Abstract
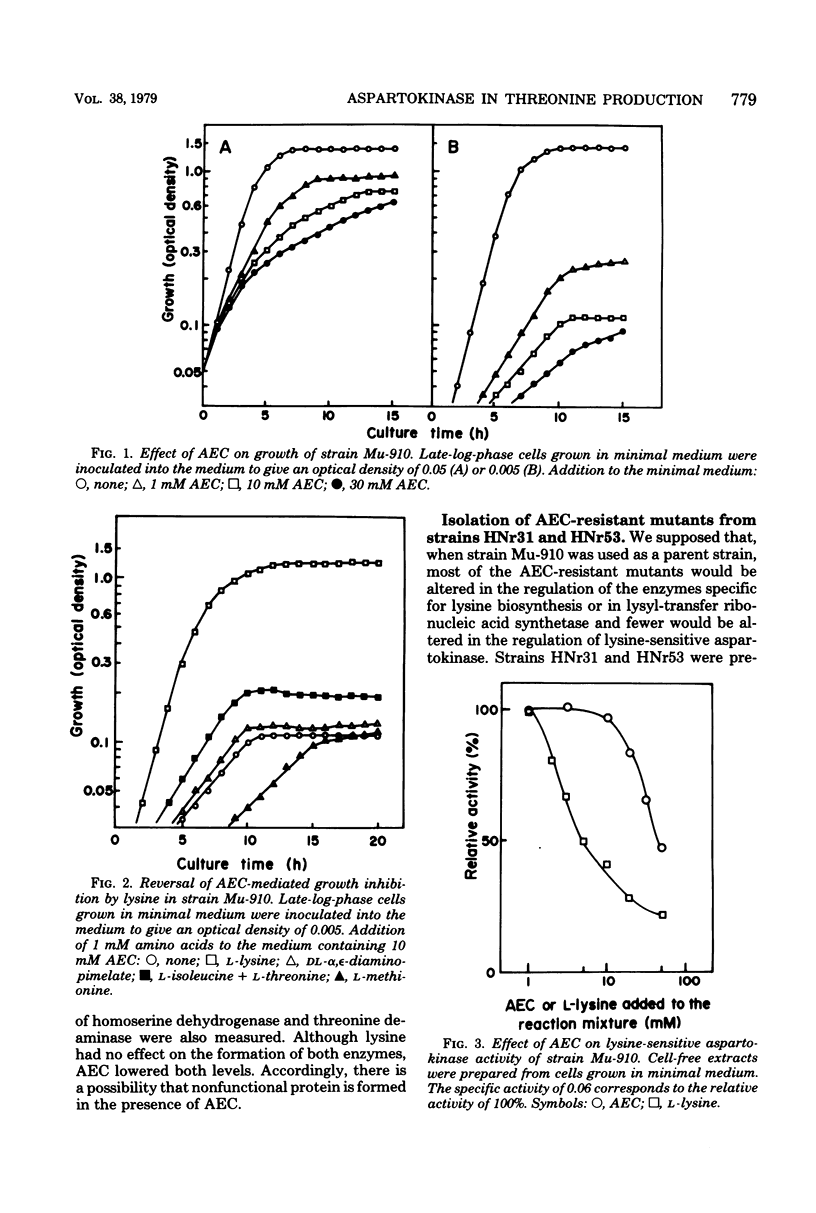
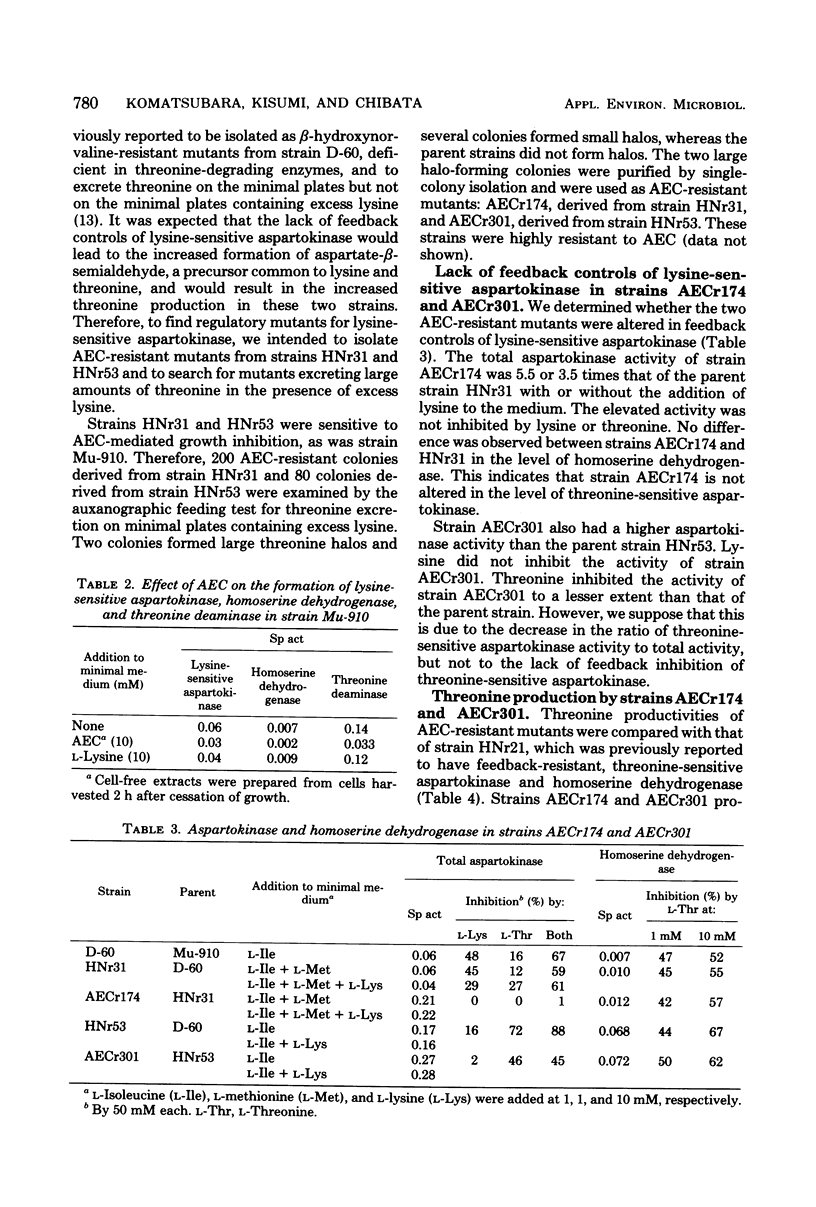
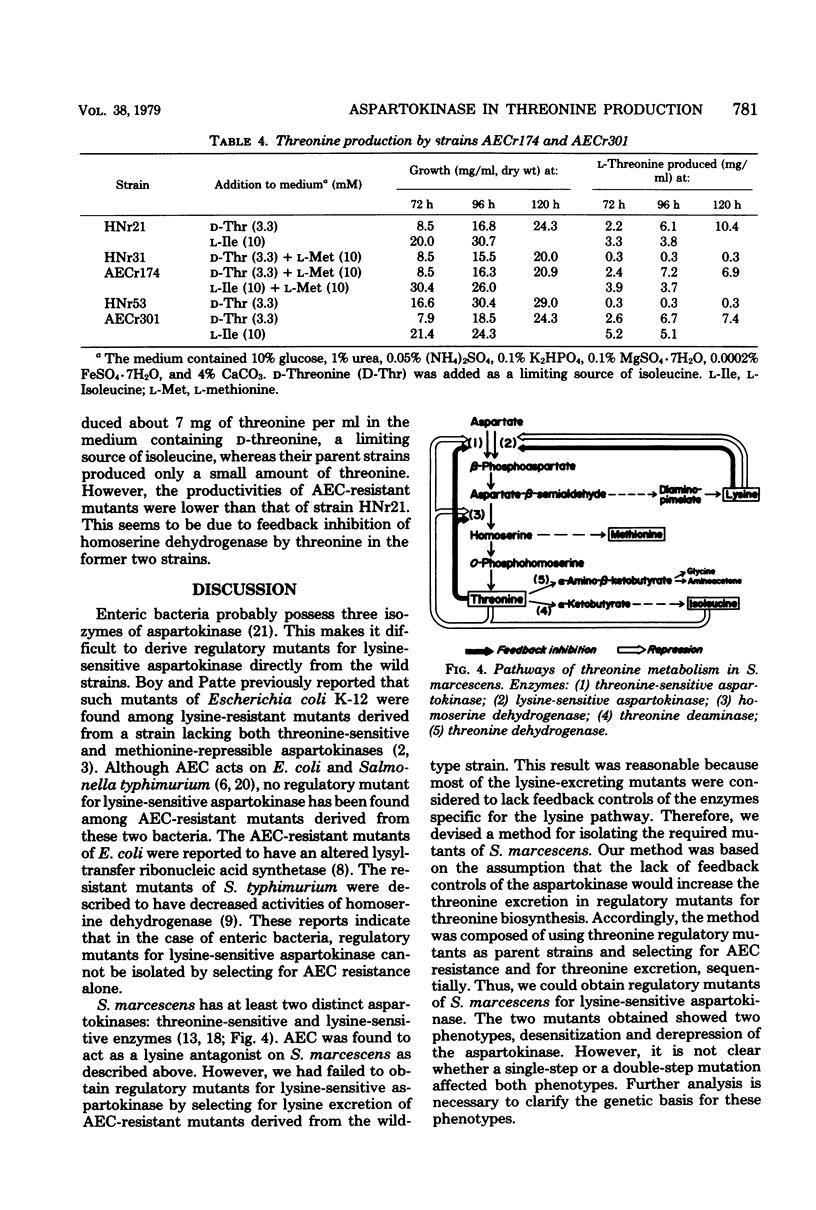
S-2-Aminoethyl cysteine (AEC) reduced both growth rate and final growth level of Serratia marcescens Sr41. The growth inhibition was completely reversed by lysine. AEC inhibited the activity of lysine-sensitive aspartokinase to a lesser extent than lysine. The AEC addition to the medium lowered not only the level of lysine-sensite aspartokinase but also those of homoserine dehydrogenase and threonine deaminase, whereas lysine repressed the aspartokinase alone. To select mutations releasing lysine-sensitive aspartokinase from feedback controls, AEC-resistant colonies were isolated from strains HNr31 and HNr53, both of which were previously found to excrete threonine on the minimal plates but not on the plates containing excess lysine. Two of 280 resistant colonies excreted large amounts of threonine. Strains AECr174 and AECr301, derived from strains HNr31 and HNr53, respectively, lacked both feedback inhibition and repression of lysine-sensitive aspartokinase. These strains produced about 7 mg of threonine per ml in the medium containing glucose and urea.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boy E., Patte J. C. Multivalent repression of aspartic semialdehyde dehydrogenase in Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):84–92. doi: 10.1128/jb.112.1.84-92.