Abstract
Context: Daily PTH administration increases bone mineral density (BMD) and reduces fracture risk. However, cost and compliance significantly limit clinical use.
Objective: Our objective was to determine whether less frequent PTH administration increases lumbar spine BMD.
Participants, Design, and Setting: Fifty postmenopausal women ages 45–70 yr with femoral neck BMD T-score between −1.0 and −2.0 participated in a double-blind, randomized, placebo-controlled trial at St. Joseph Hospital, Bangor, ME.
Intervention: Subjects received sc injections of daily PTH(1-84) (100 μg) or placebo for 1 month, followed by weekly injections (PTH or placebo) for 11 months.
Outcomes: Change in lumbar spine dual-energy x-ray absorptiometry areal BMD (primary) was assessed. Secondary outcomes included volumetric BMD at spine and hip by quantitative computed tomography, trabecular bone microarchitecture by magnetic resonance imaging of distal radius, and biochemical bone turnover markers.
Results: At 12 months, lumbar spine areal BMD increased 2.1% in PTH-treated women compared with placebo (P = 0.03). Vertebral trabecular volumetric BMD increased 3.8% in PTH-treated women compared with placebo group (P = 0.08). PTH-treated women also had higher distal radial trabecular bone volume, number, and thickness compared with placebo-treated women (P < 0.04). After 1 month of daily PTH, N-terminal propeptide of type I collagen (P1NP) was markedly increased compared with placebo (P < 0 .0001), and a difference persisted, although lessened, throughout the study. Bone resorption indices were unchanged in PTH-treated women and were reduced in the placebo group.
Conclusion: Once-weekly PTH after 1 month of daily treatment increases spine BMD, radial trabecular bone, and bone formation markers in postmenopausal women. These results suggest that less frequent alternatives to daily PTH dosing for 2 yr could be effective. Additional studies are required to define the optimal frequency of PTH administration.
In postmenopausal women with osteoporosis, once-weekly administration of PTH after one month of daily treatment increases spine bone mass density, radial trabecular bone, and bone formation markers, suggesting that less frequent alternatives to daily PTH dosing for two years could be equally effective in preventing bone loss.
Current approved treatments for postmenopausal osteoporosis include two classes of drugs: 1) antiresorptives, such as the bisphosphonates, estrogen, calcitonin, and the selective estrogen receptor modulators; and 2) anabolics, such as teriparatide [PTH(1–34)]. In contrast to the former class of drugs that prevent bone loss through inhibition of bone turnover, PTH enhances bone mass through stimulation of bone formation and remodeling. Since its approval in 2002, teriparatide has been used relatively sparingly despite its proven antifracture efficacy and its capacity to markedly increase bone mineral density (BMD) (1). High cost and the burden of daily injections for as long as 2 yr have been the most significant barriers to widespread use of PTH, particularly among primary care providers (2).
To increase clinical acceptability of PTH, various treatment regimens have been proposed that might retain the anabolic effect while reducing the duration of PTH treatment. Using PTH(1-84), we showed that 1 year of PTH followed by 1 year of alendronate resulted in larger bone density increases than 2 yr of PTH(1-84) (3,4). Cosman and colleagues (5) found that 3-month on/off cycles of PTH(1–34) in the context of ongoing alendronate therapy for 15 months yielded similar BMD increases to 15 months of continuous PTH. In another approach, once-weekly PTH(1–34) was shown to significantly increase BMD over 1 yr (6). In the largest dosage group (200 IU/wk), there was an increase in lumbar spine BMD of approximately 8%. However, because there was no placebo comparison group, it is difficult to separate the effects of the drug from longitudinal increases often seen in lumbar spine BMD due to artifactual changes in the lumbar spine with aging. To address the question of whether less frequent PTH administration could positively influence BMD, we performed a 12-month double-blind, randomized, placebo-controlled trial in postmenopausal women in a design that used a daily loading dose of PTH(1-84) for 4 wk, followed by weekly therapy.
