Abstract
Building on our previous report that osteoblasts and increased bone formation have a negative impact on myeloma cell growth in a subset of patients, we investigated the role of decorin, the main small leucine-rich proteoglycan (SLRP) expressed and produced by osteoblasts, in the antimyeloma effects of osteoblasts. In coculture experiments with osteoblasts, primary myeloma cell survival was significantly higher when decorin expression in osteoblasts was knocked down by short-hairpin RNA. Coculture experiments of myeloma cells and supporting osteoclasts in the presence of osteoblast-conditioned medium showed reduced myeloma cell survival, an effect that was attenuated by decorin-neutralizing antibody. Decorin overexpression in mesenchymal stem cells or use of recombinant decorin in coculture with osteoclasts reduced the ability of osteoclasts to support primary myeloma cell survival. The antimyeloma effect of decorin involved direct induction of apoptosis and activation of p21WAF. Decorin also inhibited myeloma cell-induced tube formation and osteoclast differentiation. Decorin expression was insignificantly lower in patients' than donors' osteoblasts and slightly increased by bortezomib. Certain SLRPs are involved in the antimyeloma effect of osteoblasts directly and indirectly through inhibition of angiogenesis and osteoclastogenesis; therefore, increasing endo-genous or exogenous SLRPs in myelomatous bone may help control myeloma.
Introduction
Changes in the cellular balance of the tumor microenvironment play critical roles in survival and proliferation of malignant cells, as well as in their escape from immunosurveillance and in metastasis development. In multiple myeloma, malignant plasma cells typically disseminate in the bone marrow through stimulation of osteoclastogenesis1 and angiogenesis2 and through suppression of osteoblastogenesis.1 Mounting evidence suggests that, although osteoclasts promote myeloma cell growth,3,4 bone-building osteoblasts have an inhibitory effect on the growth of myeloma cells in a subset of patients.5 Although much effort has been directed to identifying and validating the supportive roles of certain cytokines and extracellular matrix factors in myelomagenesis,6 little is known about potential endogenous myeloma inhibitory factors produced by cells such as osteoblasts
Mesenchymal stem cells (MSCs) have long been known to promote myeloma cell survival, at least in an in vitro setting, through production of growth factors and direct cell-cell contact.7,8 In contrast to MSCs, mature osteoblasts produce higher levels of factors necessary for building bone, including various collagens and proteoglycans.9 We hypothesized that, as certain extracellular components associated with the bone matrix may inhibit myeloma cell growth, increasing the activity of osteoblasts in myelomatous bone could shift the balance between myeloma growth-promoting and growth-inhibiting factors, resulting in impaired tumor growth.
Small leucine-rich proteoglycans (SLRPs), a group of secreted markers, are up-regulated in differentiating osteoblasts and implicated in the regulation of organic matrix assembly, remodeling of osteoid, and mineral deposition.10–12 Although no specific SLRP receptors have been identified, an SLRP such as decorin has been shown to bind to epidermal growth factor receptor (EGFR),13,14 insulin-like growth factor 1 receptor (IGF-1R),15 and the low-density lipoprotein receptor-related protein.16 Decorin,13,17–20 lumican,21,22 and biglycan23 have been shown to suppress tumor cell growth, suggesting that they could be part of the mechanism by which osteoblasts attenuate myeloma cell growth. These SLRPs have often been detected in the malignant extracellular matrix as part of the host defense mechanism against tumor invasion,24 but their expression levels in certain malignancies have been negatively associated with progression stage.25 Decorin, the most intensively studied SLRP, has been shown to inhibit tumor growth through up-regulation of the cell-cycle inhibitor p21WAF,17 binding to and sequestering of transforming growth factor-β (TGF-β),26,27 and binding to and degrading of EGFR on tumor cells,20,28,29 as well as suppression of angiogenesis.30,31 Decorin core protein was sufficient to exert most of its antitumor activities. We therefore initially focused our investigation on the role of decorin in the antimyeloma effects of osteoblasts.
Methods
Plasma cells
Myeloma plasma cells were obtained from heparinized bone marrow aspirates from 20 patients with active myeloma during scheduled clinic visits. Signed Institutional Review Board–approved informed consent forms are kept on file. Approval was obtained from the University of Arkansas for Medical Sciences institutional review board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki. Bone marrow aspirates of 4 healthy persons were purchased from Lonza Walkersville (Walkersville, MD). Cells in the bone marrow samples were separated by density centrifugation with Ficoll-Paque (specific gravity, 1.077 g/mL; GE Healthcare Bio-Sciences, Little Chalfont, United Kingdom), and the proportions of myeloma plasma cells in the light-density cell fractions were determined by CD38/CD45 flow cytometry. CD138-expressing plasma cells were isolated as previously described.32 To test the direct effect of decorin on tumor growth, primary myeloma cells were incubated in Cellgro COMPLETE medium (Mediatech, Herndon, VA) in serum-free conditions for the indicated times and decorin concentrations.
MSCs and osteoblasts
MSCs were prepared as previously described.33,34 Briefly, bone marrow mononucleate cells (2 × 106 cells/mL) from healthy donors (n = 3; Lonza Walkersville) and myeloma patients (n = 3) and from human fetal bone fragments (Advanced Bioscience Resources, Alameda, CA) were cultured in Dulbecco modified Eagle medium with low glucose (DMEM-LG) supplemented with 10% fetal bovine serum (FBS) and antibiotics (MSC medium). One-half of the medium was replaced every 4 to 6 days, and adherent cells were allowed to reach 80% confluence before they were subcultured with trypsin-ethylenediaminetetraacetic acid. The adherent MSCs expressed CD166, but not CD45 and CD34. For differentiation into osteoblasts, MSCs were incubated with DMEM-LG supplemented with 10% FBS, dexamethasone (100 nM), β-glycerophosphate (10 mM), and ascorbate (0.05 mM) (osteoblastic medium). The MSCs formed aggregates or nodules and showed increased expression of alkaline phosphatase within 1 to 2 weeks; calcium deposition followed, together with loss of CD166 expression and increased expression of osteocalcin and bone morphogenetic protein 2.5 Differentiation into mature osteoblasts was quantified after 3 to 4 weeks of incubation in osteoblastic medium with the use of Alizarin red staining. Global gene expression was profiled as described.32 Myeloma cell growth was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, and apoptotic cells were enumerated with the use of annexin V–propidium iodide (PI; Invitrogen, Carlsbad, CA) flow cytometry.3
Myeloma MSC-osteoblast cocultures
To test the effect of decorin-overexpressing MSCs on the growth of primary myeloma cells, MSCs transduced with decorin or empty vector plasmids were cultured on the backside of 1-μm filters in 24-well plates as previously described.5 Myeloma cells (0.5 × 106 cells/well) were cultured on the upper chamber of the filters for approximately 5 days. At the end of each experiment, myeloma cell viability was determined by trypan blue exclusion assay and cell growth by MTT assay.5
To study the effect of decorin knockdown on myeloma cell growth in coculture with osteoblasts, untreated MSCs (mock) or MSCs transduced with lentivirus containing decorin short-hairpin RNA (shRNA) or scrambled shRNA were differentiated into osteoblasts in 96-well plates. Primary myeloma plasma cells (1.5 × 104 cells/well in triplicate) were cultured with osteoblasts in medium lacking serum and osteoblast differentiation agents for 7 days and then subjected to MTT assay.