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassan M., Boy E., Borne F., Patte J. C. Regulation of the lysine biosynthetic pathway in Escherichia coli K-12: isolation of a cis-dominant constitutive mutant for AK III synthesis. J Bacteriol. 1975 Aug;123(2):391–399. doi: 10.1128/jb.123.2.391-399.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles F. T., Brenchley J. E. Inhibition of growth and aspartokinase activity of Salmonella typhimurium by thialysine. Biochim Biophys Acta. 1976 May 28;428(3):647–655. doi: 10.1016/0304-4165(76)90194-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Zamecnik P. C. Thiosine-resistant mutants of Escherichia coli K-12 with growth-medium-dependent lysl-tRNA synthetase activity. I. Isolation and physiological characterization. Biochim Biophys Acta. 1972 Feb 15;259(3):330–343. [PubMed] [Google Scholar]
- Jegede V. A., Spencer F., Brenchley J. E. Thialysine-resistant mutant of Salmonella typhimurium with a lesion in the thrA gene. Genetics. 1976 Aug;83(4):619–632. doi: 10.1093/genetics/83.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Enhancement of isoleucine hydroxamate-mediated growth inhibition and improvement of isoleucine-producing strains of Serratia marcescens. Appl Environ Microbiol. 1977 Dec;34(6):647–653. doi: 10.1128/aem.34.6.647-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine hydroxamate, an isoleucine antagonist. J Bacteriol. 1971 Sep;107(3):741–745. doi: 10.1128/jb.107.3.741-745.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Kisumi M., Murata K., Chibata I. Threonine production by regulatory mutants of Serratia marcescens. Appl Environ Microbiol. 