Abstract
Context: McCune-Albright syndrome (MAS) is caused by mutations in GNAS (most often R201C or R201H) leading to constitutive cAMP signaling and multiple endocrine dysfunctions, including morphological and functional thyroid involvement.
Objective: The objective of the study was to characterize the clinical and molecular features of the MAS-associated thyroid disease in a large cohort of patients.
Design: This was a retrospective analysis. Setting: The study was conducted at the National Institutes of Health Clinical Center.
Patients: The study included 100 consecutive MAS patients.
Interventions: There were no interventions.
Main Outcome Measure: Functional and morphological evaluation of the thyroid was measured. Ex vivo experiments were performed on MAS thyroid samples to study the effects of the GNAS mutations on the 5′-deiodinases. Reconstitution experiments in HEK-293 cells were performed to study the effects of GNAS mutations on the type-2 5′-deiodinase.
Results: Fifty-four patients had abnormal thyroid ultrasound findings. This group, compared with patients without abnormal findings, had higher T3 to T4 ratio, indicating an elevated 5′-deiodinase activity. Thyroid samples from MAS subjects, compared with normal tissue, showed a significant increase in both type 1 (D1) and type 2 (D2) 5′-deiodinase activity (D1 control 5.9 ± 4.5 vs. MAS 41.7 ± 26.8 fmol/min·mg, P < 0.001; D2 control 28.3 ± 13.8 vs. MAS 153.1 ± 43.7 fmol/min·mg, P < 0.001). Compared with cells transfected with the wild-type R201 allele, the basal transcriptional activity of the D2 promoter was significantly increased in both mutants (C and H) (R 10733 ± 2855, vs. C 18548 ± 4514, vs. H 19032 ± 4410 RLU ± SD, P < 0.001).
Conclusion: Thyroid pathology is a common occurrence in MAS. Consistent with the molecular etiology of the disease, the shift in T3 to T4 ratio is at least in part secondary to a cAMP-mediated intrathyroidal activation of D2 and to elevated D1 activity.
This study characterizes the clinical and molecular features of the thyroid involvement in McCune-Albright syndrome. Thyroid pathology is a common occurrence, and the changes in thyroid hormones levels are secondary to intrathyroidal activation of both type-1 and type-2 deiodinases.
McCune-Albright syndrome (MAS) is a rare disease characterized clinically by the triad of polyostotic fibrous dysplasia, café-au-lait skin spots, and hyperfunctioning endocrinopathies, including precocious puberty, hyperthyroidism, GH excess, and neonatal cortisol excess (1,2,3,4). The genetic and molecular etiology of MAS is sporadic, postzygotic activation mutations of the α-subunit of the G stimulatory protein, Gsα. Gsα is coded for by the gene GNAS and is involved in the generation of the second messenger, cAMP (5,6). More than 95% of all of the mutations in Gsα occur at the R201 position and are either R201H or R201C mutations (7). As a result of the somatic nature of the mutations, patients are mosaics, and tissues that bear the Gsα mutation (sometimes called the gsp oncogene) undergo ligand-independent cAMP signaling activation (8).
Thyroid involvement is one of the more common aspects of the disease, said to be present in up to 30–50% of the patients (9,10). Thyroid pathology includes goitrous and nongoitrous hyperthyroidism associated with elevations in serum T3, and, rarely, thyroid cancer (9,11). Generation of transgenic mice expressing the R201H mutation of Gsα in a thyroid-specific fashion mimicked the human disease in that mice developed abnormal thyroid glands with hyperfunctioning adenomas (12).
The clinical observation that MAS-associated hyperthyroidism is often characterized by a shift toward T3 toxicosis prompted us to hypothesize that, similar to patients with Graves’ disease and toxic nodules (13,14,15), an increase in 5′-deiodinase activity plays a role in the MAS-associated hyperthyroidism pathogenesis. As opposed to rodents, in which only type 1 deiodinase (D1) activity is present in thyroid, in human both D1 and type 2 deiodinase (D2) transcription and activity have been demonstrated (16). Indeed, the D2 activity is greatly increased in Graves’ disease and toxic adenoma, conditions associated with an increase in the G protein-coupled TSH receptor signaling pathway (13,17). The aim of the study was thus to describe in a large cohort of patients with MAS the functional and morphological changes of MAS-associated thyroid disease and to characterize ex vivo and in vitro the role of the 5′-deiodinases in the pathophysiology of MAS-associated hyperthyroidism.
Subjects, Materials, and Methods
Subjects
One hundred consecutive patients enrolled in ongoing National Institutes of Health (NIH) Clinical Center Institutional Review Board-approved studies of MAS were studied. All subjects or their parents gave informed consent. The diagnosis of MAS was made on clinical grounds when subjects had at least two of the classic clinical triad of typical café-au-lait skin spots, fibrous dysplasia of bone, and/or hyperfunctioning endocrinopathies (gonadotropin-independent precocious puberty, hyperthyroidism, GH excess, and/or neonatal Cushing’s syndrome). A subset of 49 subjects had GNAS mutation analysis performed by a sensitive method (18) when relevant clinical material was available for analysis.
