Abstract
The class I glutathione S-transferases (GSTs) of Anopheles gambiae are encoded by a complex gene family. We describe the genomic organization of three members of this family, which are sequentially arranged on the chromosome in divergent orientations. One of these genes, aggst1-2, is intronless and has been described. In contrast, the two A. gambiae GST genes (aggst1α and aggst1β) reported within are interrupted by introns. The gene aggst1α contains five coding exons that are alternatively spliced to produce four mature GST transcripts, each of which contains a common 5′ exon encoding the N termini of the GST protein spliced to one of four distinct 3′ exons encoding the carboxyl termini. All four of the alternative transcripts of aggst1α are expressed in A. gambiae larvae, pupae, and adults. We report on the involvement of alternative RNA splicing in generating multiple functional GST transcripts. A cDNA from the aggst1β gene was detected in adult mosquitoes, demonstrating that this GST gene is actively transcribed. The percentage similarity of the six cDNAs transcribed from the three GST genes range from 49.5% to 83.1% at the nucleotide level.
Glutathione S-transferases (GSTs) are a major family of detoxification enzymes found in most organisms. All eukaryotes possess multiple GSTs with different substrate specificities to accommodate the wide range of catalytic functions of this enzyme family. The mammalian GSTs have been classified into eight classes: alpha, mu, pi, theta, sigma, zeta, kappa, and a microsomal class (1–6). Nonmammalian GSTs have been assigned to the same classes if their amino acid sequences show >40% identity to other members of the class. However, the sequence of the majority of invertebrate GSTs is below the threshold for inclusion in the mammalian classes, and a separate classification system for these GSTs is more appropriate. Sequence data for nonmammalian GSTs are limited, but there is evidence for at least two classes of insect GSTs (class I and class II) and two classes of plant GSTs (7–9). The bacterial GSTs belong in a separate class because of their low homology with any other known eukaryotic enzyme (10).
The sequences of two insect class II GST genes have been published, aggst2-1 from Anopheles gambiae (11) and DmGST2 from Drosophila melanogaster (7), and a GenBank search identified an additional class II gene from the housefly, Musca domestica (accession no. U02616). Both DmGST2 and aggst2-1 are single-copy genes with no closely related sequences present in the genome of either species.
The insect class I GSTs, in contrast, are encoded by members of a large gene family. In D. melanogaster, eight divergent intronless genes are found within a 14-kb DNA segment (12), and at least five different class I GST genes are present in M. domestica (13). We previously have shown that A. gambiae also contains multiple class I GSTs, which are arranged sequentially in the genome (14).
The class I insect GSTs are of interest because of their role in insecticide resistance. Elevated GST activity has been detected in many resistant strains of insects (see, for example, refs. 15–17) and a subset of in vitro-expressed class I GSTs are capable of metabolizing insecticides (18, 19). GSTs are particularly important in A. gambiae, the major vector of malaria in Africa, as they represent one of only three major insecticide-resistance mechanisms to be found in this insect. We have cloned and expressed two class I GSTs, AGGST1–5 and AGGST1–6, from a 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT)-resistant strain of A. gambiae. Both catalyze the dehydrochlorination of DDT to the noninsecticidal metabolite DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene) but neither belong to the subset of A. gambiae GSTs responsible for the majority of DDT metabolism in our laboratory-resistant strain (20).
We therefore set out to investigate the A. gambiae class I GST gene family further with the aim of characterizing the genetic alterations that lead to DDT resistance in this important malaria vector. We now report the identification of three A. gambiae class I GST cDNAs and describe the genomic organization of the genes from which these GSTs are transcribed.
METHODS
Mosquito Strains.
The Suakoko 2La strain originated from Liberia and is the international reference strain for A. gambiae s.s. mapping work (21). ZAN/U was colonized from Zanzibar, Tanzania in 1982. This strain was selected with DDT for eight generations to produce a highly resistant population.
Sequencing of A. gambiae Genomic DNA.
The recombinant bacteriophage, Ag_B1 was isolated from a Suakoko 2La genomic library by screening with a partial A. gambiae class I GST cDNA, aggst1-1, as described (14). A contiguous sequence of the entire 15.3-kb insert of Ag_B1 was obtained by manual sequencing and automated sequencing using the ALF automatic sequencer (Pharmacia). The sequences were aligned by using the lasergene package (DNAstar, Madison, WI). Primers designed from this sequence were used to amplify the corresponding region of the genome from the DDT-resistant ZAN/U strain. For each primer set, separate PCRs were performed by using genomic DNA extracted from two or more individual ZAN/U mosquitoes. A contig was constructed spanning 4.4 kb of ZAN/U DNA (accession no. AF071160).