Osteoclasts and their precursors
Osteoclast precursors and bone-resorbing osteoclasts were prepared as previously described.3 Briefly, mononuclear cells were obtained from peripheral blood of myeloma patients and healthy subjects and from bone marrow samples of myeloma patients. Before stimulation to generate osteoclast precursors, the bone marrow samples were depleted of plasma cells by selection with CD138-immunomagnetic beads and then cultured for 5 days to deplete adherent stromal cells. The nonadherent mononuclear blood and bone marrow cells were cultured at 2.5 × 106 cells/mL in alpha minimum essential medium supplemented with 10% FBS, RANKL (50 ng/mL, PeproTech, Rocky Hill, NJ), macrophage colony-stimulating factor (25 ng/mL; PeproTech), and antibiotics (osteoclast medium) for 3 to 5 days, at which time the nonadherent cells were removed and the remaining adherent cells served as osteoclast precursors.3 To test the effect on osteoclast differentiation, osteoclast precursors were incubated in osteoclast medium in the absence and the presence of decorin (5-10 μg/mL) for 5 to 7 days, at which time the cultures were fixed and stained for tartrate-resistant acid phosphatase (TRAP) and the number of multinucleate TRAP-positive osteoclasts was counted as described.3 The myeloma cell–osteoclast coculture was performed as previously described.3
Construction of expression vector for decorin overexpression or decorin shRNA
To overexpress decorin in myeloma cells, decorin cDNA was cloned by polymerase chain reaction (PCR) with appropriate oligonucleotide primers from the pCMV-XL5/decorin vector containing human decorin (Origene Technologies, Rockville, MD), and the fragment was inserted into the pWPI vector, obtained from the laboratory of Dr Didier Trono (Ecole Polytechnique Federale de Lausanne, Switzerland), at the restriction site PmeI.
A group of shRNA sequences for decorin was designed with the use of software available at http://jura.wi.mit.edu/bioc/siRNAext/home.php. These sequences were submitted to a BLAST search against the human genome sequence to ensure that the sequences would not target RNA in a nonspecific manner. DNA single-stranded oligonucleotide sequences were synthesized by Integrated DNA Technologies (Coralville, IA). shRNA double-stranded oligonucleotides were formed by annealing the sense and antisense oligonucleotides at 90°C for 4 minutes, 70°C for 10 minutes, and room temperature for 15 minutes; they were then cloned into the pSUPER vector (Oligoengine, Seattle, WA) at restriction sites HindIII and BglII. These recombinant vectors were subjected to further evaluation by transient transfection to osteoblast precursors that express decorin, and the one with highest gene knockdown efficiency (sense: 5′-GATCCCCGAGTATGAGTCTTCTGTAATTCAAGAGATTACAGAAGACTCATACTCTTTTTGGAAA-3′ and antisense: 5′-AGCTTTTCCAAAAAGAGTATGAGTCTTCTGTAATCTCTTGAATTACAGAAGACTCATACTC GGG-3′) was chosen and subcloned into lentiviral pLVTH vectors (Addgene, Cambridge, MA) at the enzyme restriction sites EcoRI and ClaI. A nonsense scrambled control recombinant vector (sense: 5′-GATCCCCGACACGCGACTTGTACCACTTCAAGAGAGTGGTACAAGTCGCGTGTCTTTTTGGAAA-3′ and antisense: 5′-AGCTTTTCCAAAAAGACACGCGACTTGTACCACTCTCTTGAAGTGGTACAAGTCGCGTGTC GGG-3′)35 was subcloned into the pLVTH vector in the same way. The pWPI and pLVTH vectors contain cDNA for a green florescent protein marker to facilitate tracking of transduced cells.
Production, purification, and titer determination of lentiviral particles
All recombinant lentiviruses were produced by transient transfection of 293T cells according to a standard protocol.36 Briefly, 293T cells were incubated overnight with the transfection precipitate; afterward, culture medium was replaced and incubation for an additional 2 days followed. The media of the transfected cells were harvested, centrifuged at 3000 rpm at 4°C for 15 minutes, and filtered through a 0.22-μm pore size filter. The filtrate was layered on top of a 20% sucrose cushion and spun at 26 000 rpm at 4°C for 100 minutes in a Beckman ultracentrifuge using an SW28 rotor. The pelleted virus-like particles were suspended in DMEM and stored at −80°C. Titers of the virus stocks (titer units [TU]/mL) were determined by adding aliquots of virus suspension on monolayers of 293T cells or other appropriate cell types and then assessing the percentage of green florescent protein-positive cells by fluorescence-activated cell sorting (FACScan; BD Biosciences, San Jose, CA). Titers greater than 109 TU/mL were routinely obtained.
Lentivirus-mediated transduction of MSCs
MSCs were seeded in 24-well plates and cultured overnight before infection. For decorin overexpression, MSCs were transduced with decorin-expressing lentivirus or control empty vector; for decorin knockdown, MSCs were transduced with shRNA recombinant lentiviral particles or the control scrambled shRNA. Infection was carried out by adding the virus (MOI = 3) on cells for 18 hours. The majority (> 90%) of cultured cells were successfully transduced with shRNA vectors.
Quantitative real-time polymerase chain reaction
Briefly, total cellular RNA from each sample was extracted and purified with the Qiagen RNeasy Mini Kit as described by the manufacturer (Qiagen, Valencia, CA). Total RNA (1 μg) from each sample was reverse-transcribed with the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). The quantitative real-time polymerase chain reaction (qRT-PCR) was performed with the TaqMan gene expression assay on an ABI Prism 7000 sequence analyzer according to the manufacturer's recommended protocol (Applied Biosystems, Foster City, CA). Each reaction was run in triplicate. The comparative threshold cycle (CT) method was used to calculate the amplification fold as specified by the manufacturer. A value of 10 ng of reverse-transcribed RNA samples was amplified by using the TaqMan Universal PCR Master Mix and TaqMan gene expression assays (ID Hs00370383_m1 for human decorin and ID HS99999905_m1 for GAPDH as an endogenous control; Applied Biosystems).