All subjects had serum T3, T4, and TSH measured by enzyme immunoassay in the Department of Laboratory Medicine of the NIH, and the thyroid hormone data were also expressed as ratio T3 to T4 (nanograms per milligrams), with a ratio greater than 20 representing an index of thyrotoxicosis, as previously reported (19). All subjects underwent ultrasonographic evaluation of the thyroid, and the scans were reviewed by an experienced radiologist (T.S.), who was unaware of the hormonal status of the patients. Patients were grouped according to those who did and did not have MAS-related ultrasound abnormalities. Ultrasound abnormalities that were classified as MAS related included diffuse inhomogeneity, and hyper-and hypoechoic regions, as previously described (9). Representative clinical and ultrasound images are shown (Fig. 1). Isolated, solid nodules in subjects older than 40 yr of age and with normal thyroid function tests were considered not to be MAS related.
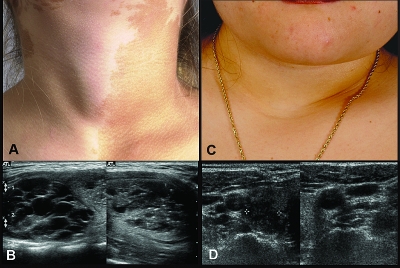
Figure 1.
Clinical images of thyroid pathology in MAS patients. Left panels, Photograph of a patient with a typical café-au-lait spot and obvious goiter; the associated ultrasound findings are shown (A and B). Right panels, MAS patient with no palpable goiter but with clear evidence of ultrasound findings is shown (C and D).
Reagents
Chemical reagents were purchased from Sigma (St. Louis, MO). DMEM, fetal bovine serum, penicillin-streptomycin, and geneticin were obtained from Life Technologies (Carlsbad, CA). 125I thyroxine was purchased from PerkinElmer (Boston, MA). AG50W chromatography columns were purchased from Bio-Rad (Hercules, CA), LH 20 chromatography columns were purchased from Pharmacia (Uppsala, Sweden).
Tissue collection
Thyroid tissue samples were collected from five MAS patients, three patients affected by toxic nodule, and from areas of normal thyroid parenchyma from five patients undergoing total thyroidectomy for thyroid cancer. Patients provided informed consent on an institutional review board-approved protocol, and materials were received via approval of research activity through the Office of Human Subject Research. Tissue samples were snap frozen in liquid nitrogen and stored in a liquid nitrogen tank.
Plasmids
The coding regions of the human GNAS gene and the R201C and R201H mutations were subcloned from an expression vector [kind gift of Dr. Larry Fisher, National Institute of Health Dental and Craniofacial Research (NIDCR)-NIH] into pcDNA3.1/His-myc from Invitrogen (Carlsbad, CA). The accuracy of the insert was confirmed by sequence of both strands; plasmid endotoxin-free stock solutions were prepared according to established methods (QIAGEN, Valencia, CA). The D2 promoter was previously cloned in pGL3 reporter vector (20), and the promoter-luciferase cassette was transferred into a pcDNA3.1 vector (pcDNA 3.1/D2pro-luc) carrying the G418 resistance gene for stable transfections selection.
Real-time PCR and sequencing
Total RNA from normal thyroid (five subjects), toxic adenoma (three subjects), and MAS (five subjects) were extracted from tissue samples using the RNAzol method, and 500 ng of sample was converted to first-strand cDNA using a reverse transcriptase kit from Marligen (Ijamsville, MD) according to the manufacturer’s instructions. The cDNA was then amplified in a TaqMan 7000 real-time PCR apparatus using β-actin, D1, and D2 TaqMan gene expression assays (Applied Biosystems, Foster City, CA). For each experiment 10 ng of cDNA samples were tested in duplicate. Real-time PCR experiments were performed in triplicate and RNA levels were expressed as arbitrary units (AU) ± 1 sd after correction for β-actin. A 241- and 221-bp fragment of GNAS gene encompassing, respectively, codons 201 and 228, were amplified from the thyroid cDNA samples using the following primers: 5′-TGAACGTGCCTGACTTTGAC-3′ (sense) and 5′-TCCACCTGGAACTTGGTCTC-3′ (antisense) and 5′-GCCCAGTACTTCCTGGACAA-3′ (sense) and 5′-ACCACGAAGATGATGGCAGT-3′ (antisense) using Platinum Taq polymerase (Invitrogen), and sequenced on both strands by BigDye terminator version 3.1 using an ABI PRISM 3730XL analyzer (Applied Biosystems).
Deiodinase assay
125I-thyroxine was purified using LH-20 columns before each experiment. Tissue samples were briefly homogenized in 0.25 m sucrose, 0.02 m Tris/HCl (pH 7.0), and 1 mm EDTA. After centrifugation at 1000 × g, the supernatant was collected and stored at −80 C. Deiodinase activity of tissue extracts was measured according to previously described methods (21). Each reaction was performed in the presence or absence of 5 mm propylthiouracil (PTU; used as a D1 inhibitor). After counting the total radioactivity with a γ-counter, the 125I released was separated by ion-exchange chromatography in AG50W-X8 columns, and the effluent was measured in a γ-counter. Each data point was measured in triplicate; data were expressed as velocity of deiodination (femtomoles per minute per milligram protein) ± 1 sd after correction for nonspecific deiodination.