Subjects and Methods
Subjects
We included women (recruitment goal was 50) between the ages of 45 and 70 yr who were at least 5 yr postmenopausal, had a total hip areal BMD (aBMD) T-score of −1.0 to −2.0, and no history of osteoporotic fractures or presence of morphometric fractures on x-ray. Exclusion criteria included current use of bisphosphonates, estrogen, raloxifene, or calcitonin or previous exposure to PTH. Previous exposure to oral bisphosphonates was limited to no more than 12 months ever and no more than 4 wk within the previous 2 yr. No previous use of iv bisphosphonates was allowed. In addition, women with 25(OH)-vitamin D levels less than 15 ng/ml, serum calcium more than 10.3 mg/dl, urine calcium/creatinine ratio more than 0.3, or creatinine clearance less than 40 ml/min were excluded. The patients were recruited from St. Joseph Hospital, Bangor, ME. The first one was screened in January 2004, and the last patient visit occurred in February 2006. Informed consent was obtained from all subjects before screening.
Trial design and treatments
This was a double-blind, randomized, placebo-controlled trial of 12 months duration with spine aBMD by dual-energy x-ray absorptiometry (DXA) as the principal outcome. After a run-in of 2 wk during which the women received placebo injections and calcium and vitamin D, women were randomized to PTH or placebo (n = 25 each). The PTH group self-administered daily sc injections of PTH(1-84) (100 μg PreOS; NPS, Salt Lake City, UT) for 4 wk, followed by weekly injections (100 μg) for the next 11 months. The placebo group self-administered placebo injections according to the same schedule. Both groups received 500 mg calcium and 400 IU vitamin D daily.
Efficacy outcome variables
Details of the methods for assessing outcome DXA and quantitative computed tomography (QCT) measurements have been previously described in detail (7) and will only be summarized here. The aBMD (grams per square centimeter) at the lumbar spine, hip, whole body, and radius was assessed by DXA (Hologic Delphi) at baseline and at 1 (spine only), 6, and 12 months. Change in lumbar spine aBMD as assessed by DXA was the primary efficacy parameter. Volumetric bone density (vBMD, grams per cubic centimeter) at the lumbar spine (L1 and L2, trabecular bone density only) and total hip (trabecular, cortical, and integral bone densities) and bone geometry were assessed by QCT, as previously described (7,8). Scans were obtained at 80 kVp using contiguous 3-mm slices.
Trabecular architecture and cortical thickness were assessed in the distal radius using magnetic resonance imaging (MRI), as previously described (9). Slices were 0.5 mm thick for the first nine patients and 0.75 mm for the remaining patients.
Trabecular and cortical bone regions were segmented using in-house developed semiautomatic routines in IDL (Research Systems Inc., Boulder, CO). Trabecular bone measures that were calculated, using two-dimensional histomorphometry on a slice by slice basis (10), included apparent bone volume fraction, apparent trabecular number, apparent trabecular spacing, and apparent trabecular thickness. The mean cortical thickness for each segmented slice was calculated using a three-dimensional distance transformation technique previously developed for trabecular bone (11). Two-dimensional trabecular bone calculations and cortical bone thicknesses were then averaged over the entire scanning region and then into 1.5-mm segments starting at 6 mm and ending at 30 mm from the endplate.
After an overnight fast, serum was drawn and stored (−70 C) at baseline and at 1, 2, 3, 6, and 12 months. Samples were stored until assayed for markers of bone metabolism [serum C-telopeptide of type I collagen (CTX), bone-specific alkaline phosphatase (BSAP), and N-terminal propeptide of type I collagen (P1NP)] in a central laboratory (P. Garnero, Synarc, Lyon, France). Assays for all time points were performed simultaneously.
Adherence, safety assessment, and adverse events (AEs)
Adherence was assessed by diaries. Full adherence to treatment each year was defined as study injections greater than 80%. Patients were queried at each visit about AEs. AEs were coded using MedDRA preferred terms (MedDRA MSSO, Reston, VA). Preferred terms were categorized in groups according to anticipated results from previous studies of PTH (7). The incidence within each category was then compared across treatments.
