Abstract
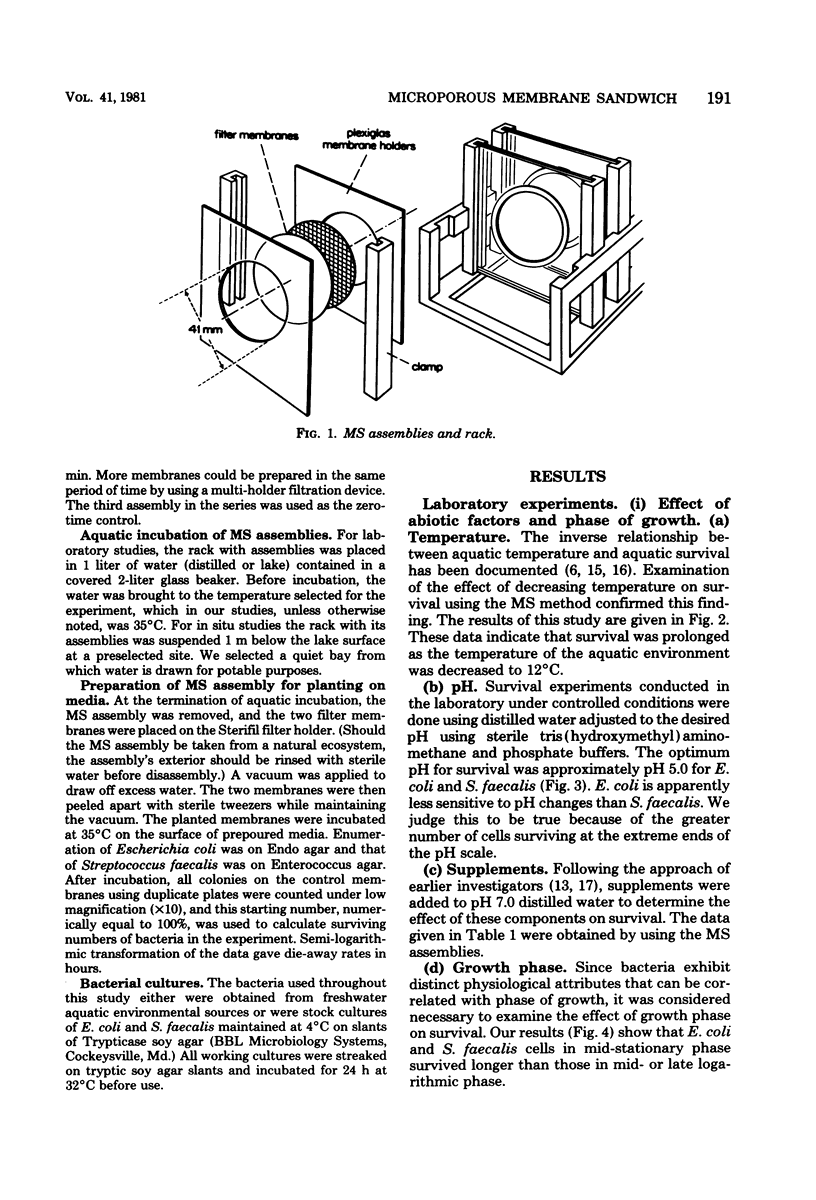
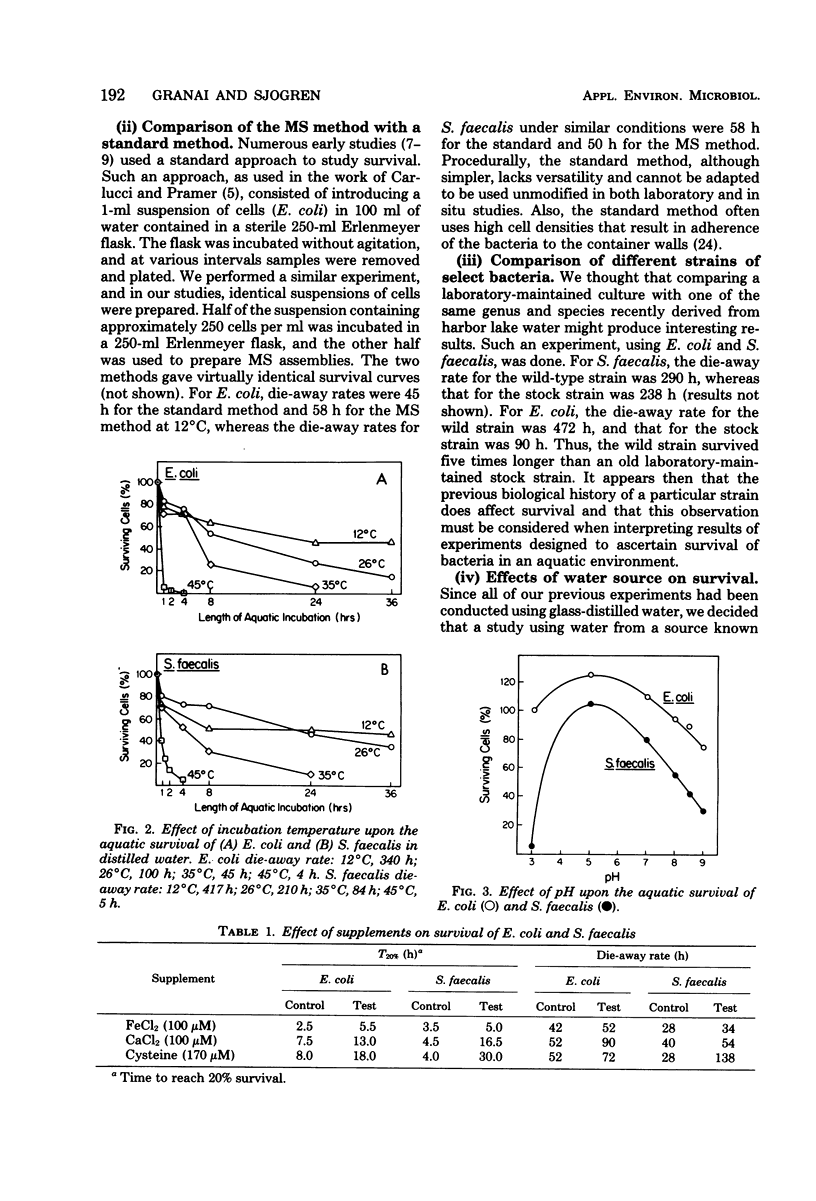
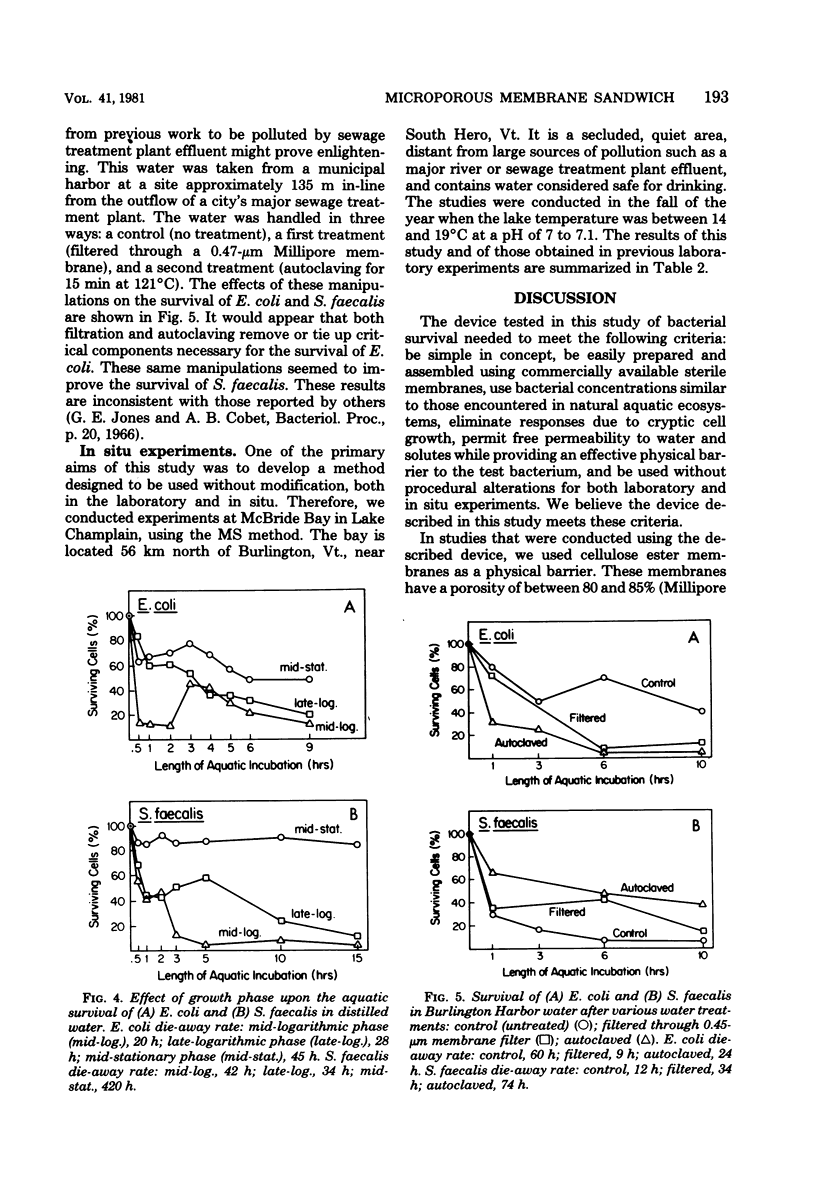
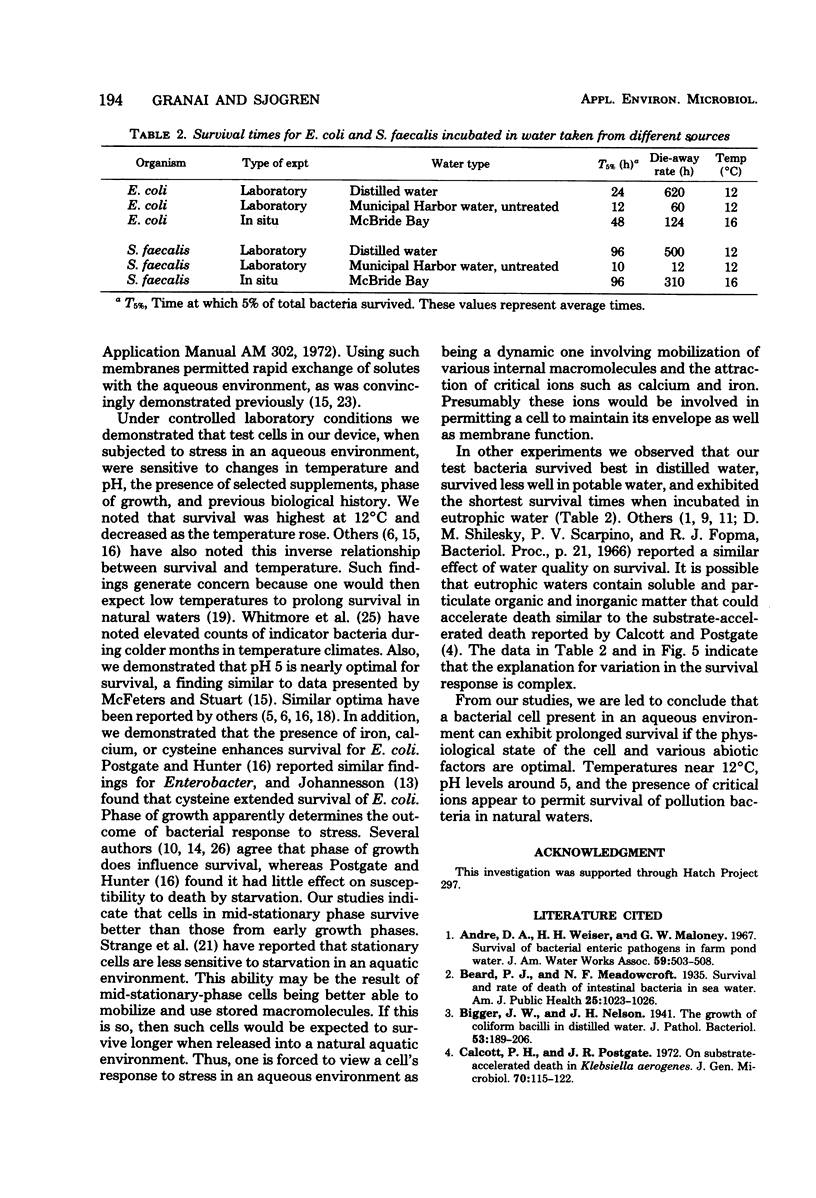
A new device and procedure for the study of bacterial survival in an aquatic environment are described. The device uses two appressed presterilized microporous membranes to expose a bacterial cell suspension to the environment at a cell concentration that closely resembles those levels found in natural aquatic ecosystems. The device has been used under laboratory controlled conditions and in situ to study and compare bacterial survival times. In laboratory studies, Escherichia coli and Streptococcus faecalis survived the longest at 12 degrees C, pH 5, and in the presence of iron or calcium ions and cysteine. Cells in mid-stationary growth phase survived longer than those in mid- or late-logarithmic phase, whereas those maintained for a year or more as stock cultures survived for shorter period of time than did recent environmental isolates. In