Cell culture
HEK-293 cells stably expressing the human D2 promoter (Hek-293/D2prom) were cultured in 75-cm2 culture flasks in DMEM supplemented with 10% fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, and 300 μg/ml geneticin. Transient transfections were performed in 60-mm dishes using Fugene6 (Roche, Indianapolis, IN) according to the manufacturers’ instructions.
Stable transfection
HEK-293 cells were used for the stable transfection. Sixty-millimeter plates were transfected with pcDNA3.1/D2pro-luc using Fugene6. Two days after transfection, the cells were harvested and seeded in 100-mm plates. Then 700 μg geneticin were added to the medium to select transfected cells (Hek-293/D2prom). Clones were then isolated and individually seeded in 96-wells plates. The transfection was confirmed by luciferase assay after forskolin treatment at different concentrations.
Luciferase assay
For each experiment, 16 60-mm plates were seeded with 2 × 105 Hek-293/D2prom. After 24 h, four plates were transfected with 1 μg of Arg201 (R), Cys201 (C), or His201 (H) or with pcDNA3.1/His-myc empty vector. After 48 h, the medium was replaced with serum-free medium, and the cells were incubated for 6 h with either forskolin or dimethylsulfoxide. After the incubation the cells were washed and harvested in cold PBS. Luciferase assay was performed using the luciferase assay system (Promega, Madison, WI). Protein concentration was measured using the bicinchoninic assay method (Pierce, Rockford, IL). Each experiment was performed in triplicate, and data were expressed as relative light units (RLU) ± 1 sd after normalization for protein concentration.
Western blot
Aliquots of cells were lysed in 150 mm NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 2 mm EDTA, 20 mm Tris-HCl, 0.1 mg/ml aprotinin, and 10 μg/ml eupeptin. Fifty micrograms of extracts were separated by gel electrophoresis using a 10% NuPAGE Bis-Tris precast gel (Invitrogen). The electrophoresis gel was blotted on a polyvinyl difluoride 0.2 μm membrane (Invitrogen). Western blot analysis was carried out using a primary antibody anti-myc (Invitrogen), and after incubation with horseradish peroxidase-conjugated secondary antibody the immunocomplex was detected by a chemiluminescence reaction using SuperSignal West Pico chemiluminescent substrate (Pierce).
Statistical analyses
Statistical analysis was performed using Prism 3.0 (GraphPad Software Inc., San Diego, CA) by unpaired t test or one-way ANOVA and Tukey post hoc analysis. Results are expressed as mean ± 1 sd; an α-error of 0.05 was considered the threshold for statistical significance. Unless otherwise reported, each experiment was performed in triplicate, and each data point represents the average of three observations.
Results
Clinical findings
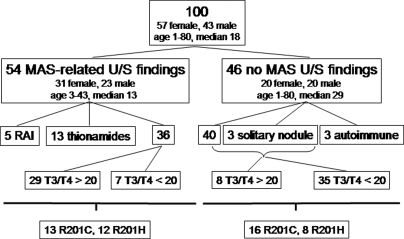
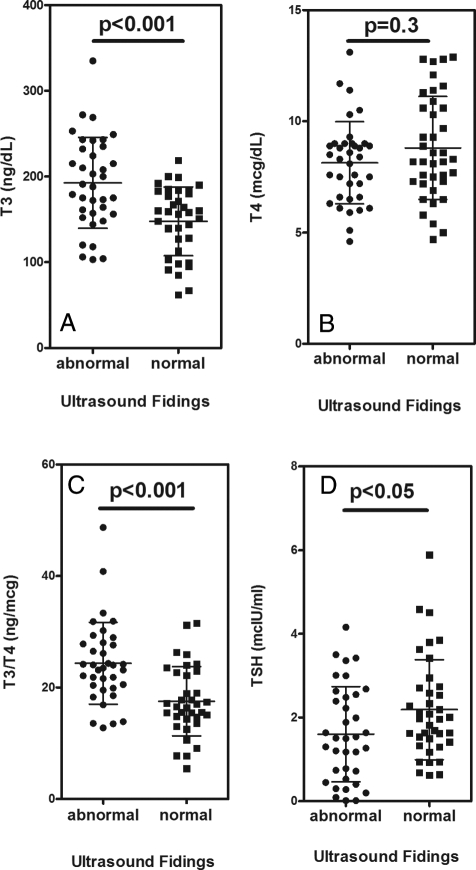
Patient demographics and characteristics are shown in Fig. 2. Fifty-four of the patients (31 females, 23 males, age range 3–43 yr, median 13 yr) had MAS-related ultrasound findings, and 46 (26 females, 20 males, age range 1–80, median 29 yr) had either a normal thyroid ultrasound or age-related abnormal ultrasound findings, i.e. older than 40 yr of age with a solitary nodule and normal thyroid function tests. The group with MAS-related ultrasound findings was significantly younger (P < 0.01). The ultrasound findings were relatively dichotomous, i.e. except for the finding of solitary nodules in three adult women, the glands were either normal or clearly involved with mixed cystic and solid lesions. Five of the patients in the MAS-related group had been previously treated with radioactive iodine and 13 were being treated with either PTU (nine subjects) or MMI (four subjects); therefore, these 18 subjects were excluded from the statistical analyses of the thyroid function tests. In addition, three patients in the non-MAS findings group who had autoimmune thyroid disease on thyroid hormone replacement therapy were not included in the statistical analyses. Seven subjects with MAS-related findings had a suppressed TSH (TSH < 0.4 mIU/liter) at presentation and were not on medication. Whereas the vast majority of patients with MAS-related ultrasound findings had a T3 to T4 ratio greater than 20 (29 of 36, 81%), the opposite was observed in the group without MAS-related ultrasound findings (eight of 43, 19%, relative risk 5.0, confidence interval 2.5–10.2, P < 0.0001). Subjects with MAS-related findings had higher T3 values and a higher T3 to T4 ratio (Fig. 3). Serum TSH levels were significantly lower in the MAS-related ultrasound findings group, compared with the non-MAS findings group (1.61 ± 1.14 vs. 2.19 ± 1.19 mIU/liter, P < 0.05; Fig. 3). There were no differences in ultrasonographic findings or thyroid function test findings between subjects with either R201C or R201H mutations.