Serum chemistries including calcium, uric acid, blood urea nitrogen, creatinine, and urinary calcium/creatinine were obtained at baseline and at 1, 2, 6, and 12 months. The 24-h urine was collected at baseline and 2 months. Algorithms used previously (7) were employed to provide clinical management for high levels of serum and urine calcium. The algorithms were triggered by serum calcium values above 10.2–11.2 mg/dl or more than 11.2 mg/dl and involved a first step of remeasurement of serum calcium and then elimination of calcium supplements if serum calcium had not returned to normal after retest. A parallel algorithm for hypercalciuria more than 0.4 mg/mg Ca/Cr was also used.
Statistical analysis
We attempted to follow randomized patients for all study visits and procedures regardless of adherence to treatment regimen. Analyses are intention-to-treat unless otherwise stated. Within-treatment group means and 95% confidence intervals for percentage changes from baseline to 12 months in DXA (anterior-posterior spine, total hip, femoral neck and radius one third site) and QCT parameters (trabecular spine and hip BMD, cortical BMD, bone mineral content (BMC), and total volume in total femur) are shown. Change in biochemical markers were compared by means of analysis of variance (loge ratio of the post-baseline value to the baseline value, with back transformation). All comparisons use a significance level of 0.05 (not adjusted for multiple comparisons), but the significance levels for comparisons that are very significant (P < 0.001) are generally noted in the text.
The study was designed with 80% power to detect a 3% difference in spine BMD between treatment groups.
Study management
The study was funded by NIAMS and was run by a Steering Committee including the two study principal investigators (D.M.B. and C.J.R.) and the NIAMS project officer (J.A.M.). The study was managed by and data collected and analyzed at the San Francisco Coordinating Center. The quality assurance and analysis for QCT were performed at Beth Israel Deaconess Medical Center, and those for MRI were performed at UCSF Department of Radiology. The trial was monitored by a Data Safety Officer appointed by NIAMS who reviewed unblinded data approximately halfway through the trial. The protocol was approved by the Institutional Review Boards at St. Joseph Hospital, Bangor, ME, and at UCSF, San Francisco, CA.
Results
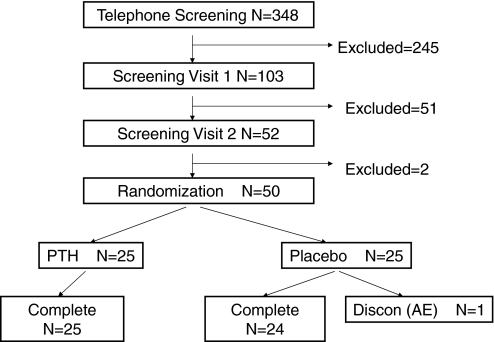
Approximately 350 women were screened. Fifty postmenopausal women between the ages of 45–70 yr were enrolled and randomly assigned to active treatment, PTH(1-84), or placebo (Fig. 1). Forty-nine women completed the trial; one was lost to follow-up (Fig. 1). At baseline, the mean age of the women was 58.2 ± 6.4 yr, and the mean body mass index was 26.8 ± 3.6 kg/m2 (Table 1). The mean T-score for aBMD at the lumbar spine was −1.0 ± 1.1 g/cm2.
Figure 1.
Study design and patient flow.
Table 1.
