Abstract
Background: Individuals with Turner syndrome (TS) are at increased risk for impaired glucose tolerance and diabetes mellitus. It is unknown whether pharmacological GH treatment commonly used to treat short stature in TS alters this risk.
Objective: Our objective was to compare adiposity and glucose tolerance in GH-treated vs. untreated girls with TS.
Methods: In a cross sectional study, GH-treated girls with TS (n = 76; age 13.6 ± 3.7 yr) were compared to girls with TS that never received GH (n = 26; age 13.8 ± 3.5 yr). Protocol studies took place in the NIH Clinical Research Center from 2001–2006 and included oral glucose tolerance tests, body composition analysis by dual-energy x-ray absorptiometry, and abdominal fat quantification by magnetic resonance imaging. GH was not given during testing.
Results: Total body fat (35 ± 8 vs. 28 ± 8%, P < 0.0001), sc abdominal fat (183 vs. 100 ml, P = 0.001), and intraabdominal fat (50 vs. 33 ml, P < 0.0001) were significantly greater in untreated girls. Fasting glucose and insulin were similar, but the response to oral glucose was significantly impaired in the untreated group (28 vs. 7% with impaired glucose tolerance, P = 0.006). A specific excess of visceral fat and insulin resistance was apparent only in postpubertal girls that had never received GH. GH-treated girls demonstrated lower adiposity compared with untreated girls for an average of 2 yr after discontinuation of GH.
Conclusions: Abdominal adiposity is significantly lower and glucose tolerance significantly better in GH-treated vs. untreated girls with TS, suggesting that beneficial effects upon body composition and regional fat deposition outweigh transient insulin antagonism associated with GH administration.
Girls with Turner syndrome treated with GH have significantly lower abdominal adiposity and greater glucose tolerance than untreated girls with the disease, implying that beneficial GH therapy effects on body composition and regional fat deposition outweigh its transient insulin antagonism.
Diabetes mellitus is increased among women with Turner syndrome (TS) (1,2). Impaired glucose tolerance (IGT) is apparent in childhood (3,4) and is associated with reduced glucose-stimulated insulin secretion (3,5,6,7) and impaired insulin sensitivity (8,9). Clarification of the metabolic phenotype in TS has been difficult for lack of appropriate control groups, because girls and women with TS usually have more body fat as well as different exposure to sex steroids compared with age-matched, 46,XX controls (8,9,10,11). Given the increased risk for diabetes, there has been concern that the widespread use of pharmacological GH treatment to increase adult stature in girls with TS may have detrimental metabolic effects, given GH’s insulin-antagonistic, diabetogenic actions (12,13).
Longitudinal studies have compared glucose challenge parameters in TS girls before initiation and after completion of GH treatment (14,15,16). These studies generally found that insulin resistance increased; however, the girls were typically prepubertal at baseline and pubertal or on estrogen treatment at the conclusion on the study, and 46,XX controls showed similar changes (14). The percentage of cases with IGT, however, did not increase overall compared with baseline (14,15,16). Only two, very short-term studies have employed age-matched TS controls to investigate GH effects on glucose tolerance, and these had somewhat divergent conclusions. In a small study of 12 TS girls, Gravholt et al. (17) found increased insulin resistance after 60 d GH treatment. Wilson et al. (18) compared glucose tolerance in untreated, GH-treated, oxandrolone-treated, and GH- and oxandrolone-treated girls with TS. Both oxandrolone groups showed increased insulin resistance after 1 yr treatment, whereas the GH-only group was no different from untreated control TS girls. All the above noted studies took place in the context of clinical research study, and it remains uncertain how GH use outside of monitored clinical trials will affect glucose tolerance and diabetes risk for girls with TS.
Subjects and Methods
Study subjects
Study subjects were participants in an NICHD Institutional Review Board-approved natural history protocol. Adults and parents of minor children gave written informed consent and minors informed assent. The protocol includes studies of bone mineral density, metabolic function, and cardiovascular imaging. Study participants were recruited through notices on the NIH website (http://turners.nichd.nih.gov/) and the Turner Syndrome Society website (www.turner-syndrome-org/). Study subjects were phenotypic females with a 50-cell peripheral karyotype in which 70% or more of cells demonstrated loss of all or part of the second sex chromosome, euthyroid, and in good general health.