Western blotting
Cytosolic and nuclear fractions were isolated with the use of the Nuclear/Cytosol Fractionation Kit (BioVision, Mountain View, CA). Equal amounts of lysate were separated by electrophoresis on 4% to 12% sodium dodecyl sulfate-polyacrylamide gels, and Western blotting was carried out according to the Western Breeze chemiluminescent immunodetection protocol as described by the manufacturer (Invitrogen). The following primary antibodies were used: antidecorin (R&D Systems, Minneapolis, MN), anti-p21WAF (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-tubulin (Zymed Laboratories, South San Francisco, CA).
Immunohistochemistry
To reduce variability between assays, untreated and manipulated MSCs or osteoblasts were cultured in the same chamber slides and were similarly processed for immunohistochemical staining. MSCs or osteoblasts grown in chamber slides were fixed with HistoChoice (Amresco, Solon, OH) for 20 minutes, thoroughly washed, and incubated in citrate buffer in a water bath (80°C, 30 minutes) for antigen retrieval. After peroxidase quenching with 3% hydrogen peroxide for 10 minutes, the slides were incubated with monoclonal antibodies against human decorin or control IgG (0.5 μg/mL; R&D Systems) for 60 minutes. The assays were completed with the use of the immunoperoxidase kit from Dako North America (Carpinteria, CA), with counterstaining with hematoxylin. An Olympus BH2 microscope (Olympus, Melville, NY) equipped with a 160×/0.17 numeric aperture objective was used to obtain images with a SPOT 2 digital camera (Diagnostic Instruments, Sterling Heights, MI). Adobe Photoshop version 10 (Adobe Systems, San Jose, CA) was used to process the images.
Tube-formation assay
BD Matrigel growth factor–reduced basement membrane matrix (BD Biosciences) was diluted on ice with DMEM (1:2 dilution factor), poured onto 96-well plates (100 μL/well), and incubated at 37°C for 30 minutes. The plates were washed with phosphate-buffered saline and stored at 4°C until the assay was performed. Human umbilical vein endothelial cells (HUVECs; ATCC, Manassas, VA) were cultured in Clonetics EBM-2 medium (Lonza Walkersville) supplemented with a cocktail of growth factors according to the manufacturer's instructions. For tube-formation assay, HUVECs were trypsinized and seeded on Matrigel-containing chamber slides (15 000 cells/well) with the indicated medium (100 μL/well) in the absence and the presence of decorin (10 μg/mL) for 3 to 4 hours. Conditioned medium collected from a 48-hour culture of primary myeloma plasma cells (1.5 × 106 cells/mL) was used to test the effect of myeloma cells on tube formation. Tube-like structures per well were counted in triplicate with the use of an Olympus phase-contrast microscope. Images were acquired with a SPOT 2 digital camera and processed with Adobe Photoshop, version 10.
Statistical analysis
Student paired t test was used to test the effect of various conditions on myeloma cell growth. Student unpaired t test was used to test the effect of decorin on tube formation and osteoclastogenesis. Values are means plus or minus SEM.
Results
SLRPs are highly expressed and produced by osteoblasts
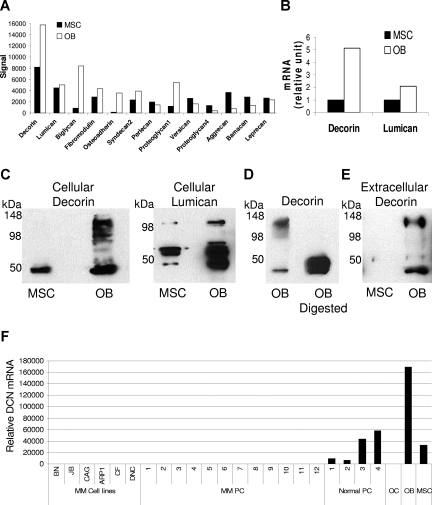
In our investigation of in vitro production and expression levels of certain SLRPs, total protein and mRNA were extracted from osteoblasts and their progeny MSCs. Global gene expression profiling revealed differential expression of various SLRPs and proteoglycans by osteoblasts and MSCs (Figure 1A). We further focused the investigation on the expression levels of decorin and lumican, 2 SLRPs implicated in bone remodeling.10–12 Expression levels of decorin and lumican, as determined by qRT-PCR, were higher in osteoblasts than MSCs (Figure 1B). Western blot analysis showed that osteoblasts produced high levels of decorin core protein (molecular weight, ∼45-48 kDa) and other fractions of these SLRPs, most likely reflecting binding of the core protein to different glycosaminoglycan chains. To confirm this assumption and the specificity of the assay, osteoblast lysate was digested with chondroitinase-ABC before being submitted for Western blotting for decorin. Indeed, the enzymatic digestion resulted in the detection of the core protein only (Figure 1D). We also looked for the presence of decorin in conditioned media of osteoblasts and MSCs grown in the absence of serum for 48 hours. This investigation showed that osteoblasts, but not MSCs, secreted a high level of decorin into cultured medium (Figure 1E). Our data also showed that, whereas normal plasma cells expressed decorin, osteoclasts and myeloma cells did not express this SLRP (Figure 1F). Taken together, these results indicate that osteoblasts produce and secrete high levels of SLRPs, including decorin.
Figure 1.
Osteoblasts express, produce, and secrete certain SLRPs. Protein and mRNA were extracted from MSCs before and after differentiation into osteoblasts (OB). (A) Global gene expression profiling demonstrated the expression of various SLRPs and proteoglycans. (B) Expression levels of decorin and lumican were validated by qRT-PCR. (C) Decorin was detected in cell lysates by Western blot. The core protein of decorin had a molecular weight less than 50 kDa, whereas the other bands reflected monomers and the binding of the core protein to different glycosaminoglycan chains. (D) Osteoblast lysate was digested with chondroitinase ABC before Western blotting for decorin was performed. It is noteworthy that only the core protein was detected after enzymatic digestion, confirming the specificity of the assay. (E) Decorin was detected in the serum-free conditioned media of osteoblasts. (F) By qRT-PCR, decorin was shown to be expressed in myeloma cells lines (MM cell lines), primary myeloma plasma cells (MM PC, n = 12), normal plasma cells (Normal PC), osteoclasts (OC), osteoblasts (OB), and MSCs. Note that decorin was expressed in normal plasma cells but not in myeloma plasma cells or osteoclasts. MSCs and osteoblasts served as positive controls.