Figure 2.
Study patients. One hundred consecutive patients with MAS were studied. Fifty-four had ultrasound (U/S) findings consistent with MAS (see text), and 46 did not. The group with MAS-related findings were younger (median age 13 vs. 29 yr, P < 0.01). Of the 54 with thyroid involvement, 18 were treated and therefore not included in the analysis of the T3 to T4 ratio (nanograms per microgram). Of the 46 without MAS-related U/S findings, three (all women > 40 yr old) had a solitary nodule, and three had antibody-positive, Hashimoto’s thyroiditis. Of the 36 patients with MAS-related U/S findings, 29 had a T3 to T4 ratio greater than 20, and seven less than 20. Of the 43 without ultrasound findings, 35 had a T3 to T4 ratio less than 20, and eight (all children) had a ratio greater than 20. Patients with MAS-related U/S findings were more likely to have a T3 to T4 ratio greater than 20 (relative risk 5.0, confidence interval 2.5–10.2, P < 0.0001). There was no difference in the prevalence of GNAS R201C or R201H mutations between the groups with and without MAS-related U/S findings.
Figure 3.
Thyroid function in MAS patients. Study subjects were grouped according to the presence or absence of ultrasound (•, abnormal; ▪, normal) findings; patients treated for hyperthyroidism or on replacement therapy were excluded form the analysis (see text for details). A, Serum total T3 levels. B, Serum total T4 levels. C Serum T3 to T4 ratio (nanograms per microgram). D Serum TSH levels.
Real-time PCR
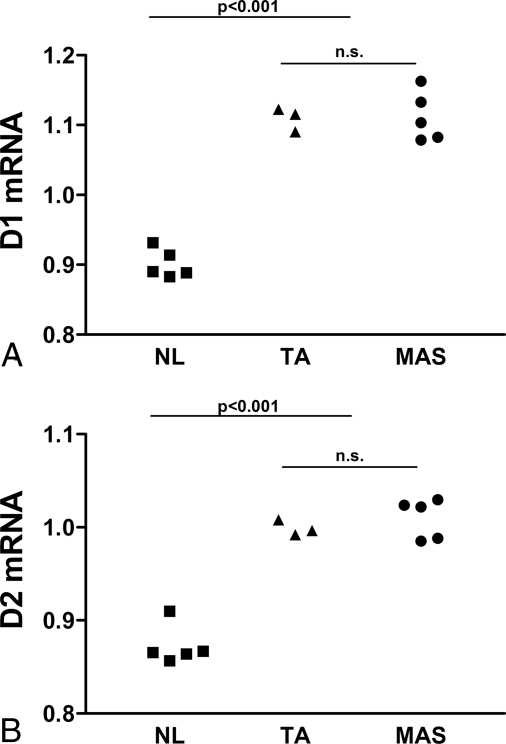
Real-time PCR experiments demonstrated that, compared with normal thyroid tissue, the MAS samples showed a modest but significant increase in both D1 and D2 mRNA levels (Fig. 4). These findings were comparable with that observed in toxic nodule control samples [D1: normal (NL) subjects 0.90 ± 0.02 vs. toxic adenoma (TA) 1.11 ± 0.02 vs. MAS 1.11 ± 0.04 AU ± sd, P < 0.0001, ANOVA, Tukey post hoc analysis, TA vs. NL and MAS vs. NL, P < 0.001, TA vs. MAS, n.s.; D2: NL 0.87 ± 0.02 vs. TA 1.00 ± 0.01 vs. MAS 1.01 ± 0.02 AU ± sd, P < 0.0001 ANOVA, Tukey post hoc analysis, MAS vs. NL, P < 0.001, TA vs. MAS, n.s.].
Figure 4.
Increased D1 and D2 expression in MAS thyroid. cDNA samples were obtained from five NL subjects, three TA patients, and five MAS patients. Thyroid samples were tested by real-time PCR for the expression of D1 (A) and D2 (B) and β-actin as reference gene. Experiments were performed in triplicate. RNA levels are expressed as arbitrary units after correction for β-actin (see text for details).
