Abstract
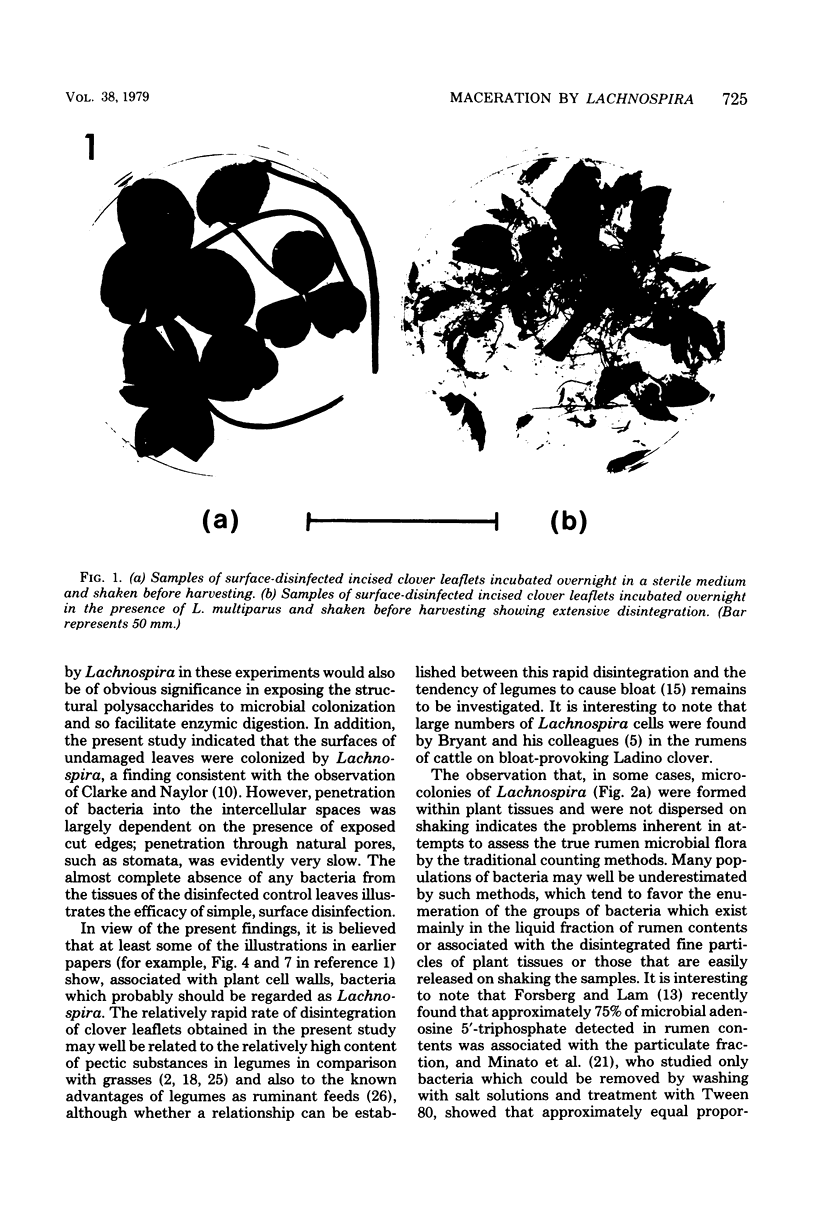
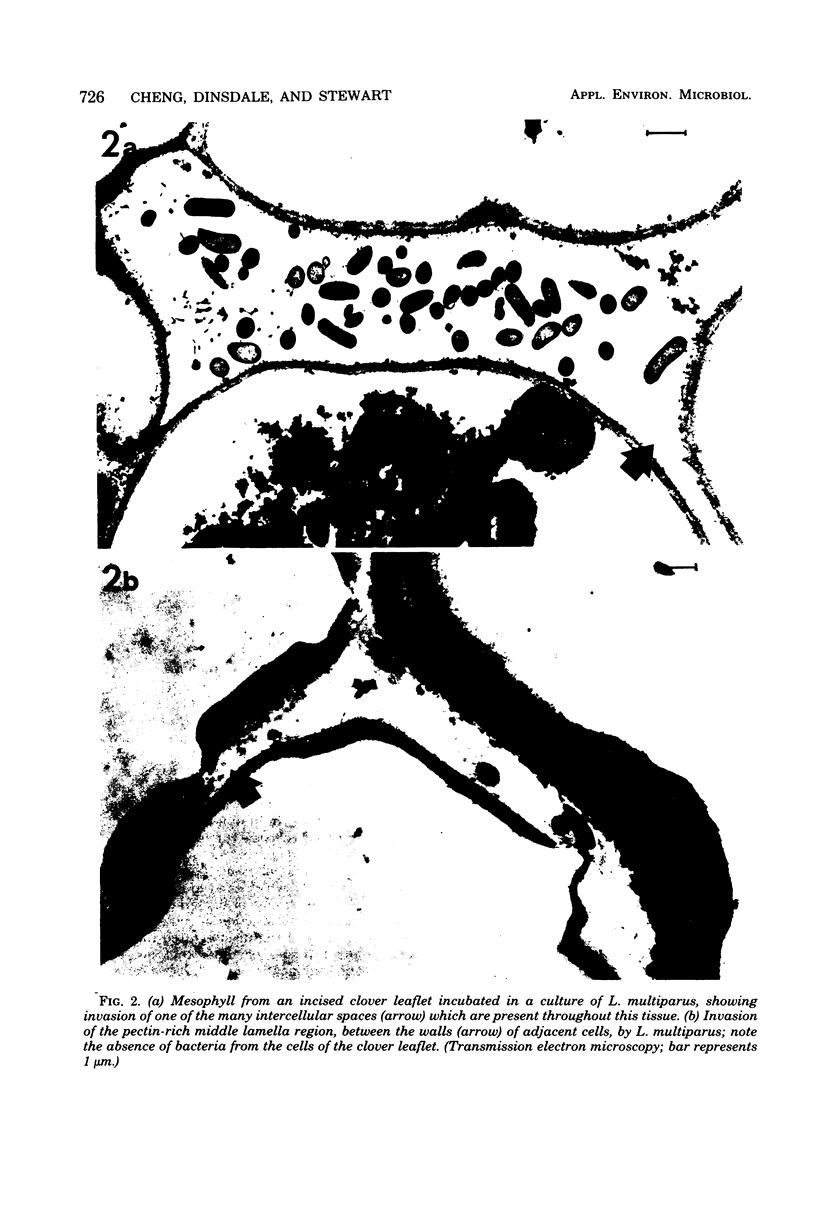
A strain of Lachnospira multiparus, a pectin-hydrolyzing bacterium from the rumen, was incubated in nutrient media in the presence of surface-disinfected clover leaflets. When the culture flasks containing the leaflets together with Lachnospira were shaken after overnight incubation, extensive maceration of the leaflets was seen, although uninoculated control leaflets remained intact during a similar treatment. Examination of inoculated leaflets by transmission electron microscopy showed extensive invasion of intercellular areas of the mesophyll tissue but only minor invasion of vascular tissue. Cutting the leaves before incubation greatly increased the ability of L. multiparus to colonize and macerate the leaflets. Similar experiments with grass leaves are also described, and the possible role of maceration in the digestion of plant material in the rumen is discussed. Although Lachnospira stains gram variable and often gram negative, the ultrastructure of the cell wall was that of a gram-positive bacterium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER F., NASR H., MORRICE F., BRUCE J. Bacterial breakdown of structural starches and starch products in the digestive tract of ruminant and non-ruminant mammals. J Pathol Bacteriol. 1950 Oct;62(4):617–638. doi: 10.1002/path.1700620412. [DOI] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N. Characteristics of two new genera of anaerobic curved rods isolated from the rumen of cattle. J Bacteriol. 1956 Jul;72(1):22–26. doi: 10.1128/jb.72.1.22-26.