Abstract
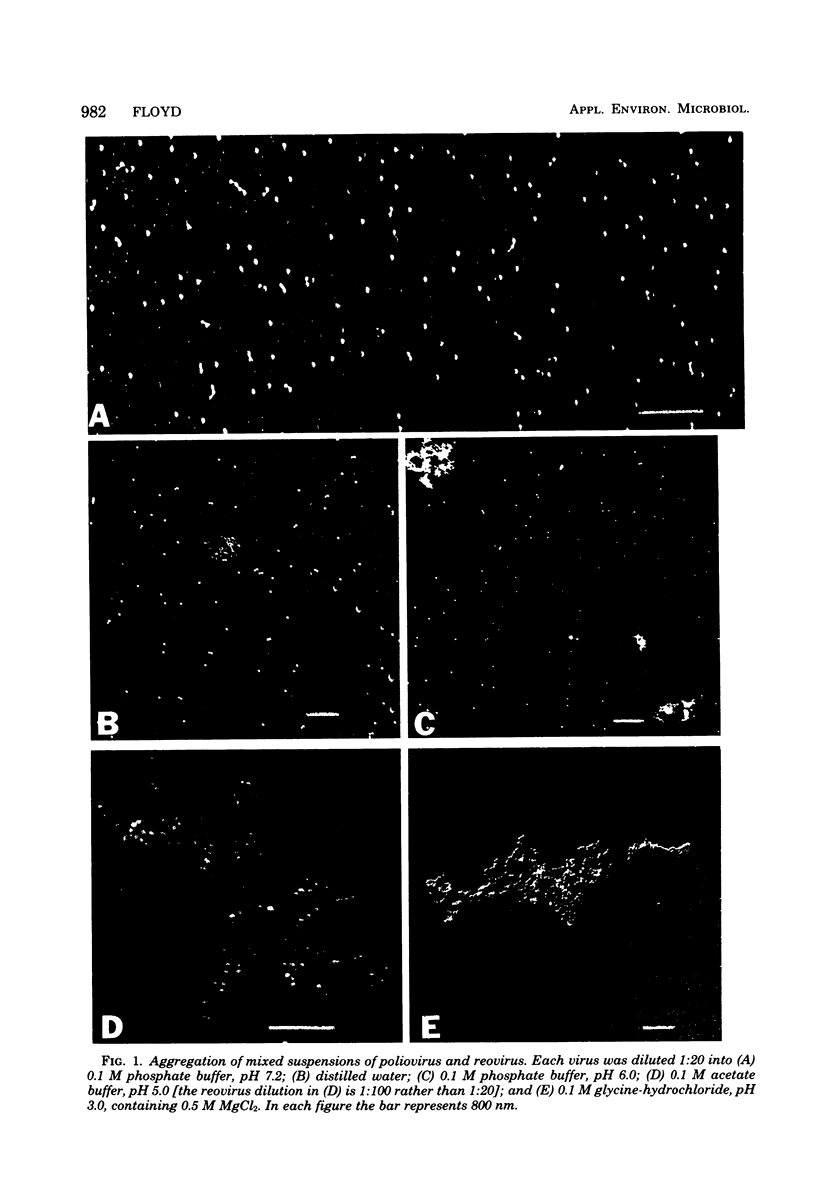
The aggregation of mixtures of two dissimilar viruses, poliovirus I (Mahoney) and reovirus III (Dearing), was followed by electron microscopy under conditions known to induce either aggregation or dispersion of each virus separately. Neither virus aggregated at pH 7 in an appropriate buffer, and no mixed aggregates were formed. Under conditions of lowered ionic strength (by dilution into distilled water) poliovirus became aggregated, whereas reovirus did not, and again no mixed aggregates were formed. At pH 6, however, poliovirus again aggregated and, although reovirus did not, it attached to poliovirus aggregates. Thus, some inducement toward aggregation was necessary to cause formation of mixed aggregates. This inducement probably took the form of a reduction of the ionic double layer surrounding the particles, which is known to occur at low pH. At pH 5 and below both viruses aggregated severely, and large mixed aggregates were formed. These mixed aggregates could be broken up by neutralization of the suspension, although small aggregates of poliovirus remained. Reovirus showed a marked tendency to attach to large clumps of poliovirus, but the reverse tendency was not observed. The results indicate that mixed aggregates may be of significance in the isolation of viruses from water or wastewater.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acute infectious nonbacterial gastroenteritis: etiology and pathogenesis. Ann Intern Med. 1972 Jun;76(6):993–1008. doi: 10.7326/0003-4819-76-6-993. [DOI] [PubMed] [Google Scholar]
- Berg G., Dahling D. R., Berman D. Recovery of small quantities of viruses from clean waters on cellulose nitrate membrane filters. Appl Microbiol. 1971 Oct;22(4):608–614. doi: 10.1128/am.22.4.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscho R., Wyatt R. G., Dolin R., Blacklow N. R., DuPont H. L., Chanock R. M. Recurrent institutional outbreaks of acute infectious nonbacterial gastroenteritis: epidemiology and etiology. Am J Epidemiol. 1973 Sep;98(3):192–198. doi: 10.1093/oxfordjournals.aje.a121548. [DOI] [PubMed] [Google Scholar]
- Cooper P. D. A genetic map of poliovirus temperature-sensitive mutants. Virology. 1968 Aug;35(4):584–596. doi: 10.1016/0042-6822(68)90287-0. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Kapikian A. Z., Purceli R. H. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973 Dec 7;182(4116):1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Floyd R., Johnson J. D., Sharp D. G. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 1976 Feb;31(2):298–303. doi: 10.1128/aem.31.2.298-303.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Aggregation of poliovirus and reovirus by dilution in water. Appl Environ Microbiol. 1977 Jan;33(1):159–167. doi: 10.1128/aem.33.1.159-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: buffer effects in the aggregation of poliovirus and reovirus at low and high pH. Appl Environ Microbiol. 1979 Sep;38(3):395–401. doi: 10.1128/aem.38.3.395-401.