Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC 1998 Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res 58:348–351 [PubMed] [Google Scholar]
- Mesiano S, Katz SL, Lee JY, Jaffe RB 1997 Insulin-like growth factors augment steroid production and expression of steroidogenic enzymes in human fetal adrenal cortical cells: implications for adrenal androgen regulation. J Clin Endocrinol Metab 82:1390–1396 [DOI] [PubMed] [Google Scholar]
- McClellan M, Brenner RM 1981 Development of the fetal adrenals in non-human primates: electron microscopy. In: Novy MJ, Resko JA, eds. Fetal endocrinology. New York: Academic Press; 383–403 [Google Scholar]
- Cross MJ, Claesson-Welsh L 2001 FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 22:201–207 [DOI] [PubMed] [Google Scholar]
- Fiedler U, Augustin HG 2006 Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 27:552–558 [DOI] [PubMed] [Google Scholar]
- Geva E, Jaffe RB 2000 Role of angiopoietins in reproductive tract angiogenesis. Obstet Gynecol Surv 55:511–519 [DOI] [PubMed] [Google Scholar]
- Geva E, Ginzinger DG, Moore 2nd DH, Ursell PC, Jaffe RB 2005 In utero angiopoietin-2 gene delivery remodels placental blood vessel phenotype: a murine model for studying placental angiogenesis. Mol Hum Reprod 11:253–260 [DOI] [PubMed] [Google Scholar]
- Vittet D, Ciais D, Keramidas M, De Fraipont F, Feige JJ 2000 Paracrine control of the adult adrenal cortex vasculature by vascular endothelial growth factor. Endocr Res 26:843–852 [DOI] [PubMed] [Google Scholar]
- Pitynski K, Litwin JA, Nowogrodzka-Zagorska M, Miodonski AJ 1996 Vascular architecture of the human fetal adrenal gland: a SEM study of corrosion casts. Ann Anat 178:215–222 [DOI] [PubMed] [Google Scholar]
- Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R 2005 Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 288:L141–L149 [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ 1999 Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284:1994–1998 [DOI] [PubMed] [Google Scholar]
- Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y 1999 Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 274:15732–15739 [DOI] [PubMed] [Google Scholar]
- Wulff C, Wilson H, Largue P, Duncan WC, Armstrong DG, Fraser HM 2000 Angiogenesis in the human corpus luteum: localization and changes in angiopoietins, tie-2, and vascular endothelial growth factor messenger ribonucleic acid. J Clin Endocrinol Metab 85:4302–4309 [DOI] [PubMed] [Google Scholar]
- Cohen B, Barkan D, Levy Y, Goldberg I, Fridman E, Kopolovic J, Rubinstein M 2001 Leptin induces angiopoietin-2 expression in adipose tissues. J Biol Chem 276:7697–7700 [DOI] [PubMed] [Google Scholar]
- Thomas M, Keramidas M, Monchaux E, Feige JJ 2004 Dual hormonal regulation of endocrine tissue mass and vasculature by adrenocorticotropin in the adrenal cortex. Endocrinology 145:4320–4329 [DOI] [PubMed] [Google Scholar]
- Thomas M, Keramidas M, Monchaux E, Feige JJ 2003 Role of adrenocorticotropic hormone in the development and maintenance of the adrenal cortical vasculature. Microsc Res Tech 61:247–251 [DOI] [PubMed] [Google Scholar]