1978 May;35(5):834–840. doi: 10.1128/aem.35.5.834-840.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsubara S., Murata K., Kisumi M., Chibata I. Threonine degradation by Serratia marcescens. J Bacteriol. 1978 Aug;135(2):318–323. doi: 10.1128/jb.135.2.318-323.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumoto H., Hosogaya S., Suzuki K., Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975 Feb;19(1):35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- SHIOTA T., FOLK J. E., TIETZE F. Inhibition of lysine utilization in bacteria by S-(beta-aminoethyl) cysteine and its reversal by lysine peptides. Arch Biochem Biophys. 1958 Oct;77(2):372–377. doi: 10.1016/0003-9861(58)90084-5. [DOI] [PubMed] [Google Scholar]
- Shailaja M. S., Rao M. R. Lysine-sensitive aspartate kinase of Serratia marcescens. Indian J Biochem Biophys. 1975 Mar;12(1):17–20. [PubMed] [Google Scholar]
- Shiio I., Miyajima R., Sano K. Genetically desensitized aspartate kinase to the concerted feedback inhibition in Brevibacterium flavum. J Biochem. 1970 Nov;68(5):701–710. doi: 10.1093/oxfordjournals.jbchem.a129403. [DOI] [PubMed] [Google Scholar]
- Stern R., Mehler A. H. Lysyl-sRNA synthetase from Escherichia coli. Biochem Z. 1965 Aug 19;342(4):400–409. [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Vold B., Szulmajster J., Carbone A. Regulation of dihydrodipicolinate synthase and aspartate kinase in Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):970–974. doi: 10.1128/jb.121.3.970-974.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



