Abstract
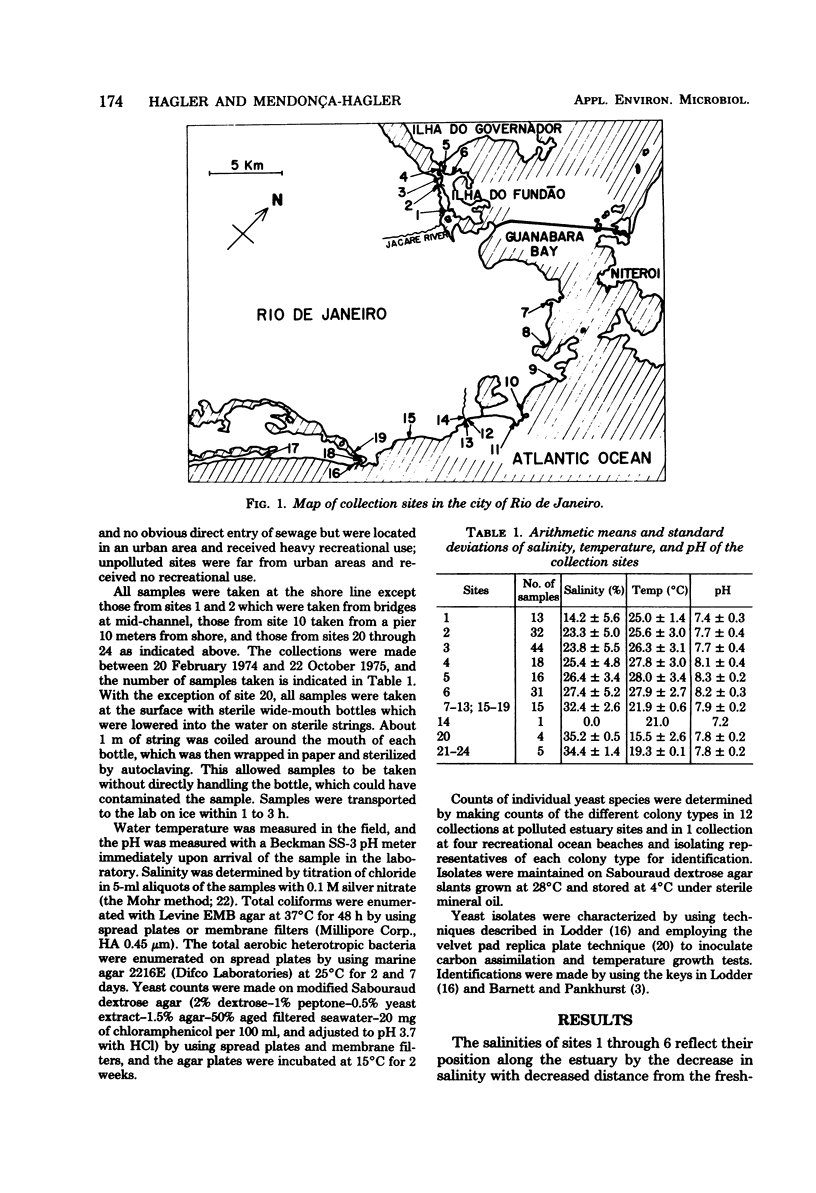
Yeast counts were made at 24 marine and estuarine sites in the vicinity of Rio de Janeiro, Brazil. Mean salinities of estuarine sites ranged from 14.2 to 27.4‰, and mean temperatures ranged from 25 to 28°C. Total coliform counts varied from 80% above 100,000 colony-forming units (CFU)/100 ml at heavily polluted sites to 100% below 100 CFU/100 ml at unpolluted sites. Total yeast counts above 100 CFU/100 ml were typical of heavily and moderately polluted water but atypical of lightly polluted and unpolluted water. Mean total yeast counts were 2,880 CFU/100 ml for heavily polluted sites, 202 CFU/100 ml for moderately polluted sites, and 3 CFU/100 ml for lightly polluted and unpolluted sites. Total yeast counts had a positive response to increased pollution levels, and Candida krusei and phenotypically similar yeasts as a group were prevalent in polluted estuarine water but rare in unpolluted seawater. The 549 strains of yeasts and yeast-like organisms isolated were grouped into 67 species, of which the 21 most prevalent made up 86% of the total yeast population. The prevalent genera in the polluted estuary were Candida, Rhodotorula, Torulopsis, Hanseniaspora, Debaryomyces, and Trichosporon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck J. D., Bubucis P. M. Membrane filter procedure for enumeration of Candida albicans in natural waters. Appl Environ Microbiol. 1978 Feb;35(2):237–242. doi: 10.1128/aem.35.2.237-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs T. J., Murchelano R. A., Jurgen F. Yeasts isolated from Long Island Sound. Mycologia. 1971 Jan-Feb;63(1):178–181. [PubMed] [Google Scholar]
- Hagler A. N., Mendonça-Hagler L. C. Taxonomic implications of Rhodotorula rubra isolates from polluted sea water in Rio de Janeiro, Brazil. Rev Bras Pesqui Med Biol. 1979 Apr;12(1):63–66. [PubMed] [Google Scholar]
- SHIFRINE M., PHAFF H. J., DEMAIN A. L. Determination of carbon assimilation patterns of yeasts by replica plating. J Bacteriol. 1954 Jul;68(1):28–35. doi: 10.1128/jb.68.1.28-35.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard R. E., Blackwood A. C. Yeasts from the St. Lawrence River. Can J Microbiol. 1971 Feb;17(2):197–203. doi: 10.1139/m71-034. [DOI] [PubMed] [Google Scholar]
- Spencer J. F., Gorin P. A., Gardner N. R. Yeasts isolated from some lakes and rivers of Saskatchewan. Can J Microbiol. 1974 Jul;20(7):949–954. doi: 10.1139/m74-146. [DOI] [PubMed] [Google Scholar]
- Spencer J. F., Gorin P. A., Gardner N. R. Yeasts occurring in the effluent disposal basins of a pulp mill in Saskatchewan. Can J Microbiol. 1974 Jul;20(7):993–998. doi: 10.1139/m74-154. [DOI] [PubMed] [Google Scholar]
- Woollett L. L., Hedrick L. R. Ecology of yeasts in polluted water. Antonie Van Leeuwenhoek. 1970;36(3):427–435. doi: 10.1007/BF02069043. [DOI] [PubMed] [Google Scholar]