PCR Amplification of GST Genes and cDNAs.
mRNA was extracted from ZAN/U larvae, pupae, and adults and was used to synthesize cDNA as described (14). Genomic DNA was extracted from individual mosquitoes by using the method of Collins et al. (22).
PCRs were performed by using the primer sets shown in Table 1 and 1–10 ng of cDNA or 1% of total genomic DNA from a single mosquito as template. Optimal PCR conditions were determined empirically for each primer pair. Taq extender PCR additive (Stratagene) was used for genomic PCRs using P2/P3D and P2/P3C primer pairs.
Table 1.
Sequences of primers used in study
| Primer name | Primer sequence | Size of PCR product expected from cDNA with P2 primer, nt | Size of PCR product expected from genomic DNA with P2 primer, nt |
|---|---|---|---|
| P2 | ATCTGCCCCGTGCCGTGC | N/A | N/A |
| Pβ1.1 | ATGACGCCAGTGCTGTATTAT | N/A | N/A |
| Pβ1.2 | CAACCCACAGCACTGCATACCTACAC | N/A | N/A |
| P3A | CCGTTCTGCGAGGGCTGG | 241 | 1,045 |
| P3B | CTTCCTTGCGAATGTTTTCGT | 546 | 2,314 |
| P3C | AACCATTTCCTCCCGCCACAA | 447 | 2,926 |
| P3D | CGGGGGCGTTTGCTTTGC | 553 | 3,700 |
Expected sizes of PCR products were calculated from Fig. 2. See text for further details. N/A, not applicable.
The PCR products were separated by gel electrophoresis and transferred to a nylon membrane. A plasmid containing the A. gambiae aggst1-6 cDNA was digested with SalI, and a 170-nt fragment, encompassing the 5′ region of the gene, was isolated and used as a template for probe construction. Probes were prepared by using the Hi Prime Labeling Kit (Boehringer Mannheim). Hybridizations were performed at 42°C overnight in formamide hybridization buffer [5× SSC, 5× Denhardt’s solution (0.02% polyvinylpyrrolidone/0.02% Ficoll/0.02% BSA), 1% SDS, 50% formamide, and 100 μg/ml of boiled, sheared salmon sperm DNA]. The filters were washed under conditions of high stringency (final wash 0.1× SSC, 0.1% SDS 65°C).
The 5′ untranslated region of aggst1-5 and aggst1-6 was amplified by PCR using DNA prepared from a cDNA library as a template. A vector primer upstream of the insert was used with antisense primers P3B or P3D.
3′ Rapid Amplification of cDNA Ends (RACE) Amplification of aggst1β Transcripts.
A cDNA transcribed from aggst1β was isolated from a single female ZAN/U adult by a modified 3′ RACE procedure. RNA was extracted from single adult mosquitoes by using TRI Reagent (Sigma). First-strand cDNA was synthesized by using Superscript II reverse transcriptase (GIBCO/BRL) and an oligo(dT) adapter primer [5′-GACTCGAGTCGACATCGA(dT)17-3′] according to the manufacturer’s protocol. Primers β1.1 and β1.2 (Table 1) were designed with 100% congruence to the genomic sequence of the predicted exon 1 of aggst1β but with sufficient mismatches to prevent them from annealing to aggst1α or aggst1-2. An initial round of PCR amplification was carried out by using primers β1.1 and the adapter primer. One microliter of this PCR was used as the template for a second round of PCR amplification using primers β1.2 and the adapter primer. A single 700-nt product was obtained, subcloned into the TA vector (Invitrogen), and sequenced.
Sequence Analysis.
Dipteran GST sequences were retrieved from the GenBank sequence database and aligned by using the clustal program (23).
RESULTS
Identification of GST–Like Sequences Within the Genomic Clone, Ag_B1.