Thyroid cDNA sequencing
Direct sequencing of the thyroid cDNA samples obtained form the thyroid specimens indicated approximately 50% proportion of the G/A alleles at codon 201, resulting in an Arg→His missense mutation in four MAS subjects. Interestingly, one sample, from a subject who was clinically indistinguishable from the other MAS patients, showed no mutation at codon 201 or 227. None of the toxic adenoma or the normal samples showed mutations codon 201 or 227. A normal sample showed a silent mutation C/A at codon 185 (Ile→Ile).
Ex vivo deiodinase activity
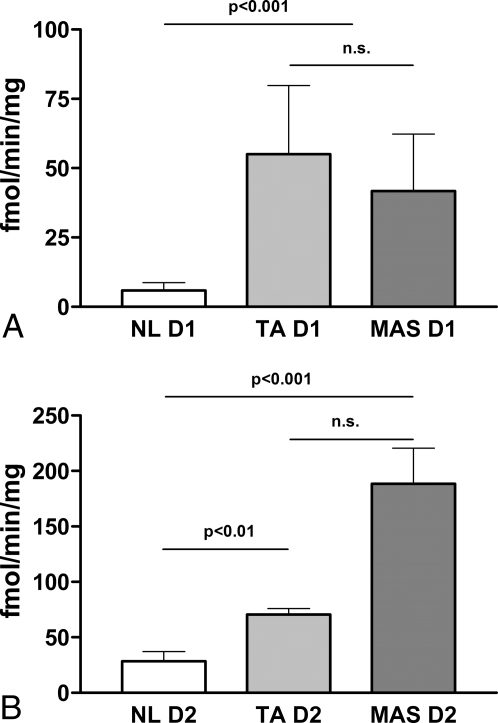
When analyzed for deiodinase activity, the MAS thyroid samples (one with an Arg→His mutation at codon 201, and one subject who was negative for mutations in either codon 201 or 227) showed a net increase, compared with normal control tissue (two subjects), in both the PTU-sensitive D1 and the PTU-resistant D2 (Fig. 5). Consistent with the real-time PCR data, the 5′-deiodinase activities in the MAS tissues were comparable with those observed in a toxic nodule control sample (one subject) (D1: NL 5.9 ± 4.5 vs. MAS 41.7 ± 26.8 vs. TA 59.1 ± 23.6 fmol/min·mg, P < 0.0001, ANOVA, Tukey post hoc analysis, NL vs. MAS and NL vs. TA, P < 0.001; D2: NL 28.3 ± 13.8 vs. MAS 153.1 ± 43.7 vs. TA 61.8 ± 9.7 fmol/min·mg, P < 0.0001, ANOVA, Tukey post hoc analysis, NL vs. MAS and MAS vs. TA, P < 0.001, NL vs. TA, P < 0.01).
Figure 5.
D1 and D2 activities are increased in MAS thyroid. Tissue samples obtained from two NL subjects, one TA patient, and two MAS patients were tested for the activity of D1 (A) and D2 (B) (see text for details).
D2 promoter activity
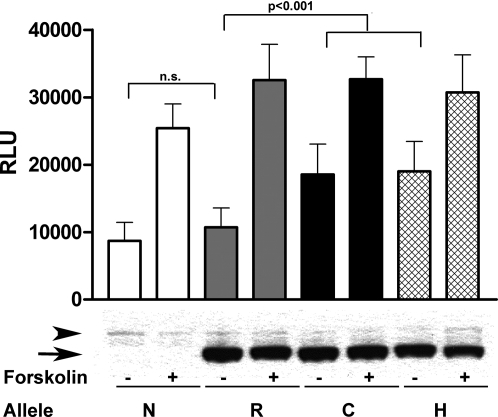
To determine in vitro the activity of the GNAS mutations on the promoter activity of the D2 gene, we performed transient transfection of GNAS expression constructs with either wild-type R or the C and H mutations in Hek-293/D2prom cells that were stably transfected with a cAMP-responsive D2 promoter/luciferase reporter construct. The data were corrected for the amount of transfected GNAS as measured by Western Blot (Fig. 6). Compared with nontransfected cells and cells transfected with the wild-type GNAS, basal transcriptional activity was significantly increased, approximately 50%, in both mutants (8712 ± 2738 N vs. 10733 ± 2855 R vs. 18548 ± 4514 C vs. 19032 ± 4410 H RLU ± sd, P < 0.0001 ANOVA, Tukey post hoc analysis, N vs. R, n.s.; R vs. C and R vs. H, P < 0.001; C vs. H, n.s.). Interestingly, with the exception of the nontransfected controls, no significant difference was observed in maximal promoters induction after forskolin treatment (25423 ± 3578 N vs. 32533 ± 5328 R vs. 32695 ± 3277 C vs. 30704 ± 5563 H RLU ± sd. Tukey post hoc analysis, N vs. R, P < 0.01; R vs. C, R vs. H, and C vs. H, n.s.).
Figure 6.
