Abstract
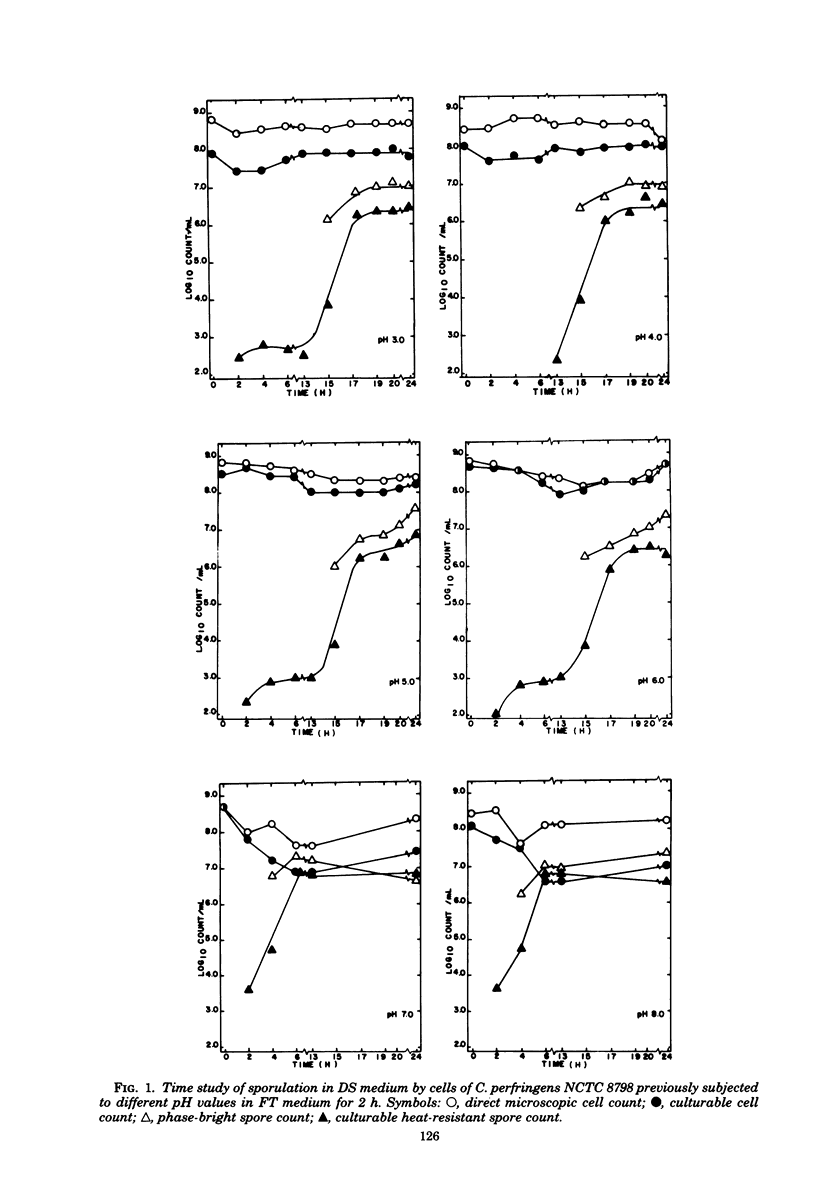
The sporulation of Clostridium perfringens NCTC 8798 was studied after exposing vegetative cells to: pH values of 1.5 to 8.0 in fluid thioglycolate broth (for 2h) and then transferring them to Duncan-Strong (DS) sporulation medium; sodium cholate or sodium deoxycholate (0.3 to 6.5 mM) in DS medium; or Rhia-Solberg medium with 0.4% (wt/wt) starch, glucose, or both added at 0 to 55 mM. At pH 1.5, no culturable heat-resistant spores were formed. For cells exposed to pH 3.0, 4.0, 5.0, or 6.0, increases in heat-resistant spores were not seen until after a lag of 12 to 13 h, whereas the lag was only 2 to 3 h for cells exposed to pH 7.0 or 8.0. Maximal spore crops were produced after only 6 to 8 h for cells exposed to pH 7 or 8, but 16 to 18 h was required for production of maximal spore crops by cells exposed to the lower-pH media. The addition of sodium cholate (3.5 to 6.5 mM) to DS medium only slightly reduced the culturable heat-resistant spore count from 1.9 X 10(7) to 3 X 10(6)/ml. The addition of 1.8 mM or more sodium deoxycholate reduced the culturable heat-resistant spore count to less than 10/ ml. When either starch or glucose alone was added to Rhia-Solberg medium there was no production of culturable heat-resistant spores, but a combination of 0.4% (wt/wt) starch and 4.4 mM glucose yielded 6 X 10(5) spores/ml. The spore production remained at this level for glucose concentrations of 6 to 22 mM, but then declined to about 3 X 10(3) spores per ml at higher concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clifford W. J., Anellis A. Clostridium perfringens. I. Sporulation in a biphasic glucose-ion-exchange resin medium. Appl Microbiol. 1971 Nov;22(5):856–861. doi: 10.1128/am.22.5.856-861.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Experimental production of diarrhea in rabbits with Clostridium perfringens. Can J Microbiol. 1969 Jul;15(7):765–770. doi: 10.1139/m69-134. [DOI] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Ileal loop fluid accumulation and production of diarrhea in rabbits by cell-free products of Clostridium perfringens. J Bacteriol. 1969 Oct;100(1):86–94. doi: 10.1128/jb.100.1.86-94.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L. Time of enterotoxin formation and release during sporulation of Clostridium perfringens type A. J Bacteriol. 1973 Feb;113(2):932–936. doi: 10.1128/jb.113.2.932-936.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLNER P. D. A medium promoting rapid quantitative sporulation in Clostridium perfringens. J Bacteriol. 1956 Apr;71(4):495–496. doi: 10.1128/jb.71.4.495-496.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUCHS A. R., BONDE G. J. The nutritional requirements of Clostridium perfringens. J Gen Microbiol. 1957 Apr;16(2):317–329. doi: 10.1099/00221287-16-2-317. [DOI] [PubMed] [Google Scholar]
- Ferguson I. R., Phillips K. D., Tearle P. V. An evaluation of the carbon dioxide component in the GasPak anaerobic system. J Appl Bacteriol. 1975 Oct;39(2):167–173. doi: 10.1111/j.1365-2672.1975.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Floch M. H., Binder H. J., Filburn B., Gershengoren W. The effect of bile acids on intestinal microflora. Am J Clin Nutr. 1972 Dec;25(12):1418–1426. doi: 10.1093/ajcn/25.12.1418. [DOI] [PubMed] [Google Scholar]
- Frieben W. R., Duncan C. L. Homology between enterotoxin protein and spore structural protein in Clostridium perfringens type A. Eur J Biochem. 1973 Nov 15;39(2):393–401. doi: 10.1111/j.1432-1033.1973.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Hauschild A. H., Niilo L., Dorward W. J. Enteropathogenic factors of food-poisoning Clostridium perfringens type A. Can J Microbiol. 1970 May;16(5):331–338. doi: 10.1139/m70-059. [DOI] [PubMed] [Google Scholar]