The study participants, age 7–21 yr, and their caregivers were queried about history of GH use, including age of initiation and duration of treatment. In addition to a questionnaire and personal interview, medical records were reviewed to confirm GH history. The information gathered included elements of socioeconomic status and reasons for using or not using GH. Girls were treated with standard doses of 0.03–0.05 mg/kg·d, administered as six or seven nightly injections per week. All subjects discontinued GH and estrogen treatment on admission to the clinical center for testing. Most girls 13 and older were on estrogen regimens. Younger girls were on transdermal patches of 14 or 25 μg estradiol/d or conjugated equine estrogen 0.325 mg; older girls were on 100-μg patches and conjugated equine estrogens (0.625–1.25 mg/d), and some on oral contraceptive. The karyotype distribution for this group was 58% 45,X; 23% mosaics for 45,X and second cell line either normal 46,XX (10 subjects) or abnormal X including mainly isoXq and ring X (13);10% 46,XiXq; 6% 46,X,delXp; and 3% 46,X,delXq.
Clinical protocol
Subjects were studied during an inpatient stay at the Clinical Research Center of the NIH. Each subject had a medical history and physical examination and underwent abdominal magnetic resonance imaging (MRI) for abdominal fat estimation, and whole-body dual energy x-ray absorptiometry (DXA) for body composition analysis. DXA was performed using the Hologic QDR2000 instrument in the pencil beam mode, as previously described (19). MR images obtained at L2–L3 and L4–L5 were processed on a GE AW4.1 work station to determine abdominal sc and intraabdominal visceral fat areas. The external body surface was outlined manually, and then a threshold criterion was applied to separate fat from other tissues. The threshold was determined visually by adjusting a slider bar. The total number of pixels meeting the fat intensity criterion within the region of interest was obtained, and an estimated total body fat volume was calculated by multiplying the number of pixels meeting the fat intensity criterion by the volume of each pixel. Then the inner margin of the sc adipose tissues was outlined, and the visceral fat within that outline was removed to retain only the sc fat. The volume of the sc fat was determined from the total volume of the remaining voxels meeting the fat intensity criterion. The volume of visceral fat was obtained by subtracting the sc fat from the total body fat. The analysis was performed on slices at L2–L3 and L4–L5. Girls that could not tolerate the MR environment did not have abdominal fat measurements. Glucose homeostasis was evaluated by measurement of fasting glucose and insulin and a standard oral glucose tolerance test (7). IGT was defined as blood glucose of 140 mg/dl or higher at the 2-h time point.
Statistics
Data are presented as means with sd or as proportions. Comparisons of group means were by ANOVA or analysis of covariance (ANCOVA) using as covariates the potentially confounding factors of age and body mass index (BMI) where appropriate. Proportions were compared by the χ2 test. Logistic regression was used to assess impact of age at diagnosis, race, and karyotype on GH use. All analyses were performed on StatView version 5.0.1 (SAS Institute Inc., Cary, NC).
Results
Study population
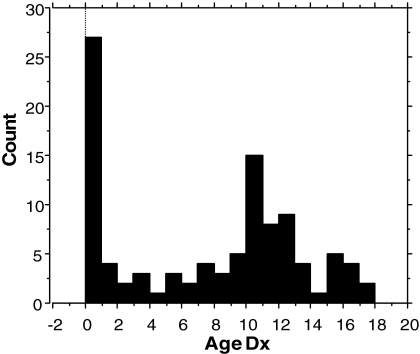
A total of 102 girls with TS participated in this study. These included all participants 21 yr old and younger. The distribution of ages at diagnosis for these girls is shown in Fig. 1. Approximately 5% were diagnosed prenatally and about 20% at birth. Another approximately 25% were diagnosed during childhood before the 10th birthday, and the remaining approximately 50% were diagnosed from 10–18 yr of age. The average age of diagnosis for the group as a whole was 7.2 with sd of 5.6 yr. Approximately 75% of the girls were GH treated for at least 6 months, and about 25% were never treated with GH. Reasons given for not using GH included a delayed diagnosis at age 13 or later, when practitioner or parents considered it too late to undertake GH treatment, were satisfied with the girl’s height, or preferred to initiate puberty as soon as possible (10 of the 26 untreated girls) or a very recent diagnosis and hadn’t yet started treatment (eight of 26); the remainder included a few parents concerned about potential adverse effects, a few that disliked the injection protocol, and two lost to medical follow-up. No individuals had medical contraindications for GH treatment, e.g. diabetes mellitus or cancer. The average age at diagnosis was 11.2 yr for the untreated group vs. 5.8 yr for the treated group (P < 0.001).
