Abstract
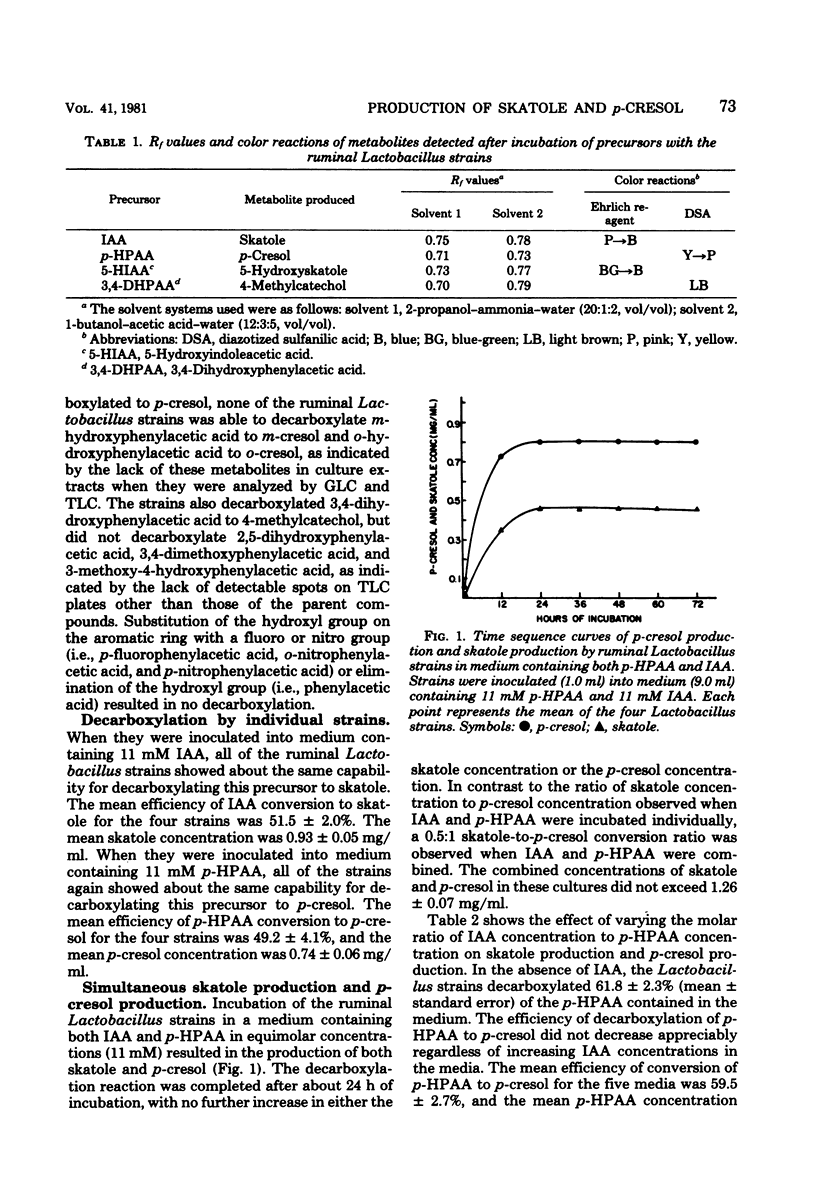
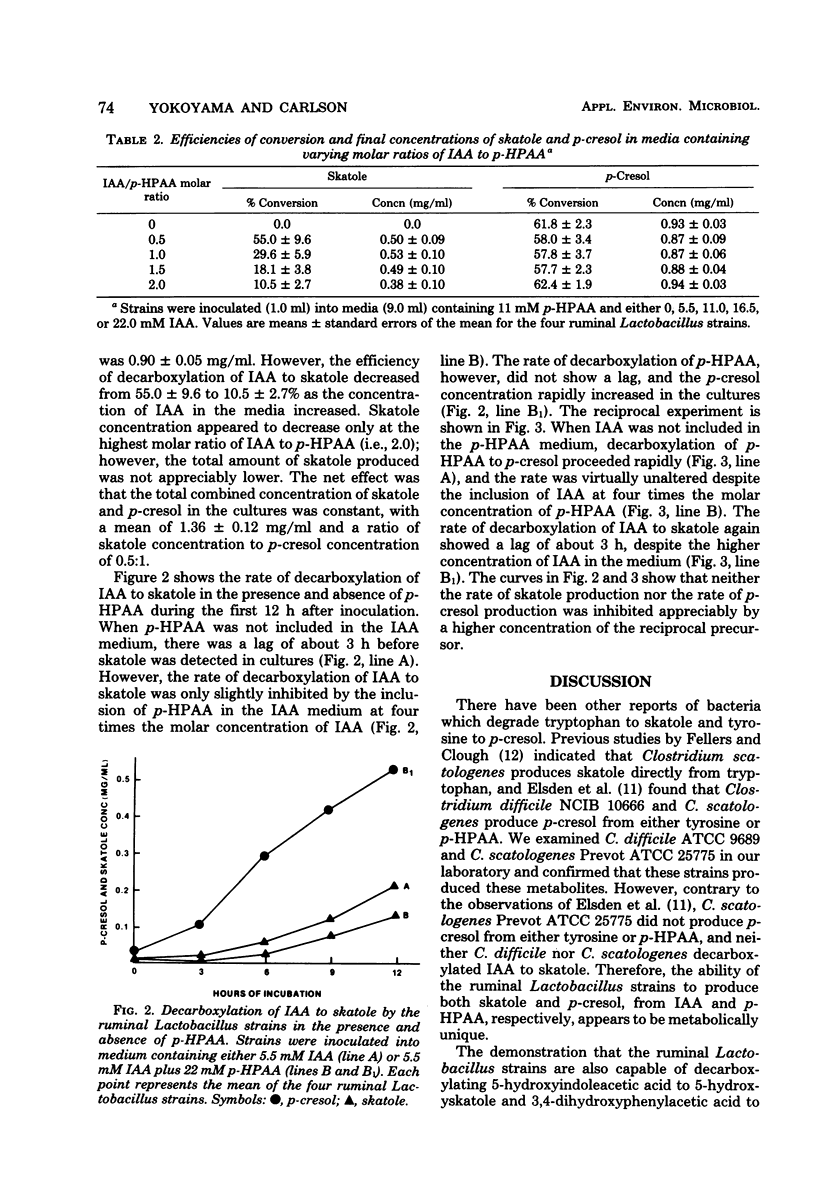
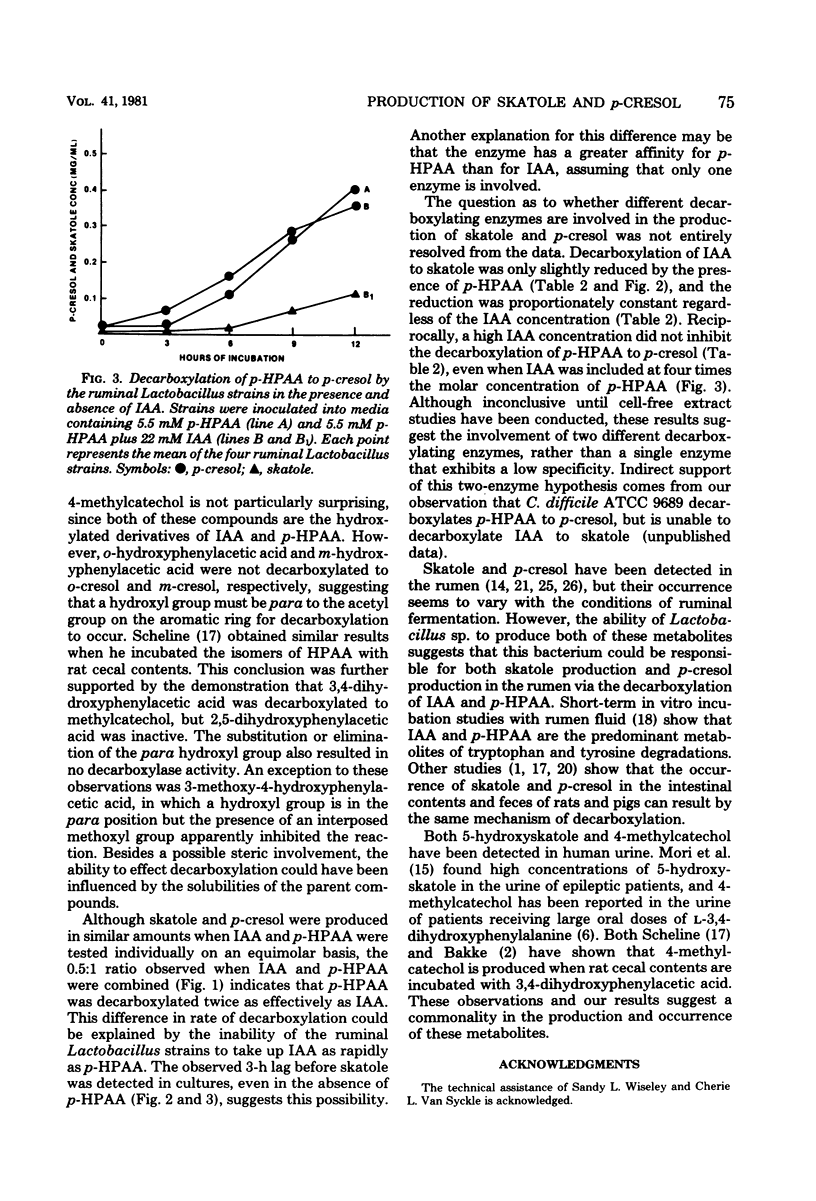
The objective of this study was to examine the substrate specificity of several ruminal strains of a Lactobacillus sp. which previously was shown to produce skatole (3-methylindole) by the decarboxylation of indoleacetic acid. A total of 13 compounds were tested for decarboxylase activity. The Lactobacillus strains produced p-cresol (4-methylphenol) by the decarboxylation of p-hydroxyphenylacetic acid, but did not produce either o-cresol or m-cresol from the corresponding hydroxyphenylacetic acid isomers. These strains also decarboxylated 5-hydroxyindoleacetic acid to 5-hydroxyskatole and 3,4-dihydroxyphenylacetic acid to methylcatechol. Skatole and p-cresol were produced in a 0.5:1 ratio, when indoleacetic acid and p-hydroxyphenylacetic acid were combined in equimolar concentrations. Competition studies with indoleacetic acid and p-hydroxyphenylacetic acid suggested that two different decarboxylating enzymes are involved in the production of skatole and p-cresol by these strains. This is the first demonstration of both skatole production and p-cresol production by a single bacterium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakke O. M. Degradation of DOPA by intestinal microorganisms in vitro. Acta Pharmacol Toxicol (Copenh) 1971;30(1):115–121. doi: 10.1111/j.1600-0773.1971.