The R201C and R201H GNAS mutants confer an increased basal transcription activity in the cAMP-responsive D2 promoter. Top, Hek-293/D2prom cells transfected with either the R201C (C) or R201H (H) mutant GNAS alleles gave a significant increase in basal promoter activity when compared with nontransfected (N) cells or cells transfected with the wild-type (R) 201 allele. No difference in basal promoter activity was observed between the C and H mutant GNAS alleles; similarly no difference in basal promoter activity was observed between N and the R, wild-type GNAS allele (− histograms). The GNAS alleles (R, C, or H) did not interfere with forskolin-stimulated induction of the D2 promoter (+ histograms). Bottom, c-myc Western blot. Aliquots of cells were separated by gel electrophoresis, and Western blot analysis was carried out using a primary antibody anti-myc and resolved by chemiluminescence. The arrowhead shows the endogenous c-myc, and the arrow shows the recombinant GNAS tagged with c-myc (see text for details).
Discussion
Using the sensitive screening test of abnormal ultrasound findings, we found that 54% of the subjects in our cohort of MAS patients had evidence of thyroid involvement. This is considerably higher than the reported prevalence in the literature of approximately 33%, (9,22). It may be secondary to an ascertainment bias in that ultrasound is a more sensitive screening test for thyroid pathology than the more commonly used finding of a suppressed TSH or palpable goiter. Alternatively, it could reflect a referral bias, in that the NIH MAS cohort may be a more severely affected group than other series reported in the literature. Nonetheless, in MAS, even in the absence of overt hyperthyroidism, the T3 to T4 ratio is increased, suggesting a constitutive intrathyroidal conversion secondary to activation of the 5′-deiodinase. This was manifested by the finding that 81% of the subjects in the MAS-related ultrasound findings group had an increased T3 to T4 ratio, and only a minority had a frankly suppressed TSH. In our MAS cohort, we found no difference in the clinical features of the patients with respect to the R201H or R201C alleles. Interestingly, these findings are similar to those described in two cases of large follicular thyroid carcinoma with paraneoplastic D2 overexpression (23). Conversely, one cannot rule out the possibility that the increased T4 conversion in T3 could result (at least in part) from the thyroidal metabolism of circulating T4 (24,25) or that other tissues carrying the GNAS mutation may contribute to the increased T3 to T4 ratio observed in MAS patients. Nonetheless, it is worth noting that MAS skeletal muscle, which is believed to be a major source of D2-mediated peripheral conversion of T4 (26), seldom harbors the GNAS mutation. When skeletal muscle is involved, the phenotype is that of an intramuscular myxoma, Mazabraud’s syndrome (27), which was seen in only two of the patients in this series (data not shown).
In humans, as opposed to rodents, both D1 and D2 are present in the thyroid gland, and their activities are proportional to the thyroid state (13,14,15). Interestingly, whereas D1 transcription is driven by thyroid hormone (28), D2 transcription and activity are regulated by a complex homeostatic mechanism (16), and the posttranslational regulation of the D2 activity plays a critical role in the maintenance of the intracellular content of T3, thereby modulating the hormonal message at a prereceptor level (29,30). In human thyroid, the TSH receptor pathway, by increasing the intracellular content of cAMP, stimulates the D2 transcription via a previously characterized cAMP-responsive element located at −766 from the ATG (31,32). Conversely, an increase in T4, the main substrate for D2, causes a net decrease in activity by stimulating the ubiquitin-mediated proteasome degradation of the enzyme, thereby generating a negative feedback mechanism (33,34). In MAS the cells affected by the GNAS activating mutation are constitutively exposed to high intracellular levels of cAMP, and thus, our ex vivo data, which showed a modest but significant increase in the transcription and activity of D2, are in keeping with the pathophysiology of MAS. One of our samples, obtained from a patient whose clinical features were indistinguishable from the others, showed no mutation in either codon 201 or 227. It has been reported that in MAS the prevalence of tissue samples negative for GNAS mutations is as high as 10% (7), so the present finding is not unexpected. To confirm the molecular mechanisms leading to the activation of D2, we performed a reconstruction experiment taking advantage of Hek-293/D2prom, a cell line that stably expresses the human D2 promoter with the functional cAMP responsive element coupled with a luciferase reporter gene. The in vitro data clearly demonstrated that in the presence of either of the two most common GNAS mutations causing MAS, the transcription of the D2 promoter-driven luciferase reporter gene is constitutively increased when compared with cells transfected with the wild-type GNAS allele. Interestingly, the cells maintained the ability to respond to forskolin, suggesting that the MAS-induced intracellular increase in cAMP, albeit sufficient to cause a shift in the function of the target cells is modest enough not to induce immediate toxicity and death. It is thus likely that in MAS the ligand-independent increase in intrathyroidal cAMP levels leading to autonomous thyroid function and cAMP-dependent D2 transcription contributes in turn to increased intracellular content of T3, ultimately driving D1 transcription (Fig. 4) and activity (Fig. 5) in a positive feedback loop. The two converging pathways cause eventually a picture of T3 toxicosis.