Baseline characteristics
| PTH (n = 25) | Placebo (n = 25) | P value | |
|---|---|---|---|
| Age at baseline (yr) | |||
| Mean | 57.6 ± 5.8 | 58.9 ± 6.9 | 0.47 |
| 45–59 | 17 (68.0) | 13 (52.0) | |
| ≥60 | 8 (32.0) | 12 (48.0) | |
| Body mass index (kg/m2) | 26.9 ± 3.5 | 26.8 ± 3.8 | 0.94 |
| Height (cm) | 161.3 ± 4.9 | 161.8 ± 6.1 | 0.74 |
| DXA aBMD (g/cm2) | |||
| Lumbar spine | 0.95 ± 0.13 | 0.93 ± 0.11 | 0.64 |
| Total hip | 0.85 ± 0.07 | 0.84 ± 0.08 | 0.85 |
| Femoral neck | 0.69 ± 0.04 | 0.67 ± 0.05 | 0.27 |
| Ultradistal radius | 0.42 ± 0.05 | 0.41 ± 0.06 | 0.74 |
| Mid radius | 0.60 ± 0.06 | 0.59 ± 0.07 | 0.71 |
| Radius 1/3 | 0.67 ± 0.06 | 0.65 ± 0.08 | 0.38 |
| DXA aBMD T-score | |||
| Lumbar spine | −0.9 ± 1.2 | −1.1 ± 1.0 | 0.64 |
| Total hip | −0.8 ± 0.6 | −0.8 ± 0.7 | 0.85 |
| Femoral neck | −1.5 ± 0.4 | −1.6 ± 0.4 | 0.27 |
| QCT vBMD, lumbar spine (g/cm3), trabecular | 0.13 ± 0.03 | 0.12 ± 0.02 | 0.16 |
| QCT vBMD, total femur (g/cm3) | |||
| Trabecular | 0.10 ± 0.02 | 0.09 ± 0.03 | 0.09 |
| Cortical | 0.50 ± 0.03 | 0.49 ± 0.03 | 0.26 |
| Integral | 0.26 ± 0.03 | 0.24 ± 0.03 | 0.02 |
| QCT vBMD, femoral neck (g/cm3) | |||
| Trabecular | 0.07 ± 0.02 | 0.06 ± 0.03 | 0.32 |
| Cortical | 0.51 ± 0.03 | 0.49 ± 0.03 | 0.07 |
| Integral | 0.27 ± 0.03 | 0.25 ± 0.02 | 0.008 |
| Biochemical markers (ng/ml) | |||
| BSAP | 14.8 ± 6.2 | 15.4 ± 4.8 | 0.69 |
| CTX | 463 ± 219 | 406 ± 139 | 0.23 |
| P1NP | 55.1 ± 23.2 | 49.4 ± 18.9 | 0.32 |
| MRI parametersa | |||
| Bone volume fraction | 0.34 ± 0.05 | 0.34 ± 0.06 | 0.93 |
| Trabecular spacing (mm) | 0.40 ± 0.06 | 0.42 ± 0.09 | 0.55 |
| Trabecular number (1/mm) | 1.69 ± 0.15 | 1.66 ± 0.19 | 0.52 |
| Trabecular thickness (mm) | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.74 |
Numbers shown are the mean ± sd. For biochemical markers, the geometric mean is shown.
Averaged across all available slices (PTH n = 22; placebo n = 21).
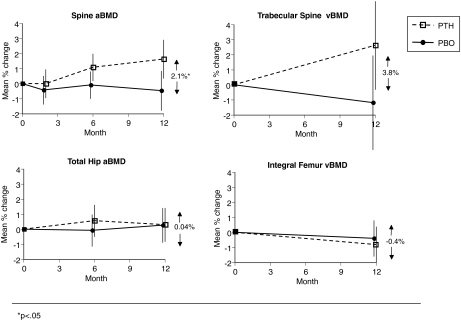
Compared with placebo-treated women, spine aBMD increased by 2.1% (P = 0.03; Table 2 and Fig. 2) and vertebral trabecular vBMD by 3.8% (P = 0.08; Table 2 and Fig. 2) in women on once-weekly PTH. Changes in total hip and femoral neck aBMD by DXA and total hip vBMD by QCT did not differ between treatments (Table 2 and Fig. 2).
Table 2.
