Abstract
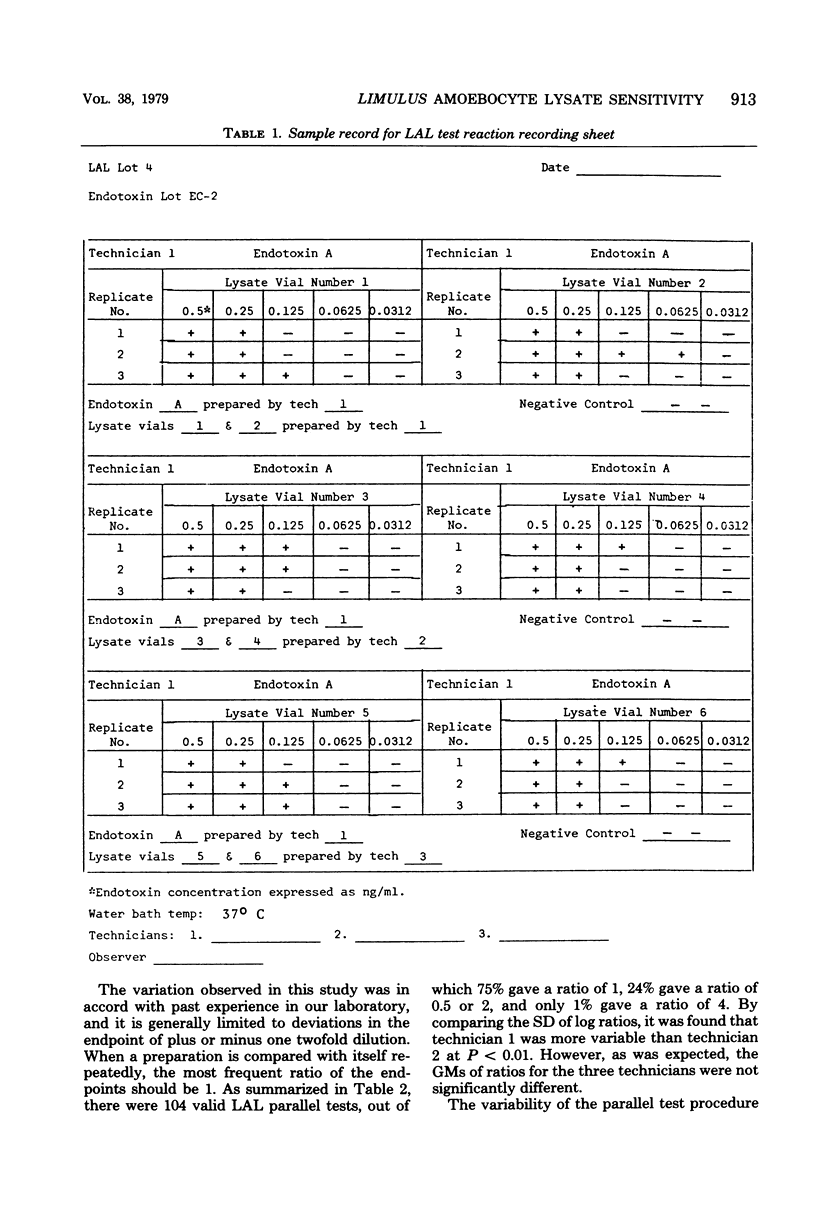
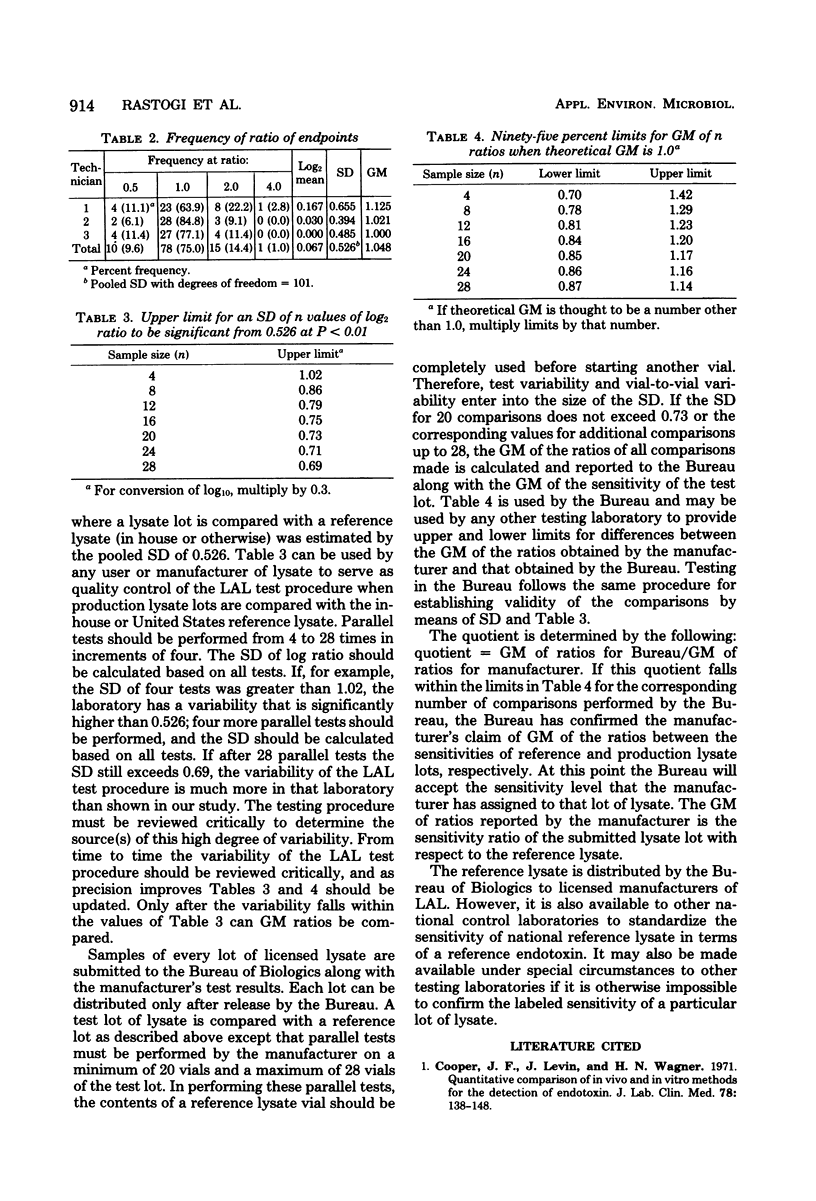
A study was designed to estimate variability of the Limulus amoebocyte lysate test by comparing a reference lysate against itself. Three technicians performed parallel tests, i.e., titrated side by side, the contents of two vials of reference lysate on 4 different days using 24 vials of the United States reference lysate and 12 vials of the United States reference endotoxin. Each parallel test was replicated three times. From the sensitivity endpoints, ratios were calculated for each parallel test. These ratios were converted to the logarithm for estimating variability among technicians and among vials of endotoxin. By using the overall variability of log ratios, a statistical procedure was developed to evaluate the sensitivity of each lot of licensed lysate submitted to the Bureau of Biologics for release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper J. F., Hochstein H. D., Seligmann E. B., Jr The Limulus test for endotoxin (Pyrogen) in radiopharmaceuticals and biologicals. Bull Parenter Drug Assoc. 1972 Jul-Aug;26(4):153–162. [PubMed] [Google Scholar]
- Cooper J. F., Levin J., Wagner H. N., Jr Quantitative comparison of in vitro and in vivo methods for the detection of endotoxin. J Lab Clin Med. 1971 Jul;78(1):138–148. [PubMed] [Google Scholar]
- Cooper J. F., Pearson S. M. Detection of endotoxin in biological products by the limulus test. Dev Biol Stand. 1977;34:7–13. [PubMed] [Google Scholar]