These findings have clinical relevance because patients affected by MAS quite often develop hyperthyroidism as children, a condition that in turn is associated with failure to thrive (35), and early growth plate closure. Thyroid surgery can be more difficult in small children, and many parents and practitioners are hesitant to treat small children with radioactive iodine. Furthermore, patients often have to endure multiple orthopedic surgical procedures. In this setting the management of the thyroid dysfunction is frequently pharmacological. Considering that PTU, unlike MMI, is a potent inhibitor of D1 at the therapeutic dosage (36,37), one should theoretically prefer this thionamide for the management of MAS-associated thyrotoxicosis, but currently no data are available to confirm or dispute this hypothesis, and in the patients studied here, there was no difference in the PTU vs. MMI group. Furthermore, the requirement of multiple daily dosing might make the use of this medication impractical in children.
In conclusion, we have demonstrated that thyroid involvement is a very common feature of MAS and that, even in the absence of overt hyperthyroidism, the activation of both D1 and D2 contributes to the increase in intrathyroidal conversion of T4 to T3. Further studies are needed to evaluate the long-term effects of an elevated T3 concentration in the absence of overt thyrotoxicosis and the theoretical superiority of PTU in the pharmacological treatment.
Acknowledgments
The authors acknowledge the generous gift of Dr. Larry Fisher (NIDCR, NIH) of the expression vectors containing the mutant and wild-type GNAS gene as well as the excellent clinical support of the nursing staff and the NIH Interinstitute Endocrine Training Program Fellows and the comments and suggestions of Douglas Forrest and Marvin C. Gershengorn (NIDDK, NIH).
Footnotes
This work was supported by the Intramural Research Program of the NIDDK-NIH and NIDCR-NIH.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 18, 2008
Abbreviations: AU, Arbitrary units; C, Cys201; D1, type 1 5′-deiodinase; D2, type 2 5′-deiodinase; H, His201; MAS, McCune-Albright syndrome; MMI, methimazole; NL, normal; PTU, propylthiouracil; R, Arg201; RLU, relative light units; TA, toxic adenoma.
References
- McCune DJ 1936 Osteitis fibrosa cystica: the case of a nine-year-old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child 52:743–744 [Google Scholar]
- Albright F, Butler AM, Hampton AO, Smith P 1937 Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation, and endocrine dysfunction, with precocious puberty in females: report of 5 cases. N Engl J Med 216:727–746 [Google Scholar]
- Collins MT 2004 McCune-Albright syndrome. In: Filetti S, ed. Paris: Orphanet [Google Scholar]
- Feuillan PP 1997 McCune-Albright syndrome. Curr Ther Endocrinol Metab 6:235–239 [PubMed] [Google Scholar]
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM 1991 Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325:1688–1695 [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Francomano CA, Levine MA 1992 Identification of a mutation in the gene encoding the α subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci USA 89:5152–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbroso S, Paris F, Sultan C 2004 Activating Gsα mutations: analysis of 113 patients with signs of McCune-Albright syndrome—a European Collaborative Study. J Clin Endocrinol Metab 89:2107–2113 [DOI] [PubMed] [Google Scholar]
- Collins MT, Shenker A 1999 McCune-Albright syndrome: new insights. Curr Opin Endocrinol Diabetes 6:119–125 [Google Scholar]
- Feuillan PP, Shawker T, Rose SR, Jones J, Jeevanram RK, Nisula BC 1990 Thyroid abnormalities in the McCune-Albright syndrome: ultrasonography and hormonal studies. J Clin Endocrinol Metab 71:1596–1601 [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Mitsiades NS, Doufas AG, Koutras DA 1997 Hyperthyroidism in McCune-Albright syndrome with a review of thyroid abnormalities sixty years after the first report. Thyroid 7:433–439 [DOI] [PubMed] [Google Scholar]
- Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, Guthrie LC, Bonat S, Robey PG, Shenker A 2003 Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs α mutations. J Clin Endocrinol Metab 88:4413–4417 [DOI] [PubMed] [Google Scholar]
- Michiels FM, Caillou B, Talbot M, Dessarps-Freichey F, Maunoury MT, Schlumberger M, Mercken L, Monier R, Feunteun J 1994 Oncogenic potential of guanine nucleotide stimulatory factor α subunit in thyroid glands of transgenic mice. Proc Natl Acad Sci USA 91:10488–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore D, Tu H, Harney JW, Larsen PR 1996 Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J Clin Invest 98:962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Araki O, Hosoi Y, Kamiya Y, Morimura T, Ogiwara T, Mizuma H, Mori M 2001 Expression and regulation of type II iodothyronine deiodinase in human thyroid gland. Endocrinology 142:2961–2967 [DOI] [PubMed] [Google Scholar]