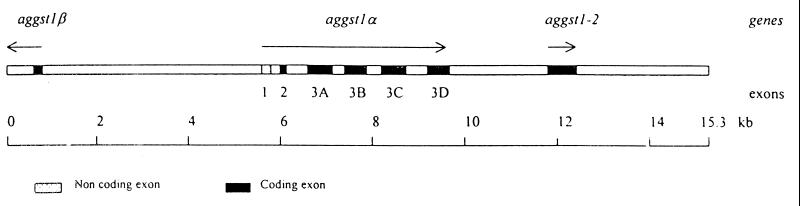
We previously have described a single, intronless class I GST gene, aggst1-2, from the A. gambiae genomic clone, Ag_B1 (14). Restriction mapping of Ag_B1 and two additional, overlapping genomic clones identified multiple fragments that hybridized to a class I GST probe. Therefore the entire insert of Ag_B1 was sequenced and compared with sequences in the database to identify putative GST genes. Six sequences, in addition to the aggst1-2 gene previously described, had high levels of homology to insect class I GST genes. The arrangement of these GST sequences is shown in Fig. 1.
Figure 1.
Arrangement of GST genes in the recombinant bacteriophage clone, Ag_B1, isolated from an A. gambiae Suakoko 2La genomic library. The orientations of the genes are indicated by arrows.
Five of these GST-like sequences are arranged sequentially on the chromosome in the same orientation. The first of these has high levels of sequence identity to the 5′ end of insect class I GSTs, while the four proceeding downstream sequences are truncated at their 5′ ends but have high sequence identities to the 3′ region of GSTs. The sixth GST-like sequence within the Ag_B1 clone is arranged in the opposite orientation and is located 4,995 bp upstream from the first of the sequences described above. This sequence has high sequence identity to the 5′ end of insect class I GSTs. The results of these homology comparisons suggest that the six sequences are exons of two divergently orientated GST genes, which we have named aggst1α and aggst1β. aggst1α is fully contained within the genomic clone Ag_B1, upstream of aggst1-2, whereas only the 5′ region of aggst1β is present in this clone.
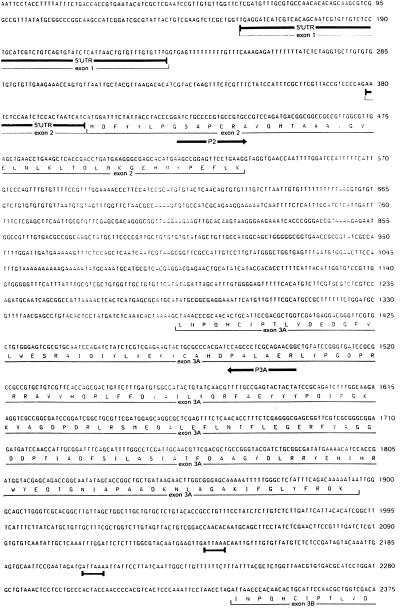
To confirm that the proposed arrangement of GST genes was not an artifact of cloning, the corresponding region of the genome from the DDT-resistant ZAN/U strain was amplified and sequenced. The sequence of this 4.4-kb region of the genome is shown in Fig. 2.
Figure 2.
Nucleotide sequence of the alternatively spliced A. gambiae class I GST gene, aggst1α. Primers designed from the sequence of the An. gambiae S2La genomic clone, Ag_B1, were used to amplify the equivalent region of the genome from the DDT-resistant ZAN/U strain. The derived amino acid sequence of the five coding exons is shown below the nucleotide sequence. The location of the primers used to detect the splice variants are marked by arrows, putative polyadenylation sites are underscored, and the sites of addition of two of the poly(A) tails are marked with #.
The ORF of exon 2 of aggst1α was aligned with the 5′ sequences of the two A. gambiae class I GST cDNAs, aggst1-5 and aggst1-6. A single substitution was found between the first 135 nt of the ORF of aggst1α and the corresponding region of aggst1-6. An additional base pair differs between the sequence of aggst1α and aggst1-5. In addition, alignment of exons 3A, 3B, 3C, and 3D of aggst1α with the A. gambiae class I GST cDNA sequences gave a near-perfect match between the sequence of exon 3B and the 3′ region of aggst1-5 (5/492 mismatched bases) and the sequence of exon 3D with the 3′ region of aggst1-6 (2/492). As aggst1-5 and aggst1-6 are highly polymorphic (H.R., unpublished observation), these high levels of sequence identity suggest that the cDNAs aggst1-5 and aggst1-6 are produced by mRNA splicing within the aggst1α gene. If all four of the exons with homology to the 3′ region end of GST genes can be used, then alternative splicing between exon 2 and either exon 3A or exon 3C should produce additional transcripts.