Percentage change (baseline to 12 months) in BMD
| PTH mean ± sd | P valuea | Placebo mean ± sd | P valuea | Comparison of PTH with placebo mean difference (95% CI) | P valueb | |
|---|---|---|---|---|---|---|
| aBMD by DXA | ||||||
| Lumbar spine | 1.63 ± 3.12 | 0.01 | −0.47 ± 3.24 | 0.47 | 2.10 (0.27, 3.93) | 0.03 |
| Total hip | 0.30 ± 3.29 | 0.59 | 0.27 ± 2.06 | 0.64 | 0.04 (−1.55, 1.62) | 0.96 |
| Femoral neck | 0.86 ± 2.83 | 0.14 | 0.95 ± 2.90 | 0.11 | −0.09 (−1.74, 1.55) | 0.91 |
| Ultradistal radius | −1.95 ± 2.94 | 0.004 | −2.26 ± 3.48 | 0.002 | 0.31 (−1.56, 2.18) | 0.74 |
| Mid radius | −2.00 ± 2.07 | <0.0001 | −1.25 ± 1.36 | 0.001 | −0.75 (−1.78, 0.28) | 0.15 |
| Radius 1/3 | −1.12 ± 2.36 | 0.01 | −0.52 ± 1.95 | 0.26 | −0.60 (−1.87, 0.66) | 0.34 |
| vBMD by QCT | ||||||
| Spine, trabecular | 2.62 ± 7.84 | 0.08 | −1.18 ± 6.14 | 0.45 | 3.80 (−0.48, 8.08) | 0.08 |
| Total femur, trabecular | −2.51 ± 5.96 | 0.07 | −2.18 ± 6.55 | 0.11 | −0.32 (−4.13, 3.49) | 0.87 |
| Total femur, cortical | −0.13 ± 1.78 | 0.73 | 0.23 ± 1.87 | 0.56 | −0.36 (−1.47, 0.75) | 0.52 |
| Total femur, integral | -0.81 ± 2.77 | 0.17 | −0.40 ± 2.75 | 0.50 | −0.41 (−2.09, 1.27) | 0.62 |
| Femoral neck, trabecular | −6.10 ± 17.11 | 0.44 | −16.7 ± 49.14 | 0.04 | 10.58 (−11.81, 32.96) | 0.35 |
| Femoral neck, cortical | 0.13 ± 2.69 | 0.83 | 0.54 ± 2.65 | 0.35 | −0.42 (−2.04, 1.21) | 0.61 |
| Femoral neck, integral | −0.74 ± 2.91 | 0.20 | −0.51 ± 2.33 | 0.37 | −0.23 (−1.83, 1.38) | 0.78 |
| MRI parametersc | ||||||
| Bone volume fraction | 2.34 ± 9.29 | 0.28 | −3.69 ± 8.07 | 0.13 | 6.03 (−0.43, 12.50) | 0.07 |
| Trabecular spacing (mm) | −2.54 ± 9.59 | 0.29 | 6.44 ± 9.75 | 0.02 | −8.98 (−16.11, −1.85) | 0.02 |
| Trabecular number (1/mm) | 2.19 ± 5.44 | 0.09 | −2.97 ± 4.60 | 0.04 | 5.16 (1.41, 8.90) | 0.01 |
| Trabecular thickness (mm) | 0.26 ± 6.72 | 0.87 | −0.95 ± 6.19 | 0.59 | 1.21 (−3.58, 6.00) | 0.61 |
CI, Confidence interval.
Within treatment.
Between treatments.
Averaged across all available slices (PTH n = 17; placebo n = 14).
Figure 2.
Changes in BMD at the spine and hip as assessed by DXA and QCT. *, P < 0.05.
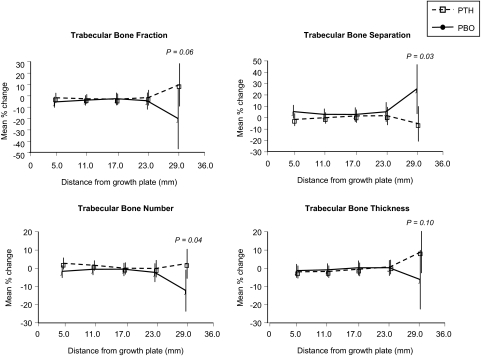
As assessed by MRI, in the more proximal regions of the distal radius, trabecular bone separation and number significantly improved (P < 0.05) in the once-weekly PTH group vs. placebo (Fig. 3). There were similar trends for trabecular bone fraction and thickness in the proximal region (P = 0.06 and P = 0.10, respectively; Fig. 3). In the more distal region, there were no differences for any trabecular parameters. No parallel trend from distal to proximal was evident for aBMD at the radius (Table 2).
Figure 3.