Figure 1.
Distribution of ages of TS diagnosis (Dx) for 102 girls participating in the NIH study from 2001–2007. The total for 0–1 yr includes five subjects diagnosed by prenatal testing.
The average age for GH treatment initiation was 8.7 ± 3.3 yr, and average treatment duration was 4.0 ± 3.31 yr. The median was 3.0 yr and range 0.5–14 yr. A few participants were treated as part of a clinical study, but most were treated by community-based pediatric endocrinologists. The group as a whole was about 82% White, 11% Hispanic, 5% Black, and 2% Asian. Because of the small number of non-White participants, statistical analyses on the role of race or ethnicity are limited, but 77% of the White participants and 61% of the non-White participants received GH treatment (P = 0.15). The karyotype distribution in GH-treated and untreated groups is shown in Table 1. Logistic regression examining the effects of age at diagnosis, race, and karyotype on GH use indicated a highly significant impact of age at diagnosis (P = 0.003), a slight effect of a mosaic karyotype (P = 0.03), and a statistically insignificant effect of race (P = 0.08).
Table 1.
Karyotype and GH treatment in TS
| GH use | 45,X | Mosaica | 46,X,iXq | 46,X,delXp | 46,X,delXq | Totals |
|---|---|---|---|---|---|---|
| No | 10 (17%) | 10 (30%) | 3 (21%) | 1 (14%) | 2 (40%) | 26 |
| Yes | 49 | 23 | 11 | 6 | 3 | 76 |
| Totals | 59 | 33 | 14 | 7 | 5 | 102 |
The percentages indicate the proportion of each karyotype group not treated with GH.
A mosaic karyotype was significantly associated with nonuse of GH in a logistic regression model including race and age at diagnosis.
Body composition and glucose tolerance
The initial goal of this study was to examine the role of adiposity in the increased prevalence of IGT reported in girls with TS (3,4,20). To this end, we measured glucose tolerance by oral glucose tolerance test, total body fat content by DXA, and central fat accumulation by abdominal MRI. At the first level of analysis (Table 2), it was apparent that adiposity and IGT were rare in GH-treated girls, including those that had discontinued as well as those currently on treatment. Thus, the study focuses on the comparison of adiposity and glucose tolerance in GH-treated vs. untreated groups. The adiposity and IGT reported for girls with TS in early studies before GH use was confirmed in our group that had never used GH but not in the GH-treated group, including girls currently on GH and those that had finished GH treatment (Table 2). These unexpected findings were quite striking, and the statistical significance was very robust even withstanding a rigorous correction for multiple comparisons.
Table 2.
GH use, body composition, and glucose tolerance in TS
| GH (76) | No GH (26) | P | |
|---|---|---|---|
| Age (yr) | 13.9 ± 3.6 | 13.6 ± 3.7 | NS |
| BMI (kg/m2) | 22.2 ± 4.7 | 25.1 ± 7.2 | 0.002a |
| Height sd | -1.93 ± 0.8 | −2.51 ± 1.3 | 0.009a |
| BF (%) | 28.2 ± 8.3 | 35 ± 7.7 | P < 0.0001b |
| SAT (ml)c | 99.5 ± 81.8 | 183.2 ± 37.5 | 0.001b |
| VAT (ml)c | 33 ± 13.7 | 49.8 ± 7.8 | 0.0009b |
| IGT | 7% | 28% | 0.006 |
SAT and VAT were measured by MRI. IGT indicates blood glucose more than 140 mg/dl on oral glucose tolerance test. Data are means ± sd or proportion. Prevalence of IGT was compared by χ 2. GH was not administered the day before or day of metabolic testing. P values remain significant at P < 0.01 after Bonferroni correction. BF, Total body fat measured by DXA.