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: effects of salts on the aggregation of poliovirus and reovirus at low pH. Appl Environ Microbiol. 1978 Jun;35(6):1084–1094. doi: 10.1128/aem.35.6.1084-1094.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: quantitation and kinetics of the aggregation of poliovirus and reovirus. Appl Environ Microbiol. 1978 Jun;35(6):1079–1083. doi: 10.1128/aem.35.6.1079-1083.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy H. M., Cooney M. K., Hatlen J. B. Adenovirus type 3 epidemic associated with intermittent chlorination of a swimming pool. Arch Environ Health. 1968 Nov;17(5):795–802. doi: 10.1080/00039896.1968.10665321. [DOI] [PubMed] [Google Scholar]
- Hill W. F., Jr, Akin E. W., Benton W. H., Metcalf T. G. Virus in water. II. Evaluation of membrane cartridge filters for recovering low multiplicities of poliovirus from water. Appl Microbiol. 1972 May;23(5):880–888. doi: 10.1128/am.23.5.880-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMB G. A., CHIN T. D., SCARCE L. E. ISOLATIONS OF ENTERIC VIRUSES FROM SEWAGE AND RIVER WATER IN A METROPOLITAN AREA. Am J Hyg. 1964 Nov;80:320–327. doi: 10.1093/oxfordjournals.aje.a120482. [DOI] [PubMed] [Google Scholar]
- Lund E., Hedström C. E., Strannegård O. A comparison between virus isolations from sewage and from fecal specimens from patients. Am J Epidemiol. 1966 Sep;84(2):282–286. doi: 10.1093/oxfordjournals.aje.a120641. [DOI] [PubMed] [Google Scholar]
- Metcalf T. G., Wallis C., Melnick J. L. Environmental factors influencing isolation of enteroviruses from polluted surface waters. Appl Microbiol. 1974 May;27(5):920–926. doi: 10.1128/am.27.5.920-926.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Recombination between conditional lethal mutants within a strain of foot-and-mouth disease virus. J Gen Virol. 1968 Jan;2(1):199–202. doi: 10.1099/0022-1317-2-1-199. [DOI] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Initial fast reaction of bromine on reovirus in turbulent flowing water. Appl Environ Microbiol. 1976 Feb;31(2):173–181. doi: 10.1128/aem.31.2.173-181.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G. Characterization and classification of virus particles associated with hepatitis A. I. Size, density, and sedimentation. J Virol. 1978 Apr;26(1):40–47. doi: 10.1128/jvi.26.1.40-47.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G. Characterization and classification of virus particles associated with hepatitis A. II. Type and configuration of nucleic acid. J Virol. 1978 Apr;26(1):48–53. doi: 10.1128/jvi.26.1.48-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Henderson M., Melnick J. L. Concentration of enteroviruses from large volumes of water. Appl Microbiol. 1973 Oct;26(4):529–534. doi: 10.1128/am.26.4.529-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of enteroviruses on membrane filters. J Virol. 1967 Jun;1(3):472–477. doi: 10.1128/jvi.1.3.472-477.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of viruses from sewage by adsorption on millipore membranes. Bull World Health Organ. 1967;36(2):219–225. [PMC free article] [PubMed] [Google Scholar]