Blocking decorin expression or activity reduces the antimyeloma effects of osteoblasts
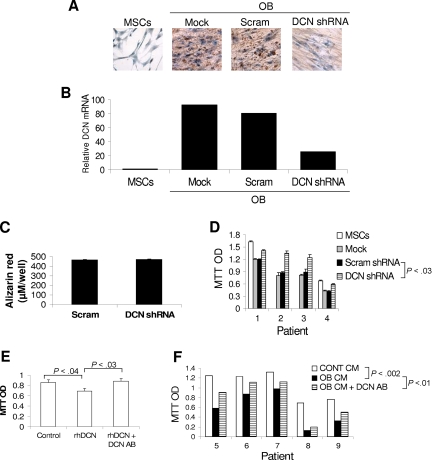
To test whether decorin was involved in the antimyeloma effects of osteoblasts, we used 2 functional assays. In the first approach, osteoblasts were prepared from unmanipulated MSCs (mock) and MSCs containing lentivirus expressing decorin shRNA or scrambled shRNA, and then cocultured them with myeloma cells from 4 patients. Decorin shRNA reduced decorin expression in differentiated osteoblasts by greater than 75% as determined by qRT-PCR and confirmed by immunohistochemical staining for decorin (Figure 2). Knockdown of decorin in MSCs had no effect on differentiation of these cells into mature osteoblasts as determined by Alizarin red staining (Figure 2C). The myeloma cells from the 4 patients who were cocultured with osteoblasts containing decorin shRNA-expressing lentivirus had an increased survival rate compared with that of unmanipulated osteoblasts or osteoblasts expressing scrambled shRNA (P < .03; Figure 2D).
Figure 2.
Blocking decorin expression or activity attenuates the inhibitory effect of osteoblasts on myeloma cell growth. (A,B) MSCs infected with lentivirus-introduced scrambled (Scram) or decorin (DCN) shRNA or left untreated (mock) were differentiated into osteoblasts. Immunohistochemical staining for decorin (panel A, brown color indicates positive staining, original magnification ×200; see “Immunohistochemistry”) and decorin expression as determined by qRT-PCR (panel B) indicate that osteoblasts produce and express high levels of decorin and that decorin expression and production are markedly reduced by specific shRNA (DCN shRNA). (C) Knockdown of decorin had no effect on the ability of MSCs to differentiate into osteoblasts as determined by Alizarin red staining. (D) Myeloma cells from 4 patients were cocultured with untreated osteoblasts (mock) or with osteoblasts infected with lentivirus-introduced scrambled (Scram) or decorin (DCN) shRNA for approximately 7 days, and then subjected to MTT assay. Note the higher myeloma cell growth rate after decorin knockdown in osteoblasts (P < .03, DCN shRNA vs Scram shRNA or mock). (E) Recombinant human decorin (rhDCN) inhibited the growth of myeloma cells in coculture with osteoclasts, an effect that was completely abrogated by decorin-neutralizing antibody (DCN AB). (F) Myeloma cells from 5 patients were cocultured with osteoclasts in the presence or absence of osteoblast-conditioned medium (OB CM) and decorin-neutralizing antibody (5 μg/mL) before being subjected to MTT assay. Osteoblast-conditioned medium inhibited myeloma cell growth (P < .002), an effect that was partially blocked by decorin-neutralizing antibody (P < .01, OB CM vs OB CM + DCN AB).
In the second approach, we neutralized decorin's effect, using decorin antibody (5 μg/mL; R&D Systems). As shown in Figure 2E, this antibody completely abrogated the inhibitory effect of recombinant decorin on the growth of primary myeloma cells in coculture with osteoclasts. To test decorin's involvement in the antimyeloma effects of osteoblasts, myeloma cells from 5 patients were cocultured with osteoclasts in medium containing 50% osteoblast-conditioned medium in the presence or absence of decorin-neutralizing antibody (5 μg/mL). In this study, osteoblast-conditioned medium reduced the myeloma cell survival rate (P < .002; Figure 2E). Addition of decorin-neutralizing antibody partially but significantly blocked the antimyeloma effect of osteoblast-conditioned medium (P < .01, osteoblast-conditioned medium vs osteoblast-conditioned medium + decorin antibody) (Figure 2E). Taken together, these results suggest that decorin is involved in the antimyeloma effects of osteoblasts.
Decorin inhibits myeloma cell growth through up-regulation of p21WAF and induction of apoptosis
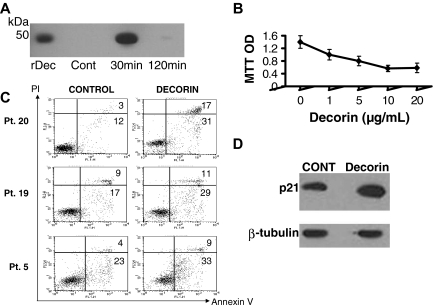
Although specific receptors for decorin and other SLRPs have not been identified, decorin has been shown to bind to and internalize known receptors, such as EGFR.14,20 We found that recombinant decorin was rapidly internalized by myeloma cells and intracellularly degraded within 2 hours (Figure 3A), suggesting that decorin directly interacts with myeloma cells. Incubation of primary myeloma cells (patient 5) with decorin (1-20 μg/mL) in serum-free conditions for approximately 36 hours resulted in reduced myeloma cell survival in a dose-dependent manner (Figure 3B). In additional experiments, myeloma cells from 3 patients were similarly cultured in the absence or presence of decorin (10 μg/mL) for approximately 36 hours and then subjected to annexin V–PI flow cytometry to detect apoptosis. The percentage of apoptotic myeloma cells was consistently increased by decorin treatment (Figure 3C). Because up-regulation of p21WAF has been reported to be involved in decorin's antitumor effect,17 we subjected myeloma cells to Western blotting and found that, as in other tumors, decorin induced up-regulation of phosphorylated p21WAF (Figure 3D). These results suggest that decorin directly inhibits myeloma cell survival and growth.
Figure 3.
Decorin is internalized and inhibits myeloma cell growth through up-regulation of p21WAF and induction of apoptosis. (A) Myeloma cells were exposed to decorin (30 μg/mL) for 30 minutes, washed, and then subjected to Western blotting for detection of decorin at the indicated times. The level of decorin was high after 30 minutes and then rapidly diminished by 120 minutes. Recombinant decorin (rDec) was used as a positive control. (B) Primary myeloma cells (patient 5) were cultured alone in the absence or presence of increasing doses of decorin (1-20 μg/mL) for approximately 36 hours. (C) Annexin V–PI flow cytometry of myeloma cells from 3 patients cultured in the absence or presence of decorin for 36 hours showed an increased percentage of apoptotic annexin V–positive myeloma cells after decorin treatment. (D) The level of phosphorylated p21WAF in myeloma cells was increased after 24-hour incubation with decorin (10 μg/mL).