Group means were compared by ANCOVA including age as covariate.
Group means were compared by ANCOVA including age and BMI as covariates.
Fifty-two GH-treated and 17 untreated girls had MRI abdominal fat measurements.
Prepubertal GH treatment, body composition, and glucose tolerance
To isolate the potentially confounding effects of estrogenization, we next examined body composition and glucose tolerance in estrogen-naive girls that had no signs of spontaneous puberty (i.e. Tanner 1 breasts) and had not started estrogen treatment (Table 3). The GH-treated group had received GH for an average of 3.0 ± 0.4 yr. These girls had about 50% less total body and sc abdominal fat tissue (SAT) compared with untreated girls. Visceral abdominal fat tissue (VAT) was slightly but not significantly lower in the GH-treated group. Most notably, about 30% of untreated girls demonstrated IGT, whereas none of the GH-treated did (P = 0.001). All of the GH-treated girls in this group were on current treatment but had not received a GH dose the night before testing.
Table 3.
Prepubertal GH treatment, body composition, and glucose tolerance
| GH (36) | No GH (18) | P | |
|---|---|---|---|
| Age (yr) | 10.7 ± 03.4 (7–16) | 12.5 ± 2.8 (7–16) | 0.02 |
| BMI (kg/m2) | 20.3 ± 3.7 | 23.2 ± 4.1 | 0.01a |
| BF (%) | 24.6 ± 3.2 | 34.4 ± 4.5 | <0.0001b |
| SAT (ml)c | 70.3 ± 12.3 | 112.0 ± 21.8 | 0.0003b |
| VAT (ml)c | 29.9 ± 7.0 | 35.9 ± 6.6 | 0.19b |
| IGT | 0 | 5/17 | 0.001 |
These data are for girls currently on GH. Data are means ± se. IGT was compared by χ 2.
Group means were compared by ANCOVA including age as covariate.
Group means were compared by ANCOVA including age and BMI as covariates.
There were 24 GH-treated and 11 untreated girls that had MRI abdominal fat measurements
Previous GH treatment, body composition, and insulin sensitivity in pubertal girls with TS
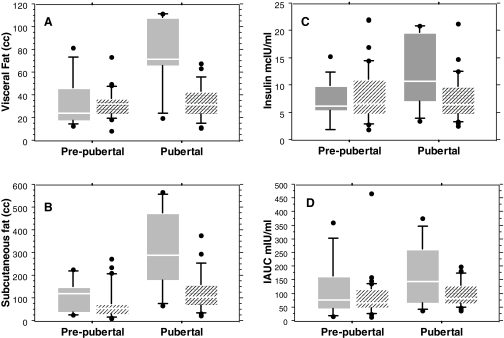
Table 4 summarizes body composition and glucose tolerance in estrogenized girls previously treated with GH, compared with estrogenized girls never treated with GH. They had discontinued GH treatment on average 2.2 yr before testing, after 5.1 ± 0.7 (1–14) yr of use. Pubertal girls with TS not treated with GH had abnormally enlarged abdominal fat depots and insulin resistance that was not seen in their GH-treated peers (Table 4). Abdominal SAT and VAT were approximately 2-fold greater in untreated pubertal girls, and this concentration of abdominal fat was significantly associated with elevated insulin levels at baseline and in response to oral glucose tolerance test. In contrast, there was little pubertal increase in visceral fat in GH-treated girls. Pre- and postpubertal changes in visceral and sc abdominal fat and insulin sensitivity in GH-treated vs. untreated groups are shown graphically in Fig. 2. IGT was found in two of eight (25%) untreated and three of 27 (11%) GH-treated girls, although this difference was not statistically significant because of small sample size. The greater sc and especially visceral adiposity was directly contributing to the development of insulin resistance, measured by elevated fasting insulin (P = 0.0002), quantitative insulin sensitivity check index (P = 0.009), and incremental area under the curve for insulin (P < 0.0001).
Table 4.