Changes in MRI-assessed trabecular parameters at the radius as a function of distance from the endplate (grouped into 6-mm categories). Approximately 30 patients contributed measurements in the regions from 5–23 mm, whereas only about six patients had information for the most proximal region. Statistical significance is shown for the most proximal region.
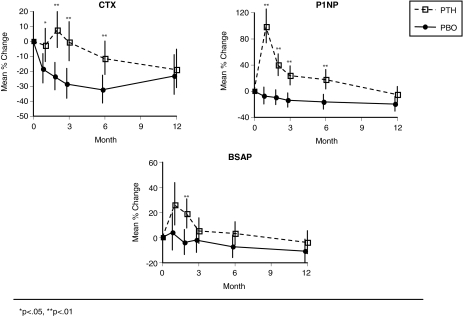
Changes in bone turnover were different between the treatment groups. After 1 month of daily PTH, P1NP increased by 98% vs. baseline (P < 0.0001; Fig. 4). Thereafter, during the period of once-weekly PTH, the decline in P1NP was relatively slow, and values did not return to baseline until the end of the 12-month study period. The modest declines in the placebo group for P1NP (and the other markers) are consistent with the mildly antiresorptive effect of calcium and vitamin D seen in placebo controls in previous trials (12,13). P1NP levels differed significantly in the PTH and placebo groups at 1, 2, 3, and 6 months of treatment (P < 0.001; Fig. 4) but not at 12 months. Bone alkaline phosphatase showed a similar pattern to P1NP with a significant increase compared with placebo by 2 months (P = 0.054 at 1 month). However, the magnitude of the increase was much less than that of P1NP. Serum CTX was initially unchanged in the PTH-treated women (Fig. 3), showed a small increase at 3 months, and then subsequently declined modestly. In the placebo group, serum CTX also showed an initial but modest decline. Differences in serum CTX between PTH and placebo were significant at 1, 2, 3, and 6 months but not at 12 months.
Figure 4.
Change in bone markers by month since randomization. *, P < 0.05; **, P < 0.01. Means and sd from logarithms of change are shown. See Subjects and Methods.
Serum calcium elevations were observed in five patients (20%) in the PTH group and none in the placebo group. Four of the five were mild (10.5–11.2 mg/dl), whereas the fifth was above 11.2 mg/dl. Four of the five occurred at 1 month at the end of the daily PTH period, and the fifth occurred at 12 months. All of those women normalized their calcium after the first retest within 1 wk; PTH was continued and calcium and vitamin D were not discontinued. Compliance with over 80% of the injections was more than 90%. There was one serious AE (chest pain) which occurred in a patient randomized to placebo.
Discussion
In this small trial, we demonstrated that 1 month of daily PTH followed by 11 months of weekly PTH had a positive effect on vertebral BMD measured by DXA and a similar trend for trabecular BMD measured by QCT. The increment in spine BMD relative to placebo-treated women was modest (+2.1% for aBMD and +3.8% for trabecular vBMD) when compared with daily PTH (1,7,14). However, the 2% difference in aBMD between treatment and placebo groups over 1 yr is not trivial in that it is similar to that previously reported for several treatments (e.g. raloxifene and risedronate) that have been shown to reduce fracture risk (12,15,16). Yet we did not see an increase in hip BMD compared with placebo as would be expected with antiresorptive drugs and seen previously with daily PTH (1,4,7). Interestingly, we observed an increase in bone formation levels that remained significant for at least 6 months with no corresponding increase in serum markers of bone resorption. Whereas the magnitudes of the BMD and biochemical marker changes were much less than those seen with daily PTH (7), the fact that significant effects could be achieved with a reduced frequency of PTH suggests that alternative regimens and dosing for PTH deserve further study.
It is still not clear whether this regimen or other nondaily dosing of PTH could reduce fracture risk. Both the increase in spine BMD and bone formation without an accompanying increase in resorption would argue for a positive impact on bone strength. On the other hand, the lack of effect on hip BMD is not supportive of an increase in bone strength at this site. Changes in BMD and markers are only weakly related to changes in fracture risk, and therefore the potential impact of this PTH dosing or some variation of intermittent PTH dosing would have to be tested in a randomized fracture trial.