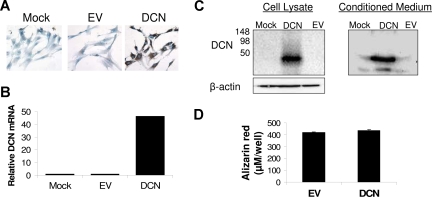
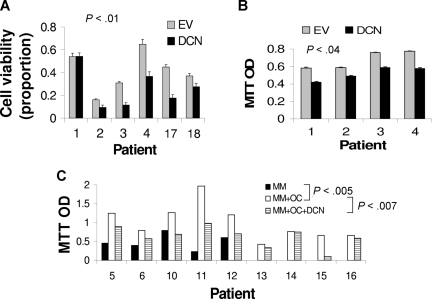
To better reproduce in vivo conditions, we exploited 2 coculture systems for testing the impact of decorin on myeloma cell growth. In the first coculture system, MSCs were infected with lentivirus containing decorin or empty vector. qRT-PCR revealed a more than 45-fold increased expression level of decorin in MSCs containing decorin-expressing lentivirus compared with unmanipulated (mock) MSCs and MSCs containing empty vector–expressing lentivirus (Figure 4). Overproduction of decorin in decorin-overexpressing MSCs was also confirmed at the protein level by immunohistochemistry (Figure 4A) and Western blot analysis (Figure 4C). Western blotting further showed that MSCs containing decorin-expressing lentivirus expressed and released decorin core protein that had no glycosaminoglycan chains (Figure 4C). Overexpression of decorin had no effect on the differentiation of MSCs into osteoblasts (Figure 4D). Myeloma cells from 6 patients cocultured with decorin-overexpressing MSCs for approximately 5 days had reduced cell viability (37% ± 7%; P < .01, n = 6; Figure 5A) and a reduced growth rate (18% ± 4%; P < .04, n = 4; Figure 5B), compared with empty vector–expressing MSCs.
Figure 4.
Overexpression of decorin in MSCs via lentivirus-introduced decorin. MSCs were left untreated or were infected with lentivirus-introduced decorin (DCN) or empty vector (EV). (A) Immunohistochemical staining for decorin (original magnification ×200). (B) As determined by qRT-PCR, decorin was overexpressed and overproduced by MSCs infected with lentivirus-introduced decorin. (C) Western blotting revealed overexpression and secretion of decorin core protein by MSCs infected with lentivirus-introduced decorin. (D) Overexpression of decorin had no effect on the ability of MSCs to differentiate into osteoblasts as determined by Alizarin red staining.
Figure 5.
Decorin reduces the stimulatory effects of MSCs and osteoclasts (OC) on myeloma cell growth. (A,B) Myeloma cells from 6 patients were cocultured for approximately 5 days with MSCs infected with lentivirus expressing empty vector (EV) or decorin (DCN). At the end of each experiment, (A) myeloma cell viability was determined and (B) MTT assay was performed with the use of cells from 4 of those patients. Decorin-expressing MSCs demonstrated reductions in myeloma cell viability and growth. (C) Myeloma cells were cultured alone (n = 5) or cocultured with osteoclasts (n = 9) in the presence or absence of decorin (10 μg/mL). Osteoclasts supported long-term growth of myeloma cells (P < .005, MM vs MM + OC), and decorin inhibited this effect in 6 of 9 experiments (P < .007, MM + OC vs MM + OC + DCN).
In the second coculture system, myeloma cells were cultured alone (n = 5) or cocultured with osteoclasts (n = 9) in the presence or absence of decorin (10 μg/mL) for 7 days. As previously demonstrated, incubation of primary myeloma cells alone resulted in a reduced survival rate, whereas coculture with osteoclasts promoted the survival of the myeloma cells (P < .005; Figure 5C).3 Addition of decorin to myeloma cell–osteoclast cocultures resulted in an antitumor response in 6 of the 9 experiments. Overall, decorin reduced the growth of myeloma cells by 35% plus or minus 8% compared with control myeloma cells in coculture with osteoclasts (P < .007; Figure 5C). Both recombinant decorin (R&D Systems) and decorin purified from bovine articular cartilage (Sigma-Aldrich, St Louis, MO) resulted in antimyeloma effects (data not shown). Moreover, decorin, as assessed by MTT assay, had no effect on the viability of mature osteoclasts, indicating that the antimyeloma effect of this SLRP was not a consequence of a reduced number of osteoclasts (data not shown). These studies suggest that decorin inhibits growth and survival of myeloma cells directly and in the presence of supporting microenvironmental cells, such as osteoclasts and MSCs.
Decorin inhibits angiogenesis and osteoclastogenesis
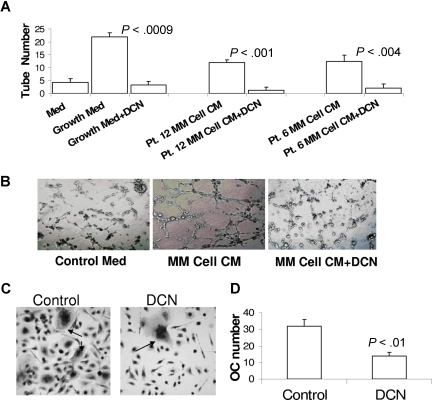
In our investigation of the effect of decorin on the stimulation of angiogenesis and osteoclastogenesis, 2 typical manifestations were associated with myeloma cell growth in the bone marrow.1,2 To study decorin's effect on angiogenesis, we used the tube-formation assay of HUVECs on Matrigel and initially demonstrated decorin's ability to inhibit tube formation in medium supplemented with a cocktail of growth factors (Figure 6A,B; see “Tube-formation assay”). Next, we demonstrated that conditioned medium from culture of myeloma cells stimulated tube formation by HUVECs (P < .01) and that decorin significantly attenuated this effect (P < .004; Figure 6A,B).
Figure 6.
Decorin inhibits myeloma cell-induced tube formation and osteoclastogenesis. HUVECs were seeded on Matrigel for 3 to 4 hours with the indicated media and conditions. (A) Medium containing a cocktail of growth factors (see “Tube-formation assay”) or 48-hour conditioned medium from culture of myeloma cells from 2 patients induced tube formation by HUVECs, an effect that was completely abrogated by decorin (DCN, 10 μg/mL). (B) Photomicrographs show representative tube formation after incubation with medium lacking growth factors (control medium) and with myeloma cell-conditioned medium in the absence or presence of decorin (original magnification ×10). (C,D) Osteoclast (OC) precursors were incubated with osteoclast medium in the absence or presence of decorin. The number of TRAP-expressing multinucleate osteoclasts shown in panel C (→; original magnification ×20) was counted and plotted in panel D.
To test whether decorin affects osteoclastogenesis, osteoclast precursors3 were cultured in osteoclastic medium in the presence or absence of decorin for 5 to 7 days. During this incubation period, many multinucleate osteoclasts formed in control but not decorin-treated cultures (Figure 6C,D). Altogether, these experiments indicate that SLRPs such as decorin are effective inhibitors of angiogenesis and osteoclastogenesis.