Previous GH treatment, body composition, and glucose tolerance in pubertal girls with TS
| GH (27) | No GH (8) | P | |
|---|---|---|---|
| Age (yr) | 17.4 ± 2.4 (13–21) | 16.9 ± 3.5 (15–19) | 0.5 |
| BMI (kg/m2) | 24.4 ± 3.8 | 30.7 ± 5.3 | 0.01a |
| BF (%) | 33.7 ± 1.8 | 39.5 ± 3.4 | 0.03b |
| SAT (ml)c | 142.5 ± 22.4 | 313.8 ± 75.6 | <0.0001b |
| VAT (ml)c | 36.1 ± 4.1 | 75.3 ± 13.8 | <0.0001b |
| Fasting insulin (μIU/ml) | 7.3 ± 2.8 | 12.5 ± 4.4 | 0.005b |
| IAUC (IU/min·ml) | 9.9.1 ± 2.9 | 17.3 ± 4.2 | 0.0001b |
These girls had all discontinued GH or were never on GH. Data are means ± se. The number of GH-treated girls in Tables 3 and 4 does not equal 76 because some were still on GH during puberty (either spontaneous or estrogen treated, n = 10) and some had discontinued GH but not started estrogen (n = 3). P values for SAT, VAT, fasting insulin, and incremental area under the curve for insulin (IAUC) remain significant at <0.008 after the Bonferroni correction.
Group means were compared by ANCOVA including age as covariate.
Group means were compared by ANCOVA including age and BMI as covariates.
Twenty-three GH-treated and six untreated girls had MRI abdominal fat measurements.
Figure 2.
Pre- and postpubertal effects of GH treatment on visceral (A) and sc (B) abdominal fat and insulin sensitivity (C and D) in girls with TS. Hatched boxes indicate GH-treated groups and solid boxes nontreated groups. The line dividing each box is the median, and the box itself encompasses 25–75th percentiles, with the ends of the whiskers marking 10th and 90th percentiles.
Potential bias in GH-treated vs. untreated groups
Because groups were not randomized for GH use, we tried to determine whether untreated girls might preferentially come from lower socioeconomic or minority groups with limited access to healthcare. However, there was no significant difference in racial distribution in GH-treated vs. nontreated groups (see above), and parental educational levels were similar in treated vs. untreated groups (e.g. maternal years education were 13.8 ± 1.7 for GH users vs. 15.08 ± 1.8 for nonusers). Participants specifically denied economic reasons for not using GH. Another potential source of bias is that more severely affected (e.g. shortest) girls may be more likely to get an early diagnosis and GH treatment. Consistent with this view, 45,X karyotypes were more common in the GH-treated group (Table 1). However, the possibility that GH users are more severely affected makes the present findings of better body composition and glucose homeostasis in GH-treated girls more remarkable.
Discussion
There are some unique aspects of the present study’s design that are worth mentioning. First, this is the only sizeable study investigating the effects of GH on glucose homeostasis and body composition in TS with a contemporaneous untreated control group and the only study specifically examining abdominal fat accumulation. Second, the girls in this study were receiving community-based care, in contrast to previous studies all based on highly structured and closely supervised clinical trials. We found that GH-treated girls were leaner, with less abdominal fat and normal glucose tolerance compared with never-treated girls in the current study and compared with TS girls in studies before the era of GH treatment (3,5,21,22,23,24). The untreated group demonstrated a striking accumulation of intraabdominal fat, or VAT, that was not seen in the GH-treated group. Visceral fat does not normally increase to this extent in pubertal girls, although it may in boys (25). The excessive abdominal adiposity in untreated girls was associated with reduced insulin sensitivity and IGT. Thus, girls treated with GH during childhood seemed to avoid the development of central, abdominal adiposity and the adverse metabolic phenotype typical of girls with TS. The present findings are novel and remarkable because it was predicted by some that GH treatment would increase insulin resistance and risk for diabetes in girls with TS. To the contrary, this study suggests that untreated girls may be at greater risk for insulin resistance and diabetes due to their excessive adiposity.