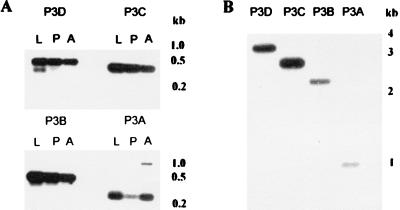
To assess this possibility, a common forward primer complementary to aggst1α exon 2 was used with antisense primers specific to aggst1α exons 3A, 3B, 3C, or 3D in PCRs using cDNA extracted from ZAN/U larvae, pupae, or adults as templates. The location of these primers is shown in Fig. 2, and their sequence and expected size of product is given in Table 1. Fig. 3A shows that all four cDNAs were detected in all life stages of ZAN/U A. gambiae. The cDNAs amplified by primer sets P2/P3B and P2/P3D correspond to the previously published cDNAs aggst1-5 and aggst1-6, respectively, whereas the cDNAs amplified by primer sets P2/P3A and P2/P3C, designated aggst1–3 and aggst1–4, have not previously been characterized. The exon compositions of the four mature transcripts of aggst1α are summarized in Table 2.
Figure 3.
PCR amplification of aggst1α. PCRs were performed on cDNAs from A. gambiae ZAN/U fourth-instar larvae (L), pupae (P), and 1-day-old adults (A) and A. gambiae genomic DNA (B) using the primer sets indicated. The PCR products were separated by gel electrophoresis and transferred to nylon membranes. The DNA was hybridized with a 32P-labeled 5′ GST probe as described in Methods. The final posthybridization wash was at 65°C in 0.1× SSC and 0.1% (wt/vol) SDS.
Table 2.
Exon composition and splice sites of alternative transcripts of the A. gambiae aggst1α gene
| cDNA | Exon composition* | 5′ splice site (exon/intron) | 3′ splice site (intron/exon) |
|---|---|---|---|
| Consensus | AG/GTRAGT | YnNYAG/NN | |
| exon 1 … 2 | TG/GTGAGT | CCCCAG/AA | |
| aggst1-3 | exon 2 … 3A | AG/GTAGGT | AAAG/CT |
| aggst1-5 | exon 2 … 3B | CCTAG/AT | |
| aggst1-4 | exon 2 … 3C | TTTGTAG/CT | |
| aggst1-6 | exon 2 … 3D | TTTTCTAG/CT |
(R = A or G, Y = C or T, N = A, C, G or T).
The presence of the noncoding exon, exon 1, has been confirmed in aggst1-5 and aggst1-6 only.
Because the results shown in Fig. 3A do not preclude the interpretation that expression of the different transcripts is heterogeneous within the ZAN/U population, RNA was extracted from individual adult male and female mosquitoes and used as templates for reverse transcription–PCRs. All four alternatively spliced transcripts were detected in each individual, demonstrating that agggst1-3, aggst1-4, aggst1-5, and aggst1-6 are simultaneously expressed (data not shown).
Confirmation of the Role of mRNA Splicing.
To verify that the cDNAs described above were produced by splicing and were not transcribed from additional gene sequences elsewhere in the genome, the same primer sets were used in PCRs with genomic DNA as the template. The expected size of genomic PCR product for each of the PCR sets was calculated from Fig. 2 and is shown in Table 1. With each primer set, a band of the expected size was obtained. Frequently, several additional PCR products were present, but they failed to hybridize to a 5′ class I GST probe (Fig. 3B).
Analysis of Nontranslated Regions and Exon Intron Boundaries.
The nontranslated upstream sequences of aggst1-5 and aggst1-6 were amplified from DNA extracted from a cDNA library. The sequences obtained matched the published 5′ untranslated region sequence of the partial cDNA aggst1-1, which was obtained by 5′ RACE (14). Comparison of the cDNA sequences with the genomic sequence upstream of aggst1α revealed the presence of a 146-nt intron 24 nt upstream from the initiator ATG and identified an additional noncoding exon, exon 1, within aggst1α (Fig. 2). The exon-intron boundaries within aggst1α are compared with the consensus sequence in Table 2. As the distance from the 5′ splice site increases, the agreement of the 3′ splice site to the consensus sequence also increases.