Effect of bortezomib on decorin expression in donor and patient osteoblasts
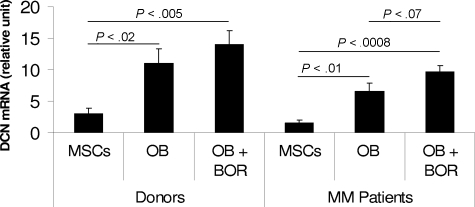
Bortezomib is a novel pharmaceutical agent with promising efficacy, even in patients with relapsed refractory myeloma.37 Recent clinical observations indicate that bortezomib significantly increases levels of markers of bone formation38–40 and number of osteoblasts41 in patients with myeloma. We therefore generated MSCs from 3 healthy donors and 3 patients with myeloma and differentiated these MSCs into osteoblasts in the absence or presence of bortezomib (2.5 nM)41 for 2 weeks. Decorin expression was insignificantly higher in MSCs and osteoblasts from healthy donors than patients with myeloma (Figure 7).
Figure 7.
Expression of decorin in donor and patient MSCs and osteoblasts, and after bortezomib treatment. MSCs generated from healthy donors (n = 3) and patients with myeloma (n = 3) were differentiated into osteoblasts (OB) in the absence or presence of bortezomib (BOR, 2 nM) for 2 weeks. Decorin expression was determined by qRT-PCR amplification. Note the increased expression of decorin after differentiation of donor and patient MSCs into osteoblasts. Decorin expression was insignificantly lower in patient than in donor MSCs and osteoblasts and higher after bortezomib treatment.
Decorin expression was significantly increased after the differentiation of donor (P < .02) and patient (P < .01) MSCs into osteoblasts (Figure 7). Bortezomib slightly increased decorin expression in the differentiated osteoblasts, particularly in patient osteoblasts, for which the P value reached a near-significant level (P < .07). These insignificant values are probably the result of the small number of samples tested, and study is ongoing to evaluate the expression of decorin in donor and patient osteoblasts, along with bortezomib's impact, in a large number of samples.
Discussion
This study provides the first insight into the molecular mechanisms associated with the antimyeloma effect of osteoblasts, which, as we previously showed, impede the growth of myeloma cells in a subset of patients while having no effect on or stimulating the survival of myeloma cells in other subsets of patients.5 In this study, we demonstrate that osteoblasts, in contrast to MSCs, express and produce high levels of various SLRPs and proteoglycans. The focus in this study was the involvement of decorin in the antimyeloma effects of osteoblasts because decorin has been implicated as a tumor suppressor18,20,28,29,42 and its expression level has been correlated with disease progression.25,43
We showed that blocking decorin activity with neutralizing antibody or inhibiting its expression with the use of shRNA partially but significantly reduced the inhibitory effect of osteoblasts on myeloma cell growth and survival. Moreover, recombinant decorin directly induced myeloma cell apoptosis and attenuated the stimulatory effect of osteoclasts on myeloma cells. Overexpression of decorin in MSCs lessened the ability of these cells to support myeloma cell survival. For the first time, we also showed the ability of decorin to suppress osteoclast differentiation and myeloma cell–induced tube formation by HUVECs. In accordance with our previous findings demonstrating the inhibitory effect of increased osteoblastogenesis and bone formation on myeloma progression in vivo,5,44–46 this study suggests that osteogenic cells exert their antitumor effect directly, as well as indirectly through modulation of the myeloma bone marrow microenvironment by the production of high levels of decorin and probably other SLRPs.
Intriguingly, myeloma cells, in contrast to normal plasma cells, did not express decorin. Furthermore, decorin expression had a tendency to be lower in myeloma patients' than in healthy donors' MSCs and the osteoblasts that were differentiated from these cells. An ongoing study using a larger number of samples will determine whether decorin expression is significantly different in MSCs and osteoblasts from normal and myeloma patients and in myeloma patients at different stages of disease. Similarly, bortezomib treatment, which has been clinically associated with increased osteoblastic activity in myeloma patients,38–41 had a tendency to increase decorin expression in osteoblasts, particularly those generated from patients with myeloma. These studies suggest that bortezomib has direct effects on myeloma cells, as well as mediating the antimyeloma response through an increase in the number of osteoblasts and through expression of antimyeloma factors, such as SLRPs, in myelomatous bone.
The mechanisms by which decorin impedes myeloma cell growth are partially understood. We have demonstrated that decorin is internalized by myeloma cells, induces up-regulation of p21WAF, and directly promotes apoptosis in primary CD138-selected myeloma cells, confirming similar effects in other malignancies.17,20,28,29 Decorin may mediate cellular processes by binding to and regulating the activity of a number of growth factors, including TGF-β,47 tumor necrosis factor α,48 and platelet-derived growth factor,49 and by binding to EGFR14 and IGF-1R.15 Decorin is also involved in extracellular matrix assembly through interactions with extracellular matrix proteins, including certain collagen types,50–53 fibronectin,54 thrombospondin 1,55 and tenascin-X.56 The consequences of these actions and interactions may lead to inhibition or stimulation of cell adhesion, survival, proliferation, and migration, depending on the physiologic or pathologic conditions.
Intensive studies by Iozzo et al suggest that a major antitumor mechanism of decorin is mediated by binding to EGFR and induction of phosphorylation and rapid degradation of the receptor in carcinoma cells.13,20,28,29 Recent studies emphasize the role of heparin-binding EGF ligands as myeloma cell growth factors by demonstrating that the majority of primary myeloma cells (but not myeloma cell lines) express EGFR and respond to growth stimulation by heparin-binding EGF ligands through autocrine and paracrine mechanisms.57,58 Whether the antimyeloma effect of decorin is also mediated via inhibition of EGF signaling is a matter for future studies. We speculate that the heterogeneous response of myeloma cells to decorin and/or osteoblasts may depend on properties of the myeloma cells, including level of dependency on the bone marrow microenvironment, proliferation rate, and cytogenetic abnormalities. Indeed, the majority of transformed myeloma cell lines do not respond to decorin (data not shown). This suggests that decorin or osteoblasts have an impact on myeloma cells that are highly bone marrow–dependent and thus likely to be susceptible to growth inhibition by drugs that interfere with the myeloma cell–microenvironment interaction, prevent bone disease, or stimulate bone formation.
Decorin's only partial involvement in the antimyeloma effects of osteoblasts in this study suggests that additional factors mediate these effects. Other SLRPs produced by osteoblasts, such as lumican21,22 and biglycan,23 have been associated with antitumor activity. Bone morphogenetic proteins, also produced by osteoblasts, have been shown to inhibit myeloma cell growth.59–61 Our unpublished data (S.Y., January 2007) show that osteoblasts produce high levels of various fibulins, some of which are known to inhibit angiogenesis and tumorigenesis.62 Secretion of certain collagens, such as XVIII, IVA1, and IVA2, by osteoblasts during bone formation may lead to the production of antiangiogenic and antitumorigenic fragments, such as endostatin, arresten, and canstatin.63,64 It is noteworthy, however, that mesenchymal osteoblasts, particularly immature osteoblasts, also secrete various cytokines and adhesion molecules recognized as myeloma cell growth factors, such as interleukin-6 and osteopontin.65–69 Thus, the inhibitory or stimulatory effects of osteoblastogenesis on myeloma cells may be influenced by the differentiation stage of osteoblasts and the balance between growth and inhibitory factors produced by osteoblasts in the bone marrow milieu or cocultures, as well as by the susceptibility of myeloma cells to growth modula-tion by these factors. Additional studies are required to elucidate the molecular mechanisms associated with myeloma cell–osteoblast interactions.