These findings suggest that GH’s salutary effects on body composition outweigh acute effects of insulin antagonism in girls with TS. GH’s antiinsulin effects stem from its role as a counterregulatory hormone, mobilizing lipids from adipose tissue to provide muscle-sparing energy substrate. The GH-induced acute elevation of free fatty acids impairs insulin-stimulated glucose uptake by muscle. These GH effects may be beneficial evolutionarily because they promote extraction of fat for energy utilization and sparing of muscle during fasting or famine. GH administered at bedtime stimulates intense lipolysis with liberation of free fatty acids that are elevated through the morning hours (26). Previous diagnoses of insulin resistance associated with GH treatment were based on metabolic studies carried out the morning after a bedtime GH dose (14,15,17,27). In the present study and in an earlier study by Wilson et al. (18), GH was not administered the night before testing, and neither study found insulin resistance in GH-treated girls compared with contemporaneous TS controls. In fact, both studies found fewer IGT cases in GH-treated groups. These observations suggest that insulin resistance noted in proximity to GH dosing is without lasting adverse effects.
The metabolic phenotype of postpubertal TS girls is very similar to that of GH-deficient patients, i.e. excessive abdominal adiposity associated with insulin resistance that is reversed with GH treatment (28). Girls with TS are treated with pharmacological GH to increase final height but are not usually thought to be GH deficient as part of the syndrome. Evaluation of the GH-IGF system is complicated, however, by differences in body composition and estrogen status of girls and women with TS compared with conventional controls (29). GH treatment may be helpful in non-GHD individuals with visceral obesity by reducing abdominal fat and improving insulin sensitivity (28). As noted above, the accumulation of visceral fat around the time of puberty in girls with TS is more typical of males than females (25). This trait may be related to the fact that the majority of girls with TS, similar to males, carry a single normal maternal X-chromosome, which is associated with excessive visceral adiposity, independent of sex steroid effects (30).
The persistence of the beneficial effects on body composition among girls with TS in the years after cessation of GH therapy was unexpected. In older adults, GH’s anabolic effects are generally transient and recede within months after discontinuation of GH. It is possible, as a matter of speculation, that GH may have more persistent effects on body composition in children than in older adults. For instance, GH may reduce adipocyte cell number or volume and/or increase myocyte mass. Alternatively, or in addition, it is likely that improved physical fitness and self-esteem associated with GH treatment may lead to adoption of a healthy active lifestyle with ongoing salutary effects. In addition, GH-treated girls were probably under closer medical surveillance than untreated girls and may have benefited from medical advice on nutrition and weight control. Clearly, more study with long-term follow-up of treated vs. untreated girls is essential to clarify our observations.
Potential selection bias is an important concern in any nonrandomized study. In this case, one might consider that obese girls are less likely to be diagnosed early or less likely to be offered GH treatment. However, no study subject reported not being offered or advised against the use of GH because of obesity or any other medical concern. Moreover, obesity should attract more rather than less medical attention. However, overweight is associated with taller height in many children, so they might have been less likely to be identified because of short stature. The fact that a high prevalence of obesity (22,23,24) and IGT (3,4,5) was found among (non-GH-treated) girls with TS years ago suggests that our observations in untreated girls reflect the typical metabolic phenotype in TS and that the healthy body composition and glucose tolerance in the GH-treated groups is due to GH treatment rather than selection bias. Because GH treatment is known to reduce adiposity and improve body composition in other disorders, the improved body composition in our GH-treated groups is biologically plausible.
Clearly, these interesting and novel findings need further investigation, with ongoing longitudinal study to determine the longevity of the relative protection from abdominal adiposity in GH-treated girls.
Footnotes
This research was entirely supported by NICHD Division of Intramural Research.
Disclosure Statement: N.W., V.B., S.H., and C.B. have nothing to declare.
First Published Online March 18, 2008
Abbreviations: ANCOVA, Analysis of covariance; BMI, body mass index; DXA, dual-energy x-ray absorptiometry; IGT, impaired glucose tolerance; MRI, magnetic resonance imaging; SAT, sc adipose fat tissue; TS, Turner syndrome; VAT, visceral adipose fat tissue.