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Bakke O. M. Studies on the degradation of tyrosine by rat caecal contents. Scand J Gastroenterol. 1969;4(7):603–608. [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne D. B., Karoum F., Ruthven C. R., Sandler M. The metabolism of orally administered L-Dopa in Parkinsonism. Br J Pharmacol. 1969 Sep;37(1):57–68. doi: 10.1111/j.1476-5381.1969.tb09522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. R., Dickinson E. O., Yokoyama M. T., Bradley B. Pulmonary edema and emphysema in cattle after intraruminal and intravenous administration of 3-methylindole. Am J Vet Res. 1975 Sep;36(9):1341–1347. [PubMed] [Google Scholar]
- Carlson J. R., Yokoyama M. T., Dickinson E. O. Induction of pulmonary edema and emphysema in cattle and goats with 3-methylindole. Science. 1972 Apr 21;176(4032):298–299. doi: 10.1126/science.176.4032.298. [DOI] [PubMed] [Google Scholar]
- Elsden S. R., Hilton M. G., Waller J. M. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976 Apr 1;107(3):283–288. doi: 10.1007/BF00425340. [DOI] [PubMed] [Google Scholar]
- Fellers C. R., Clough R. W. INDOL AND SKATOL DETERMINATION IN BACTERIAL CULTURES. J Bacteriol. 1925 Mar;10(2):105–133. doi: 10.1128/jb.10.2.105-133.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A., Yasaka Y., Masamoto K., Hiramatsu M. Gas chromatography of 5-hydroxy-3-methylindole in human urine. Clin Chim Acta. 1978 Mar 1;84(1-2):63–68. doi: 10.1016/0009-8981(78)90477-1. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Metabolism of phenolic acids by the rat intestinal microflora. Acta Pharmacol Toxicol (Copenh) 1968;26(2):189–205. doi: 10.1111/j.1600-0773.1968.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Ward P. F., Dawson R. M. The formation and metabolism of phenyl-substituted fatty acids in the ruminant. Biochem J. 1964 Jan;90(1):12–24. doi: 10.1042/bj0900012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. T., Carlson J. R., Dickinson E. O. Ruminal and plasma concentrations of 3-methylindole associated with tryptophan-induced pulmonary edema and emphysema in cattle. Am J Vet Res. 1975 Sep;36(9):1349–1352. [PubMed] [Google Scholar]
- Yokoyama M. T., Carlson J. R. Dissimilation of tryptophan and related indolic compounds by ruminal microorganisms in vitro. Appl Microbiol. 1974 Mar;27(3):540–548. doi: 10.1128/am.27.3.540-548.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. T., Carlson J. R., Holdeman L. V. Isolation and characteristics of a skatole-producing Lactobacillus sp. from the bovine rumen. Appl Environ Microbiol. 1977 Dec;34(6):837–842. doi: 10.1128/aem.34.6.837-842.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. T., Carlson J. R. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am J Clin Nutr. 1979 Jan;32(1):173–178. doi: 10.1093/ajcn/32.1.173. [DOI] [PubMed] [Google Scholar]