We saw an increase in bone formation without an increase in bone resorption, evidence of a so-called uncoupling of the two processes. It has been suggested that such an improved balance between formation and resorption could signal a much improved ability of a therapy to increase bone strength. Daily PTH therapy yields a temporary uncoupling; formation increases during the first 3 months, whereas resorption increases do not fully occur until about 3–6 months (7,17). This period has been termed the anabolic window, and it has been hypothesized to be important to the bone-building effect of anabolic treatments. A similar uncoupling was suggested in a study using the combination of PTH(1–34) and raloxifene, where formation for the combination was similar to that for PTH alone, but the resorption increases for the combination were only about half of those seen with PTH alone (18). A similar qualitative uncoupling has been claimed for strontium ranelate, but the magnitude of the increase in formation (about 5%) and decrease in resorption (about 8%) are very small compared with much larger effects seen with antiresorptive (40–80%) and anabolic treatments (100–200%). Using some combination of cyclic and nondaily anabolic treatment, possibly in combination with a mild antiresorptive, may be possible to maximize and extend the period of increased formation while minimizing resorption increases and therefore maximizing the increases in bone strength. Specific possibilities for alternative PTH dosing include a longer loading period (perhaps 3 months), a more frequent follow-up dosing (perhaps twice per week), or a second 1-month loading period (perhaps after 6 months of weekly PTH). Generally, these findings, along with the recent study of 3-month cyclic PTH (5), provide a rationale for further exploration of alternative PTH dosing regimes.
Our MRI results suggested a trend toward an improvement in trabecular architecture, especially in the more proximal regions of the radius. The magnitude of the differences was modest, mirroring modest changes in trabecular BMD in the spine, but suggests that our treatment regimen had a positive effect on trabecular bone. The only other published treatment study that has used MRI to analyze trabecular bone examined the effects of nasal calcitonin on the distal radius (9), and notably, this study found a similar trend, whereby differences in trabecular architecture between treatment groups were more prominent in the more proximal regions of the distal radius. Whether our findings truly reflect greater trabecular changes with PTH in the more proximal regions or whether MRI simply has a greater ability to detect changes in more proximal regions requires further study. Trials of daily PTH show that radius aBMD decreases relative to placebo (1,7) and that these losses are greater in the more proximal regions of the radius, presumably reflecting greater cortical loss in these areas. These complex changes in the radius, together with fracture risk reduction observed for PTH(1–34), confirm that areal BMD does not reflect changes in bone architecture, and that future trials should include measurements that can define specific changes in the cortical and trabecular compartments at various skeletal sites. Such measurements may provide greater insight into the antifracture efficacy of osteoporosis treatments.
There were several limitations to this study. First, we used a daily loading dose of PTH for 1 month. Thus, we cannot be certain that once-weekly PTH for 12 months without a loading dose is more effective than placebo. Rather, we believe the loading dose of PTH is essential to activate lining cells and osteoblasts and elicit a true anabolic effect. Second, we did not directly compare once-weekly with daily PTH; hence, most of our comparisons with daily PTH are based on previously published studies using either the 1-34 or 1-84 preparation (1,7,17). Third, the 100-μg dose of PTH(1-84) used in this study, and administered weekly, is identical to the daily dose approved in Europe (4). However, the only other study of weekly PTH used PTH(1–34) at a maximum dose of 60 μg, three times the daily dose of teriparatide, and found a larger effect on spine BMD (+8% at 1 yr) (6). Thus, it is possible that a dose higher than 100 μg PTH(1-84) used once per week would have a larger impact. It should also be noted that we studied a group of relatively young women without severe osteoporosis; hence, our results may not be generalizable to older patients with more severe disease. Lastly, this study was very small and not powered to see subtle differences in many of the measurements used, particularly MRI and QCT. The trends we noted will have to be examined in larger multicenter trials.
Nonetheless, we have shown that a daily loading dose for 1 month followed by weekly administration of PTH has an anabolic effect on the skeleton. Taken together with other recent results on cyclical use of PTH, these results suggest that it may not be necessary to use PTH daily for an extended period to receive the full anabolic benefit; less frequent or shorter-duration use of PTH may be as good as or better than daily PTH for 2 yr in strengthening bone and reducing fracture risk. Future trials are warranted to define the optimal frequency and duration of PTH administration that can increase bone strength and ultimately reduce fracture risk.