References
- Forbes AP, Engel E 1963 The high incidence of diabetes mellitus in 41 patients with gonadal dysgenesis, and their close relatives. Metabolism 12:428–439 [PubMed] [Google Scholar]
- Gravholt CH, Juul S, Naeraa RW, Hansen J 1998 Morbidity in Turner syndrome. J Clin Epidemiol 51:147–158 [DOI] [PubMed] [Google Scholar]
- Polychronakos C, Letarte J, Collu R, Ducharme JR 1980 Carbohydrate intolerance in children and adolescents with Turner syndrome. J Pediatr 96:1009–1014 [DOI] [PubMed] [Google Scholar]
- Cicognani A, Mazzanti L, Tassinari D, Pellacani A, Forabosco A, Landi L, Pifferi C, Cacciari E 1988 Differences in carbohydrate tolerance in Turner syndrome depending on age and karyotype. Eur J Pediatr 148:64–68 [DOI] [PubMed] [Google Scholar]
- AvRuskin TW, Crigler JF, Jr., Soeldner JS 1979 Turner’s syndrome and carbohydrate metabolism. I. Impaired insulin secretion after tolbutamide and glucagon stimulation tests: evidence of insulin deficiency. Am J Med Sci 277:145–152 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Naeraa RW, Nyholm B, Gerdes LU, Christiansen E, Schmitz O, Christiansen JS 1998 Glucose metabolism, lipid metabolism, and cardiovascular risk factors in adult Turner’s syndrome. The impact of sex hormone replacement. Diabetes Care 21:1062–1070 [DOI] [PubMed] [Google Scholar]
- Bakalov VK, Cooley MM, Quon MJ, Luo ML, Yanovski JA, Nelson LM, Sullivan G, Bondy CA 2004 Impaired insulin secretion in the Turner metabolic syndrome. J Clin Endocrinol Metab 89:3516–3520 [DOI] [PubMed] [Google Scholar]
- Caprio S, Boulware S, Diamond M, Sherwin RS, Carpenter TO, Rubin K, Amiel S, Press M, Tamborlane WV 1991 Insulin resistance: an early metabolic defect of Turner’s syndrome. J Clin Endocrinol Metab 72:832–836 [DOI] [PubMed] [Google Scholar]
- Salgin B, Amin R, Yuen K, Williams RM, Murgatroyd P, Dunger DB 2006 Insulin resistance is an intrinsic defect independent of fat mass in women with Turner’s syndrome. Horm Res 65:69–75 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Naeraa RW 1997 Reference values for body proportions and body composition in adult women with Ullrich-Turner syndrome. Am J Med Genet 72:403–408 [DOI] [PubMed] [Google Scholar]
- Ostberg JE, Thomas EL, Hamilton G, Attar MJH, Bell JD, Conway GS 2005 Excess visceral and hepatic adipose tissue in Turner syndrome determined by magnetic resonance imaging: estrogen deficiency associated with hepatic adipose content. J Clin Endocrinol Metab 90:2631–2635 [DOI] [PubMed] [Google Scholar]
- Fowelin J, Attvall S, von Schenck H, Smith U, Lager I 1991 Characterization of the insulin-antagonistic effect of growth hormone in man. Diabetologia 34:500–506 [DOI] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO, Moller J, Orskov L, Ovesen P, Schmitz O, Christiansen JS, Orskov H 1995 Metabolic effects of growth hormone in humans. Metabolism 44:33–36 [DOI] [PubMed] [Google Scholar]
- Radetti G, Pasquino B, Gottardi E, Boscolo Contadin I, Aimaretti G, Rigon F 2004 Insulin sensitivity in Turner’s syndrome: influence of GH treatment. Eur J Endocrinol 151:351–354 [DOI] [PubMed] [Google Scholar]
- Van Pareren YK, De Muinck Keizer-Schrama SM, Stijnen T, Sas TC, Drop SL 2002 Effect of discontinuation of long-term growth hormone treatment on carbohydrate metabolism and risk factors for cardiovascular disease in girls with Turner syndrome. J Clin Endocrinol Metab 87:5442–5448 [DOI] [PubMed] [Google Scholar]