Each of the four alternative 3′ exons is preceded by, and terminates in, an in-frame stop codon. The intragenic distances between them range from 130 nt (exons 3B and 3C) to 440 nt (exons 3A and 3B), and a putative polyadenylation sequence is located between each exon. The exact lengths of the 3′ nontranslated sequences of aggst1-5 and aggst1-6 as determined by 3′ RACE are 116 nt and 105 nt, respectively (Fig. 2).
Detection of an Additional GST cDNA by 3′ RACE PCR.
A nested 3′ RACE PCR using forward primers specific to the putative 5′ GST exon of aggst1β, amplified a cDNA, designated aggst1–7, with high levels of sequence identity to the A. gambiae class I GST genes. No match to the 3′ sequence of this cDNA was found within the genomic clone Ag_B1, and attempts to amplify the gene from A. gambiae genomic DNA by using primer sets specific for the cDNA sequence aggst1-7 were unsuccessful, suggesting that this gene is interrupted by one or more large introns.
An alignment of the predicted amino acid sequences of the six A. gambiae class I GSTs described in this study is shown in Fig. 4. The degree of similarity at the amino acid level ranges from 87.9% for aggst1-5 vs. aggst1-6 to 46.3% for aggst1-2 vs. aggst1-7 (Table 3).
Figure 4.
Amino acid alignment of A. gambiae class I GST cDNAs. Gaps introduced to maximize sequence identity are shown by a horizontal dash. ∗ indicates the presence of an identical residue in all six A. gambiae GSTs. Residues in bold are shared by all 27 Dipteran class I GSTs within GenBank. The five residues in bold italics are shared by all known insect GSTs. A vertical arrow marks the junction between exon 2 and exon 3 in aggst1-3, aggst1-4, aggst1-5, and aggst1-6.
Table 3.
Pairwise distances between A. gambiae class I GST cDNAs
| aggst1-2 | aggst1-3 | aggst1-4 | aggst1-5 | aggst1-6 | aggst1-7 | |
|---|---|---|---|---|---|---|
| aggst1-2 | 0.391 | 0.411 | 0.394 | 0.391 | 0.537 | |
| aggst1-3 | 0.432 | 0.284 | 0.267 | 0.269 | 0.503 | |
| aggst1-4 | 0.452 | 0.318 | 0.242 | 0.233 | 0.476 | |
| aggst1-5 | 0.457 | 0.293 | 0.310 | 0.121 | 0.468 | |
| aggst1-6 | 0.450 | 0.294 | 0.312 | 0.169 | 0.442 | |
| aggst1-7 | 0.505 | 0.464 | 0.471 | 0.452 | 0.430 |
Mean amino acid character differences are shown above the diagonal, mean nucleotide character differences below the diagonal.
DISCUSSION
Genomic Organization of Class I GSTs.
We have identified three cDNA clones that encode distinct A. gambiae GSTs. These cDNAs are in addition to the single class II GST and three class I GSTs (aggst1-5, aggst1-6, and aggst1-2) described previously (11, 14, 20). The additional sequences are classified as class I GSTs because they share 50–83% sequence identity with the previously characterized class I GSTs but are only distantly related to the class II GST (approximately 33% nucleotide identity). A serine near the N terminus is characteristic of mammalian theta GSTs and insect class I GSTs (24); this residue, which plays a vital role in the catalytic mechanism of the GSTs, is substituted by a tyrosine residue in all other GST classes (25). All of the A. gambiae class I GSTs sequenced to date possess this N-terminal serine (position 11 in Fig. 4).
Four of the six A. gambiae class I GSTs use a common 5′ exon, which is spliced to one of four alternative 3′ exons to produce mature transcripts. The translation products of these transcripts are therefore identical at the N termini (residues 1–45) but highly variable at the C termini. Crystal structures of mammalian and insect GSTs show that the majority of the active site residues involved in the binding and activation of GSH are found within the N terminal and hence this region of the protein is highly conserved between GSTs (24, 26). The divergence in the C-terminal domain confers the variation in substrate specificities of different GSTs. Therefore, the genomic organization of the A. gambiae GSTs provides a mechanism of increasing the diversity of GSTs produced and consequently expanding their substrate range, with a minimal increase in the length of the genome.