situ studies indicate that 5% of the starting number of E. coli and S. faecalis cells may survive longer than 96 h at 16 degrees C in potable lake water, whereas survival times in polluted lake water were approximately 12 h.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P. J., Meadowcroft N. F. Survival and Rate of Death of Intestinal Bacteria in Sea Water. Am J Public Health Nations Health. 1935 Sep;25(9):1023–1026. doi: 10.2105/ajph.25.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. An evaluation of factors affecting the survival of Escherichia coli in sea water. II. Salinity, pH, and nutrients. Appl Microbiol. 1960 Jul;8:247–250. doi: 10.1128/am.8.4.247-250.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcott P. H., Postgate J. R. On substrate-accelerated death in Klebsiella aerogenes. J Gen Microbiol. 1972 Apr;70(1):115–122. doi: 10.1099/00221287-70-1-115. [DOI] [PubMed] [Google Scholar]
- Cohen B. Disinfection Studies : The Effects of Temperature and Hydrogen Ion Concentration upon the Viability of Bact. coli and Bact. typhosum in Water. J Bacteriol. 1922 Mar;7(2):183–230. doi: 10.1128/jb.7.2.183-230.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORRILL R. H., McNEIL E. M. The effect of cold diluent on the viable count of Pseudomonas pyocyanea. J Gen Microbiol. 1960 Apr;22:437–442. doi: 10.1099/00221287-22-2-437. [DOI] [PubMed] [Google Scholar]
- Geldreich E. E., Best L. C., Kenner B. A., Van Donsel D. J. The bacteriological aspects of stormwater pollution. J Water Pollut Control Fed. 1968 Nov;40(11):1861–1872. [PubMed] [Google Scholar]
- Hendricks C. W. Enteric bacterial growth rates in river water. Appl Microbiol. 1972 Aug;24(2):168–174. doi: 10.1128/am.24.2.168-174.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANNESSON J. K. Nature of the bactericidal agent in sea water. Nature. 1957 Aug 10;180(4580):285–286. doi: 10.1038/180285a0. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Schultz J. S., Gerhardt P. Dialysis culture of microorganisms: design, theory, and results. Bacteriol Rev. 1969 Mar;33(1):1–47. doi: 10.1128/br.33.1.1-47.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin R. S., Dutka B. J. Comparison of the surface structure, metal binding, and fecal coliform recoveries of nine membrane filters. Appl Environ Microbiol. 1977 Jul;34(1):69–79. doi: 10.1128/aem.34.1.69-79.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos G. J., Swartz R. G. Survival of bacteria in seawater using a diffusion chamber apparatus in situ. Appl Environ Microbiol. 1976 Jun;31(6):913–920. doi: 10.1128/aem.31.6.913-920.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman S. A., Carey C. L. Decomposition of Organic Matter in Sea Water by Bacteria: I. Bacterial Multiplication in Stored Sea Water. J Bacteriol. 1935 May;29(5):531–543. doi: 10.1128/jb.29.5.531-543.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C. E., Walker H. H. THE EARLIER PHASES OF THE BACTERIAL CULTURE CYCLE. Bacteriol Rev. 1939 Dec;3(2):147–186. doi: 10.1128/br.3.2.147-186.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]