- Sas T, de Muinck Keizer-Schrama S, Aanstoot HJ, Stijnen T, Drop S 2000 Carbohydrate metabolism during growth hormone treatment and after discontinuation of growth hormone treatment in girls with Turner syndrome treated with once or twice daily growth hormone injections. Clin Endocrinol (Oxf) 52:741–747 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Naeraa RW, Brixen K, Kastrup KW, Mosekilde L, Jorgensen JOL, Christiansen JS 2002 Short-term growth hormone treatment in girls with Turner syndrome decreases fat mass and insulin sensitivity: a randomized, double-blind, placebo-controlled, crossover study. Pediatrics 110:889–896 [DOI] [PubMed] [Google Scholar]
- Wilson DM, Frane JW, Sherman B, Johanson AJ, Hintz RL, Rosenfeld RG 1988 Carbohydrate and lipid metabolism in Turner syndrome: effect of therapy with growth hormone, oxandrolone, and a combination of both. J Pediatr 112:210–217 [DOI] [PubMed] [Google Scholar]
- Ari M, Bakalov VK, Hill S, Bondy CA 2006 The effects of growth hormone treatment on bone mineral density and body composition in girls with Turner syndrome. J Clin Endocrinol Metab 91:4302–4305 [DOI] [PubMed] [Google Scholar]
- Rasio E, Antaki A, Van Campenhout J 1976 Diabetes mellitus in gonadal dysgenesis: studies of insulin and growth hormone secretion. Eur J Clin Invest 6:59–66 [DOI] [PubMed] [Google Scholar]
- Ross JL, Feuillan P, Long LM, Kowal K, Kushner H, Cutler Jr GB 1995 Lipid abnormalities in Turner syndrome. J Pediatr 126:242–245 [DOI] [PubMed] [Google Scholar]
- Hanaki K, Ohzeki T, Ishitani N, Motozumi H, Matsuda-Ohtahara H, Shiraki K 1992 Fat distribution in overweight patients with Ullrich-Turner syndrome. Am J Med Genet 42:428–430 [DOI] [PubMed] [Google Scholar]
- Lu PW, Cowell CT, Jimenez M, Simpson JM, Silink M 1991 Effect of obesity on endogenous secretion of growth hormone in Turner’s syndrome. Arch Dis Child 66:1184–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianfarani S, Vaccaro F, Pasquino AM, Marchione SA, Passeri F, Spadoni GL, Bernardini S, Spagnoli A, Boscherini B 1994 Reduced growth hormone secretion in Turner syndrome: is body weight a key factor? Horm Res 41:27–32 [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Clark PA, Walter K, Patrie J, Weltman A, Rogol AD 2000 Pubertal alterations in growth and body composition. V. Energy expenditure, adiposity, and fat distribution. Am J Physiol Endocrinol Metab 279:E1426–E1436 [DOI] [PubMed] [Google Scholar]
- Kousta E, Chrisoulidou A, Lawrence NJ, Anyaoku V, Al-Shoumer KA, Johnston DG 2000 The effects of growth hormone replacement therapy on overnight metabolic fuels in hypopituitary patients. Clin Endocrinol (Oxf) 52:17–24 [DOI] [PubMed] [Google Scholar]
- Sas TC, Muinck Keizer-Schrama SM, Stijnen T, Aanstoot HJ, Drop SL 2000 Carbohydrate metabolism during long-term growth hormone (GH) treatment and after discontinuation of GH treatment in girls with Turner syndrome participating in a randomized dose-response study. Dutch Advisory Group on Growth Hormone. J Clin Endocrinol Metab 85:769–775 [DOI] [PubMed] [Google Scholar]
- Attallah H, Friedlander AL, Hoffman AR 2006 Visceral obesity, impaired glucose tolerance, metabolic syndrome, and growth hormone therapy. Growth Horm IGF Res 16:62–67 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Veldhuis JD, Christiansen JS 1998 Increased disorderliness and decreased mass and daily rate of endogenous growth hormone secretion in adult Turner syndrome: the impact of body composition, maximal oxygen uptake and treatment with sex hormones. Growth Horm IGF Res 8:289–298 [DOI] [PubMed] [Google Scholar]
- Van PL, Bakalov VK, Zinn AR, Bondy CA 2006 Maternal X chromosome, visceral adiposity, and lipid profile. JAMA 295:1373–1374 [DOI] [PubMed] [Google Scholar]