- Hsu E. J., Ordal Z. J. Sporulation of Clostridium thermosaccharolyticum under conditions of restricted growth. J Bacteriol. 1969 Mar;97(3):1511–1512. doi: 10.1128/jb.97.3.1511-1512.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabara J. J., Vrable R. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids. 1977 Sep;12(9):753–759. doi: 10.1007/BF02570908. [DOI] [PubMed] [Google Scholar]
- Labbe R. G., Duncan C. L. Influence of carbohydrates on growth and sporulation of Clostridium perfringens type A. Appl Microbiol. 1975 Mar;29(3):345–351. doi: 10.1128/am.29.3.345-351.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R. G., Duncan C. L. Sporulation and enterotoxin production by Clostridium perfringens type A under conditions of controlled pH and temperature. Can J Microbiol. 1974 Nov;20(11):1493–1501. doi: 10.1139/m74-233. [DOI] [PubMed] [Google Scholar]
- Labbe R. G., Rey D. K. Raffinose increases sporulation and enterotoxin production by Clostridium perfringens type A. Appl Environ Microbiol. 1979 Jun;37(6):1196–1200. doi: 10.1128/aem.37.6.1196-1200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R., Somers E., Duncan C. Influence of starch source on sporulation and enterotoxin production by Clostridium perfringens type A. Appl Environ Microbiol. 1976 Mar;31(3):455–457. doi: 10.1128/aem.31.3.455-457.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey T. D. Intestinal microecology. Introduction: the villus in chemostat man. Am J Clin Nutr. 1974 Nov;27(11):1266–1276. doi: 10.1093/ajcn/27.11.1266. [DOI] [PubMed] [Google Scholar]
- Muhammed S. I., Morrison S. M., Boyd W. L. Nutritional requirements for growth and sporulation of Clostridium perfringens. J Appl Bacteriol. 1975 Jun;38(3):245–253. doi: 10.1111/j.1365-2672.1975.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Riha W. E., Jr, Solberg M. Chemically defined medium for the growth of Clostridium perfringens. Appl Microbiol. 1971 Oct;22(4):738–739. doi: 10.1128/am.22.4.738-739.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks L. E., Thompson P. A. Clear, defined medium for the sporulation of Clostridium perfringens. Appl Environ Microbiol. 1978 Feb;35(2):405–410. doi: 10.1128/aem.35.2.405-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald M., Costilow R. N. Minimal growth requirements for Clostridium perfringens and isolation of auxotrophic mutants. Appl Microbiol. 1975 Jan;29(1):1–6. doi: 10.1128/am.29.1.1-6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S. A., Ferguson A. R. New quantitative, qualitative, and confirmatory media for rapid analysis of food for Clostridium perfringens. Appl Microbiol. 1971 Mar;21(3):500–506. doi: 10.1128/am.21.3.500-506.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D. H., Duncan C. L., Perna G. Clostridium perfringens Type A Food Poisoning II. Response of the Rabbit Ileum as an Indication of Enteropathogenicity of Strains of Clostridium perfringens in Human Beings. Infect Immun. 1971 Jan;3(1):171–178. doi: 10.1128/iai.3.1.171-178.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting M. N., Fung D. Y. Chemically defined medium for growth and sporulation of Clostridium perfringens. Appl Microbiol. 1972 Nov;24(5):755–759. doi: 10.1128/am.24.5.755-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Torres-Anjel M. J., Riemann H. P. Improved culture techniques and sporulation medium for enterotoxin production by Clostridium perfringens type A. Taiwan Yi Xue Hui Za Zhi. 1974 Jul;73(7):404–409. [PubMed] [Google Scholar]
- Tsai C., Riemann H. P. Relation of enterotoxigenic Clostridium perfringens type A to food poisoning. II. Acid exposure and storage conditions affecting enterotoxigenesis of C. perfringens. Taiwan Yi Xue Hui Za Zhi. 1974 Dec;73(12):703–708. [PubMed] [Google Scholar]
- Uemura T., Genigeorgis C., Riemann H. P., Franti C. E. Antibody against Clostridium perfringens type A enterotoxin in human sera. Infect Immun. 1974 Feb;9(2):470–471. doi: 10.1128/iai.9.2.470-471.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