Our findings suggest that decorin may affect myeloma cell growth indirectly by controlling angiogenesis and osteoclastogenesis. Decorin has previously been shown to inhibit tumor angiogenesis30 and tube formation,31 although other work has proposed an important role for decorin in wound healing.70 These studies suggest that decorin's effects on microvasculature depend on specific physiologic and pathologic conditions. The effect of decorin on osteoclast formation has not been reported. Mice deficient in SLRPs often develop osteoporosis because of impaired bone formation rather than increased osteoclast activity.12 Bone-building osteoblasts have been shown to produce a high level of osteoprotegerin, known to inhibit osteoclastogenesis,71,72 and other antiosteoclastogenic factors, such the ephrin receptor EphB4.73 It is therefore not unexpected that certain SLRPs may help control osteoclastogenesis during the process of bone formation.
In conclusion, we have demonstrated that SLRPs such as decorin are involved in the antimyeloma effects of osteoblasts through direct mechanisms, as well as indirectly by interfering with the stimulatory effects of osteoclasts and MSCs on myeloma cell growth and by inhibiting myeloma-induced angiogenesis and osteoclastogenesis. This study suggests that increased osteoblastic activity and bone formation may help control myeloma by increasing levels of antitumor factors in myelomatous bone marrow, including certain SLRPs and other as-yet-unidentified factors produced by osteoblasts.
Acknowledgments
The authors thank the members of Dr Yaccoby's laboratory: Rinku Saha, Wen Ling, Sharmin Khan, Jianmei Chen, and Paul Perkins, as well as the faculty, staff, and patients of the Myeloma Institute for Research and Therapy for their support, and the Office of Grants and Scientific Publications at the University of Arkansas for Medical Sciences for editorial assistance during the preparation of this manuscript.
This work was supported by grants from the National Cancer Institute (CA-93897; S.Y.) and from the Multiple Myeloma Research Foundation (Senior and Translational Research Award; S.Y.). A.P. was supported by a grant from Fondazione Catanese per la Studio e la Cura delle Malattie Neoplastiche del Sangue (FON.CA.NE.SA), Catania, Italy.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.L. performed research, analyzed and interpreted the data, and wrote the paper; A.P. performed research, interpreted the data, and wrote the paper; S.Y. performed research, conceptualized the work, analyzed and interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shmuel Yaccoby, Myeloma Institute for Research and Therapy, Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, 4301 W Markham, Slot 776, Little Rock, AR 72205; e-mail: yaccobyshmuel@uams.edu.
References
- 1.Bataille R, Chappard D, Marcelli C, et al. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88:62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vacca A, Ribatti D, Roncali L, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- 3.Yaccoby S, Wezeman MJ, Henderson A, et al. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 4.Abe M, Hiura K, Wilde J, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 5.Yaccoby S, Wezeman MJ, Zangari M, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsiades CS, Mitsiades NS, Munshi NC, et al. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: interplay of growth factors, their receptors and stromal interactions. Eur J Cancer. 2006;42:1564–1573. doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Grigorieva I, Thomas X, Epstein J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Exp Hematol. 1998;26:597–603. [PubMed] [Google Scholar]
- 8.Bloem AC, Lamme T, de Smet M, et al. Long-term bone marrow cultured stromal cells regulate myeloma tumour growth in vitro: studies with primary tumour cells and LTBMC-dependent cell lines. Br J Haematol. 1998;100:166–175. doi: 10.1046/j.1365-2141.1998.00517.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 10.Waddington RJ, Roberts HC, Sugars RV, et al. Differential roles for small leucine-rich proteoglycans in bone formation. Eur Cell Mater. 2003;6:12–21. doi: 10.22203/ecm.v006a02. [DOI] [PubMed] [Google Scholar]
- 11.Balint E, Lapointe D, Drissi H, et al. Phenotype discovery by gene expression profiling: mapping of biological processes linked to BMP-2-mediated osteoblast differentiation. J Cell Biochem. 2003;89:401–426. doi: 10.1002/jcb.10515. [DOI] [PubMed] [Google Scholar]
- 12.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 13.Moscatello DK, Santra M, Mann DM, et al. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iozzo RV, Moscatello DK, McQuillan DJ, et al. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 15.Schonherr E, Sunderkotter C, Iozzo RV, et al. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 16.Brandan E, Retamal C, Cabello-Verrugio C, et al. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J Biol Chem. 2006;281:31562–31571. doi: 10.1074/jbc.M602919200. [DOI] [PubMed] [Google Scholar]
- 17.De Luca A, Santra M, Baldi A, et al. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 18.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 19.Reed CC, Waterhouse A, Kirby S, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 20.Zhu JX, Goldoni S, Bix G, et al. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 21.Naito Z. Role of the small leucine-rich proteoglycan (SLRP) family in pathological lesions and cancer cell growth. J Nippon Med Sch. 2005;72:137–145. doi: 10.1272/jnms.72.137. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Aoki T, Mori Y, et al. Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res. 2004;64:7058–7064. doi: 10.1158/0008-5472.CAN-04-1038. [DOI] [PubMed] [Google Scholar]
- 23.Weber CK, Sommer G, Michl P, et al. Biglycan is overexpressed in pancreatic cancer and induces G1-arrest in pancreatic cancer cell lines. Gastroenterology. 2001;121:657–667. doi: 10.1053/gast.2001.27222. [DOI] [PubMed] [Google Scholar]
- 24.Leygue E, Snell L, Dotzlaw H, et al. Lumican and decorin are differentially expressed in human breast carcinoma. J Pathol. 2000;192:313–320. doi: 10.1002/1096-9896(200011)192:3<313::AID-PATH694>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Troup S, Njue C, Kliewer EV, et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res. 2003;9:207–214. [PubMed] [Google Scholar]
- 26.Huijun W, Long C, Zhigang Z, et al. Ex vivo transfer of the decorin gene into rat glomerulus via a mesangial cell vector suppressed extracellular matrix accumulation in experimental glomerulonephritis. Exp Mol Pathol. 2005;78:17–24. doi: 10.1016/j.yexmp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Stander M, Naumann U, Wick W, et al. Transforming growth factor-beta and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999;296:221–227. doi: 10.1007/s004410051283. [DOI] [PubMed] [Google Scholar]