Acknowledgments
We acknowledge the efforts of the Steering Committee, including the two study principal investigators (D.M.B and C.J.R.) and the NIAMS project officer (J.A.M); the Beth Israel Deaconess Medical Center, Boston, MA; the UCSF Department of Radiology, San Francisco, CA; the Data Safety Officer appointed by NIAMS; the Institutional Review Boards at St. Joseph Hospital, Bangor, ME, and at UCSF, San Francisco, CA. We also thank NPS Pharmaceuticals for the donation of the study drug and placebo.
Footnotes
This study was supported by the NIAMS under the following grant numbers: N01 AR32268 and NIAMS-092.
Disclosure Statement: D.M.B. received grant support from Merck (1997 to 2006) and Novartis (2001 to 2011). D.M.B., M.L.B., and S.M. consult for GlaxoSmithKlineand Merck. M.L.B. received grant support (January 2005 to February 2006) from NPS. M.L.B. received lecture fees from Eli Lilly, Novartis, Roche, Procter & Gamble, and Merck. S.M. received lecture fees from Merck. L.P., J.A.M., D.C.N., E.R., and C.J.R. have nothing to declare.
First Published Online March 18, 2008
Abbreviations: aBMD, Areal BMD; AE, adverse event; BMD, bone mineral density; BSAP, bone-specific alkaline phosphatase; CTX, C-telopeptide of type I collagen; DXA, dual-energy x-ray absorptiometry; MRI, magnetic resonance imaging; P1NP, N-terminal propeptide of type I collagen; QCT, quantitative computed tomography; vBMD, volumetric BMD.
References
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Liu H, Michaud K, Nayak S, Karpf DB, Owens DK, Garber AM 2006 The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med 166:1209–1217 [DOI] [PubMed] [Google Scholar]
- Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ 2005 One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med 353:555–565 [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, Blosch CM, Mathisen AL, Morris SA, Marriott TB 2007 Effect of Recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146:326–339 [DOI] [PubMed] [Google Scholar]
- Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R 2005 Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 353:566–575 [DOI] [PubMed] [Google Scholar]
- Fujita T, Inoue T, Morii H, Morita R, Norimatsu H, Orimo H, Takahashi HE, Yamamoto K, Fukunaga M 1999 Effect of an intermittent weekly dose of human parathyroid hormone (1–34) on osteoporosis: a randomized double-masked prospective study using three dose levels. Osteoporos Int 9:296–306 [DOI] [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ 2003 The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215 [DOI] [PubMed] [Google Scholar]
- Lang TF, Li J, Harris ST, Genant HK 1999 Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr 23:130–137 [DOI] [PubMed] [Google Scholar]
- Chesnut 3rd CH, Majumdar S, Newitt DC, Shields A, Van Pelt J, Laschansky E, Azria M, Kriegman A, Olson M, Eriksen EF, Mindeholm L 2005 Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the QUEST study. J Bone Miner Res 20:1548–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newitt DC, van Rietbergen B, Majumdar S 2002 Processing and analysis of in vivo high-resolution MR images of trabecular bone for longitudinal studies: reproducibility of structural measures and micro-finite element analysis derived mechanical properties. Osteoporos Int 13:278–287 [DOI] [PubMed] [Google Scholar]
- Laib A, Ruegsegger P 1999 Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-micron-resolution microcomputed tomography. Bone 24:35–39 [DOI] [PubMed] [Google Scholar]
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut 3rd CH, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD 1999 Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352 [DOI] [PubMed] [Google Scholar]
- Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA 2004 Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199 [DOI] [PubMed] [Google Scholar]
- McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF 2005 Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768 [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR 1999 Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE 2005 Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151 [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA 2003 The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17 [DOI] [PubMed] [Google Scholar]
- Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, Glass EV, Myers SL, Krege JH 2005 Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 20:1905–1911 [DOI] [PubMed] [Google Scholar]