It is unlikely that the number of alternative 3′ exons within aggst1α exceeds the four reported here because no additional candidate sequences were found within the 2,285 nt of sequence separating the termination codon of the most distal 3′ exon, 3D, and the start of the intronless gene aggst1-2. However, the full extent of the A. gambiae class I GST family remains to be determined. Eight distinct fractions of GST activity were resolved by sequential column chromatography of A. gambiae homogenates, all of which contained multiple GSTs, which suggests that many more A. gambiae GST genes remain to be cloned (15). In this study we report the amplification of a single full-length GST transcript, aggst1-7, from an adult female mosquito, which we predict is produced by the splicing of exon 1 of aggst1β to one or more 3′ exons. We currently are sequencing overlapping genomic clones to investigate whether alternative splice variants of this gene also exist.
The organization of class I GSTs in A. gambiae contrasts with that in M. domestica. In this species multiple genes encode each class I GST, and additional loci code for fusion GSTs comprising the 5′ half of one GST gene joined to the 3′ half of a different GST gene (13). In addition, while the class I GST genes of A. gambiae and the intronless class I GST genes of D. melanogaster are tightly linked, the multiple loci that encode the housefly GSTs appear to be dispersed throughout the genome.
Choice of Splice Site.
The factors determining the choice of a 3′ splice site remain to be determined. Different subsets of GSTs are responsible for DDT resistance in A. gambiae larvae and adults (27). We therefore investigated whether exon choice was developmentally regulated, but the results of reverse transcription–PCR showed that all four splice variants, aggst1-3, aggst1-4, aggst1-5, and aggst1-6, are simultaneously expressed in fourth-instar larvae, pupae, and 1-day-old adults. Similarly, all exons are expressed in males and females. It seems likely that the choice of 3′ splice site is tissue dependent with specific factors regulating GST expression in each tissue.
Tissue-specific expression of GST isozymes is well documented in mammals (28), but little is known about the tissue distribution of insect GSTs. By using antisera raised against housefly GSTs, the class II enzyme, MdGST2, was shown to be expressed in the indirect flight muscles of the thorax and in the central nervous system, whereas the class I GSTs were uniformly distributed in the hemolymph cells (29). However, the anti-GST-1 antisera used in the M. domestica study was raised against biochemically purified class I GSTs (8), and the housefly class I GST subsequently was shown to be a complex enzyme family consisting of at least five independent subunits (13). Whether this antisera detects all members of this class has not been reported and hence the tissue distribution of insect class I GSTs remains unclear. It should now be possible to address this question for the A. gambiae class I GSTs, either by reverse transcription–PCR using the primer sets described in this study, or by expressing the various 3′ exons in vitro and raising specific antisera to the carboxyl termini of each GST isozyme that could be used in immunohistochemical studies to locate the GSTs in vivo.
The multiple substitutions within the C-terminal domain of the different A. gambiae class I GSTs are predicted to lead to variations in substrate specificities. We have shown this to be the case in an earlier report in which we expressed and characterized two members of this group (20). These GSTs, despite sharing the highest levels of sequence identity within the A. gambiae class I GST family, had markedly different catalytic properties. Further characterization of the substrate specificities, sites of expression, and regulatory mechanisms of the various A. gambiae class I GSTs will lead to a better understanding of the normal physiological role of these enzymes and their role in insecticide resistance.
CONCLUSIONS
This study reports the involvement of alternative RNA splicing in generating multiple functional GST transcripts. Two alternatively spliced transcripts of a human mu GST have been isolated from a cDNA library, but both of these clones are incomplete and do not encode functional GSTs (30). The presence of this splicing mechanism contributes to the high level of GST class I variation reported in biochemical studies of A. gambiae insecticide resistance-associated GSTs. The class I GSTs of M. domestica and D. melanogaster are also highly variable and encoded by complex gene families but the genomic organization of the insect class I GST genes is dramatically different between the insect species. The variability of the insect class I GSTs is in sharp contrast to the class II GSTs, which are present as single genes in these three insect species.
Acknowledgments
We thank Dr. Julian Marchesi for assistance with primer design. This work was funded by a Wellcome Trust Prize Studentship to H.R.