- 28.Csordas G, Santra M, Reed CC, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin: a mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 29.Seidler DG, Goldoni S, Agnew C, et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 30.Grant DS, Yenisey C, Rose RW, et al. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 31.Davies CL, Melder RJ, Munn LL, et al. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res. 2001;62:26–42. doi: 10.1006/mvre.2001.2311. [DOI] [PubMed] [Google Scholar]
- 32.Zhan F, Hardin J, Kordsmeier B, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002;99:1745–1757. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- 33.Ge Y, Zhan F, Barlogie B, et al. Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. Br J Haematol. 2006;133:83–92. doi: 10.1111/j.1365-2141.2006.05976.x. [DOI] [PubMed] [Google Scholar]
- 34.Yaccoby S. The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clin Cancer Res. 2005;11:7599–7606. doi: 10.1158/1078-0432.CCR-05-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyake K, Flygare J, Kiefer T, et al. Development of cellular models for ribosomal protein S19 (RPS19)-deficient diamond-blackfan anemia using inducible expression of siRNA against RPS19. Mol Ther. 2005;11:627–637. doi: 10.1016/j.ymthe.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Zufferey R, Nagy D, Mandel RJ, et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 37.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 38.Zangari M, Esseltine D, Lee CK, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131:71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 39.Heider U, Kaiser M, Muller C, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77:233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 40.Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135:688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 41.Giuliani N, Morandi F, Tagliaferri S, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 42.Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem. 2000;275:35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 43.Matsumine A, Shintani K, Kusuzaki K, et al. Expression of decorin, a small leucine-rich proteoglycan, as a prognostic factor in soft tissue tumors. J Surg Oncol. 2007;96:411–418. doi: 10.1002/jso.20745. [DOI] [PubMed] [Google Scholar]
- 44.Yaccoby S, Ling W, Zhan F, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards CM, Edwards JR, Lwin ST, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiang YW, Shaughnessy JD, Jr, Yaccoby S. Activation of canonical Wnt signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood. 2008 Mar 14; doi: 10.1182/blood-2007-10-120253. DOI: 10.1182/blood-2007-10-120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hildebrand A, Romaris M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tufvesson E, Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002;530:124–128. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 49.Nili N, Cheema AN, Giordano FJ, et al. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol. 2003;163:869–878. doi: 10.1016/S0002-9440(10)63447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown DC, Vogel KG. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix. 1989;9:468–478. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- 51.Bidanset DJ, Guidry C, Rosenberg LC, et al. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267:5250–5256. [PubMed] [Google Scholar]
- 52.Font B, Aubert-Foucher E, Goldschmidt D, et al. Binding of collagen XIV with the dermatan sulfate side chain of decorin. J Biol Chem. 1993;268:25015–25018. [PubMed] [Google Scholar]
- 53.Tenni R, Viola M, Welser F, et al. Interaction of decorin with CNBr peptides from collagens I and II: evidence for multiple binding sites and essential lysyl residues in collagen. Eur J Biochem. 2002;269:1428–1437. doi: 10.1046/j.1432-1033.2002.02784.x. [DOI] [PubMed] [Google Scholar]
- 54.Lewandowska K, Choi HU, Rosenberg LC, et al. Fibronectin-mediated adhesion of fibroblasts: inhibition by dermatan sulfate proteoglycan and evidence for a cryptic glycosaminoglycan-binding domain. J Cell Biol. 1987;105:1443–1454. doi: 10.1083/jcb.105.3.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winnemoller M, Schon P, Vischer P, et al. Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment. Eur J Cell Biol. 1992;59:47–55. [PubMed] [Google Scholar]
- 56.Elefteriou F, Exposito JY, Garrone R, et al. Binding of tenascin-X to decorin. FEBS Lett. 2001;495:44–47. doi: 10.1016/s0014-5793(01)02361-4. [DOI] [PubMed] [Google Scholar]
- 57.Mahtouk K, Jourdan M, De Vos J, et al. An inhibitor of the EGF receptor family blocks myeloma cell growth factor activity of HB-EGF and potentiates dexamethasone or anti-IL-6 antibody-induced apoptosis. Blood. 2004;103:1829–1837. doi: 10.1182/blood-2003-05-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahtouk K, Hose D, Reme T, et al. Expression of EGF-family receptors and amphiregulin in multiple myeloma: amphiregulin is a growth factor for myeloma cells. Oncogene. 2005;24:3512–3524. doi: 10.1038/sj.onc.1208536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawamura C, Kizaki M, Ikeda Y. Bone morphogenetic protein (BMP)-2 induces apoptosis in human myeloma cells. Leuk Lymphoma. 2002;43:635–639. doi: 10.1080/10428190290012182. [DOI] [PubMed] [Google Scholar]
- 60.Hjertner O, Hjorth-Hansen H, Borset M, et al. Bone morphogenetic protein-4 inhibits proliferation and induces apoptosis of multiple myeloma cells. Blood. 2001;97:516–522. doi: 10.1182/blood.v97.2.516. [DOI] [PubMed] [Google Scholar]
- 61.Ro TB, Holt RU, Brenne AT, et al. Bone morphogenetic protein-5, -6 and -7 inhibit growth and induce apoptosis in human myeloma cells. Oncogene. 2004;23:3024–3032. doi: 10.1038/sj.onc.1207386. [DOI] [PubMed] [Google Scholar]
- 62.Gallagher WM, Currid CA, Whelan LC. Fibulins and cancer: friend or foe? Trends Mol Med. 2005;11:336–340. doi: 10.1016/j.molmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 65.Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 66.Barille S, Collette M, Bataille R, et al. Myeloma cells upregulate interleukin-6 secretion in osteoblastic cells through cell-to-cell contact but downregulate osteocalcin. Blood. 1995;86:3151–3159. [PubMed] [Google Scholar]
- 67.Karadag A, Oyajobi BO, Apperley JF, et al. Human myeloma cells promote the production of interleukin 6 by primary human osteoblasts. Br J Haematol. 2000;108:383–390. doi: 10.1046/j.1365-2141.2000.01845.x. [DOI] [PubMed] [Google Scholar]
- 68.McKee MD, Nanci A. Osteopontin: an interfacial extracellular matrix protein in mineralized tissues. Connect Tissue Res. 1996;35:197–205. doi: 10.3109/03008209609029192. [DOI] [PubMed] [Google Scholar]
- 69.Standal T, Hjorth-Hansen H, Rasmussen T, et al. Osteopontin is an adhesive factor for myeloma cells and is found in increased levels in plasma from patients with multiple myeloma. Haematologica. 2004;89:174–182. [PubMed] [Google Scholar]
- 70.Jarvelainen H, Puolakkainen P, Pakkanen S, et al. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 71.Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 72.Qiang YW, Chen Y, Stephens O, et al. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008 Feb 27; doi: 10.1182/blood-2008-01-132134. DOI: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]