- Jorgensen J. H., Smith R. F. Rapid detection of contaminated intravenous fluids using the Limulus in vitro endotoxin assay. Appl Microbiol. 1973 Oct;26(4):521–524. doi: 10.1128/am.26.4.521-524.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandan R., Brown D. R. An improved in vitro pyrogen test: to detect picograms of endotoxin contamination in intravenous fluids using limulus amoebocyte lysate. J Lab Clin Med. 1977 Apr;89(4):910–918. [PubMed] [Google Scholar]
- Rastogi S. C., Hochstein H. D., Seligmann E. B., Jr Quality control procedures for testing limulus amebocyte lysate sensitivity using Reference Endotoxin 1b. Bull Parenter Drug Assoc. 1977 May-Jun;31(3):120–127. [PubMed] [Google Scholar]
- Rastogi S. C., Hochstein H. D., Seligmann E. B., Jr Statistical determination of endotoxin content in influenza virus vaccine by the limulus amoebocyte lysate test. J Clin Microbiol. 1977 Aug;6(2):144–148. doi: 10.1128/jcm.6.2.144-148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Akiya F. I., Elin R. J., Hochstein H. D., Luoma M. K., Milner E. C., Milner K. C., Thomas K. R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976 Jan;3(1):21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel R. E., Tsuji K. Comparison of limulus amebocyte lysates and correlation with the United States Pharmacopeial pyrogen test. Appl Environ Microbiol. 1977 Jun;33(6):1265–1269. doi: 10.1128/aem.33.6.1265-1269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