- Brtko J, Bobalova J, Podoba J, Schmutzler C, Kohrle J 2002 Thyroid hormone receptors and type I iodothyronine 5′-deiodinase activity of human thyroid toxic adenomas and benign cold nodules. Exp Clin Endocrinol Diabetes 110:166–170 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- Weetman AP, Shepherdley CA, Mansell P, Ubhi CS, Visser TJ 2003 Thyroid over-expression of type 1 and type 2 deiodinase may account for the syndrome of low thyroxine and increasing triiodothyronine during propylthiouracil treatment. Eur J Endocrinol 149:443–447 [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Majolagbe A, Kuznetsov SA, Collins MT, Mankani MH, Corsi A, Bone HG, Wientroub S, Spiegel AM, Fisher LW, Robey PG 2000 Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res 15:120–128 [DOI] [PubMed] [Google Scholar]
- Amino N, Yabu Y, Miyai K, Fujie T, Azukizawa M, Onishi T, Kumahara Y 1978 Differentiation of thyrotoxicosis induced by thyroid destruction from Graves’ disease. Lancet 2:344–346 [DOI] [PubMed] [Google Scholar]
- Coppotelli G, Summers A, Chidakel A, Ross JM, Celi FS 2006 Functional characterization of the 258 A/G (D2-ORFa-Gly3Asp) human type-2 deiodinase polymorphism: a naturally occurring variant increases the enzymatic activity by removing a putative repressor site in the 5′ UTR of the gene. Thyroid 16:625–632 [DOI] [PubMed] [Google Scholar]
- St. Germain DL 1988 Dual mechanisms of regulation of type I iodothyronine 5′-deiodinase in the rat kidney, liver, and thyroid gland. Implications for the treatment of hyperthyroidism with radiographic contrast agents. J Clin Invest 81:1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel MD, Schwindinger WF, Levine MA 1996 Clinical implications of genetic defects in G proteins. The molecular basis of McCune-Albright syndrome and Albright hereditary osteodystrophy. Medicine (Baltimore) 75:171–184 [DOI] [PubMed] [Google Scholar]
- Kim BW, Daniels GH, Harrison BJ, Price A, Harney JW, Larsen PR, Weetman AP 2003 Overexpression of type 2 iodothyronine deiodinase in follicular carcinoma as a cause of low circulating free thyroxine levels. J Clin Endocrinol Metab 88:594–598 [DOI] [PubMed] [Google Scholar]
- Laurberg P, Vestergaard H, Nielsen S, Christensen SE, Seefeldt T, Helleberg K, Pedersen KM 2007 Sources of circulating 3,5,3′-triiodothyronine in hyperthyroidism estimated after blocking of type 1 and type 2 iodothyronine deiodinases. J Clin Endocrinol Metab 92:2149–2156 [DOI] [PubMed] [Google Scholar]
- Laurberg P 1986 Thyroxine entering the thyroid gland via the vascular bed may leave the gland as triiodothyronines. Studies with perfused dog thyroid lobes. Endocrinology 118:895–900 [DOI] [PubMed] [Google Scholar]
- Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR 2005 Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MT 2006 Spectrum and natural history of fibrous dysplasia of bone. J Bone Miner Res 21(Suppl 2):P99–P104 [DOI] [PubMed] [Google Scholar]
- Jakobs TC, Schmutzler C, Meissner J, Kohrle J 1997 The promoter of the human type I 5′-deiodinase gene—mapping of the transcription start site and identification of a DR+4 thyroid-hormone-responsive element. Eur J Biochem 247:288–297 [DOI] [PubMed] [Google Scholar]
- Curcio-Morelli C, Zavacki AM, Christofollete M, Gereben B, de Freitas BC, Harney JW, Li Z, Wu G, Bianco AC 2003 Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest 112:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, Nissim S, Mornon JP, Zavacki AM, Zeold A, Capelo LP, Curcio-Morelli C, Ribeiro R, Harney JW, Tabin CJ, Bianco AC 2005 The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol 7:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha T, Kim SW, Salvatore D, Gereben B, Tu HM, Harney JW, Rudas P, Larsen PR 2000 Characterization of the 5′-flanking and 5′-untranslated regions of the cyclic adenosine 3′,5′-monophosphate-responsive human type 2 iodothyronine deiodinase gene. Endocrinology 141:229–237 [DOI] [PubMed] [Google Scholar]
- Canettieri G, Celi FS, Baccheschi G, Salvatori L, Andreoli M, Centanni M 2000 Isolation of human type 2 deiodinase gene promoter and characterization of a functional cyclic adenosine monophosphate response element. Endocrinology 141:1804–1813 [DOI] [PubMed] [Google Scholar]
- Kim BW, Zavacki AM, Curcio-Morelli C, Dentice M, Harney JW, Larsen PR, Bianco AC 2003 Endoplasmic reticulum-associated degradation of the human type 2 iodothyronine deiodinase (D2) is mediated via an association between mammalian UBC7 and the carboxyl region of D2. Mol Endocrinol 17:2603–2612 [DOI] [PubMed] [Google Scholar]
- Gereben B, Salvatore D 2005 Pretranslational regulation of type 2 deiodinase. Thyroid 15:855–864 [DOI] [PubMed] [Google Scholar]
- Segni M, Leonardi E, Mazzoncini B, Pucarelli I, Pasquino AM 1999 Special features of Graves’ disease in early childhood. Thyroid 9:871–877 [DOI] [PubMed] [Google Scholar]
- Leonard JL, Rosenberg IN 1978 Thyroxine 5′-deiodinase activity of rat kidney: observations on activation by thiols and inhibition by propylthiouracil. Endocrinology 103:2137–2144 [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaplan MM, Leonard JL, Larsen PR 1983 Evidence for two pathways of iodothyronine 5′-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest 71:992–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]