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Akin D. E., Costerton J. W. Rumen bacteria: interaction with particulate dietary components and response to dietary variation. Fed Proc. 1977 Feb;36(2):193–197. [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of Butyrivibrio fibrisolvens: a gram-positive bacterium. J Bacteriol. 1977 Mar;129(3):1506–1512. doi: 10.1128/jb.129.3.1506-1512.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Pectin-fermenting bacteria isolated from the bovine rumen. J Bacteriol. 1969 Jul;99(1):189–196. doi: 10.1128/jb.99.1.189-196.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale D., Morris E. J., Bacon J. S. Electron microscopy of the microbial populations present and their modes of attack on various cellulosic substrates undergoing digestion in the sheep rumen. Appl Environ Microbiol. 1978 Jul;36(1):160–168. doi: 10.1128/aem.36.1.160-168.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Lam K. Use of adenosine 5'-triphosphate as an indicator of the microbiota biomass in rumen contents. Appl Environ Microbiol. 1977 Mar;33(3):528–537. doi: 10.1128/aem.33.3.528-537.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradel C. M., Dehority B. A. Fermentation of isolated pectin and pectin from intact forages by pure cultures of rumen bacteria. Appl Microbiol. 1972 Feb;23(2):332–340. doi: 10.1128/am.23.2.332-340.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGOWSKI J. M., SELL H. M., HUFFMAN C. F., DUNCAN C. W. The carbohydrates in alfalfa Medicago sativa. I. General composition, identification of a nonreducing sugar and investigation of the pectic substances. Arch Biochem Biophys. 1958 Aug;76(2):306–316. doi: 10.1016/0003-9861(58)90156-5. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]