ABBREVIATIONS
- DDT
1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane
- GST
glutathione S-transferase
- RACE
rapid amplification of cDNA ends
Footnotes
References
- 1.Mannervik B. Adv Enzymol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- 2.Meyer D J, Coles B, Pemble S E, Gilmore K S, Fraser G M, Ketterer B. Biochem J. 1991;274:409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer D J, Thomas M. Biochem J. 1995;311:739–742. doi: 10.1042/bj3110739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Board P J, Baker R T, Chelvanayagam G, Jermiin L S. Biochem J. 1997;328:929–935. doi: 10.1042/bj3280929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pemble S E, Wardle A F, Taylor J B. Biochem J. 1996;319:749–754. doi: 10.1042/bj3190749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jong J L, Morgenstern R, Jornvall H, DePierre J W, Tu C-P D. J Biol Chem. 1988;263:8430–8436. [PubMed] [Google Scholar]
- 7.Beall C, Fyrberg C, Song S, Fyrberg E. Biochem Genet. 1992;30:515–527. doi: 10.1007/BF01037590. [DOI] [PubMed] [Google Scholar]
- 8.Fournier D, Bride J M, Poire M, Berge J B, Plapp F W. J Biol Chem. 1992;267:1840–1845. [PubMed] [Google Scholar]
- 9.Marrs KA. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- 10.Mignogna G, Allocati N, Aceto A, Piccolomini R, Ilio C D, Barra D, Martini F. Eur J Biochem. 1993;211:421–425. doi: 10.1111/j.1432-1033.1993.tb17566.x. [DOI] [PubMed] [Google Scholar]
- 11.Reiss R A, James A A. Insect Mol Biol. 1993;2:25–32. doi: 10.1111/j.1365-2583.1993.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 12.Toung Y-P S, Hsieh T, Tu C-P D. J Biol Chem. 1993;268:9737–9746. [PubMed] [Google Scholar]
- 13.Zhou Z-H, Syvanen M. Mol Gen Genet. 1997;256:187–194. doi: 10.1007/s004380050560. [DOI] [PubMed] [Google Scholar]
- 14.Ranson H, Cornel A J, Fournier D, Vaughan A, Collins F H, Hemingway J. J Biol Chem. 1997;272:5464–5468. doi: 10.1074/jbc.272.9.5464. [DOI] [PubMed] [Google Scholar]
- 15.Prapanthadara L, Hemingway J, Ketterman A J. Pestic Biochem Physiol. 1993;47:119–133. [Google Scholar]
- 16.Grant D F, Dietze E C, Hammock B D. Insect Biochem. 1991;4:421–433. [Google Scholar]
- 17.Ottea J A, Plapp F W. Pestic Biochem Physiol. 1984;22:203–208. [Google Scholar]
- 18.Tang A H, Tu C P D. J Biol Chem. 1994;269:27876–27884. [PubMed] [Google Scholar]
- 19.Syvanen M, Zhou Z, Wharton J, Goldsbury C, Clark A. J Mol Evol. 1996;43:236–240. doi: 10.1007/BF02338831. [DOI] [PubMed] [Google Scholar]
- 20.Ranson H, Prapanthadara L, Hemingway J. Biochem J. 1997;324:97–102. doi: 10.1042/bj3240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L, Saunders R D C, Fortini D, Della Torre A, Coluzzi M, Glover D M, Kafatos F C. Proc Natl Acad Sci USA. 1991;88:11187–11191. doi: 10.1073/pnas.88.24.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins F H, Mendez M A, Rasmussen M O, Mehaffey P C, Besansky N J, Finnerty V. Am J Trop Med Hyg. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- 23.Higgins D G, Sharp P M. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 24.Wilce M C J, Board P G, Feil S C, Parker M W. EMBO J. 1995;14:2133–2143. doi: 10.1002/j.1460-2075.1995.tb07207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenberg G, Board P G, Mannervik B. FEBS Lett. 1991;293:153–155. doi: 10.1016/0014-5793(91)81174-7. [DOI] [PubMed] [Google Scholar]
- 26.Dirr H, Reinemer P, Huber R. Eur J Biochem. 1994;220:645–661. doi: 10.1111/j.1432-1033.1994.tb18666.x. [DOI] [PubMed] [Google Scholar]
- 27.Prapanthadara L, Hemingway J, Ketterman A. Bull Entomol Res. 1995;85:267–274. [Google Scholar]
- 28.Hayes J D, Pulford D J. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 29.Franciosa H, Berge J B. Insect Biochem Mol Biol. 1995;25:311–317. doi: 10.1016/0965-1748(94)00053-k. [DOI] [PubMed] [Google Scholar]
- 30.Ross V L, Board P G. Biochem J. 1993;294:373–380. doi: 10.1042/bj2940373. [DOI] [PMC free article] [PubMed] [Google Scholar]