Abstract
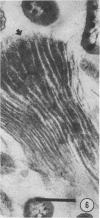
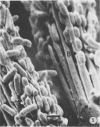
The location and morphology of the bacteria associated with the gastrointestinal tract of Acheta domestica were studied, and these bacteria were partially characterized. Bacteria were associated with the peritrophic membrane in the midgut and with the gut wall and cuticular structures of the hindgut. No bacteria were associated with the fat bodies. Colony-forming unit determinations indicated that there were three times more cultivatable bacteria in the hindgut than in the midgut. Of these bacteria, 40 to 85% cleared uric acid anaerobically, and 90 to 100% cleared uric acid aerobically. Of the 25 isolates obtained, 21 belonged to the genera Citrobacter, Klebsiella, Yersinia, Bacteroides, and Fusobacterium.
Full text
PDF



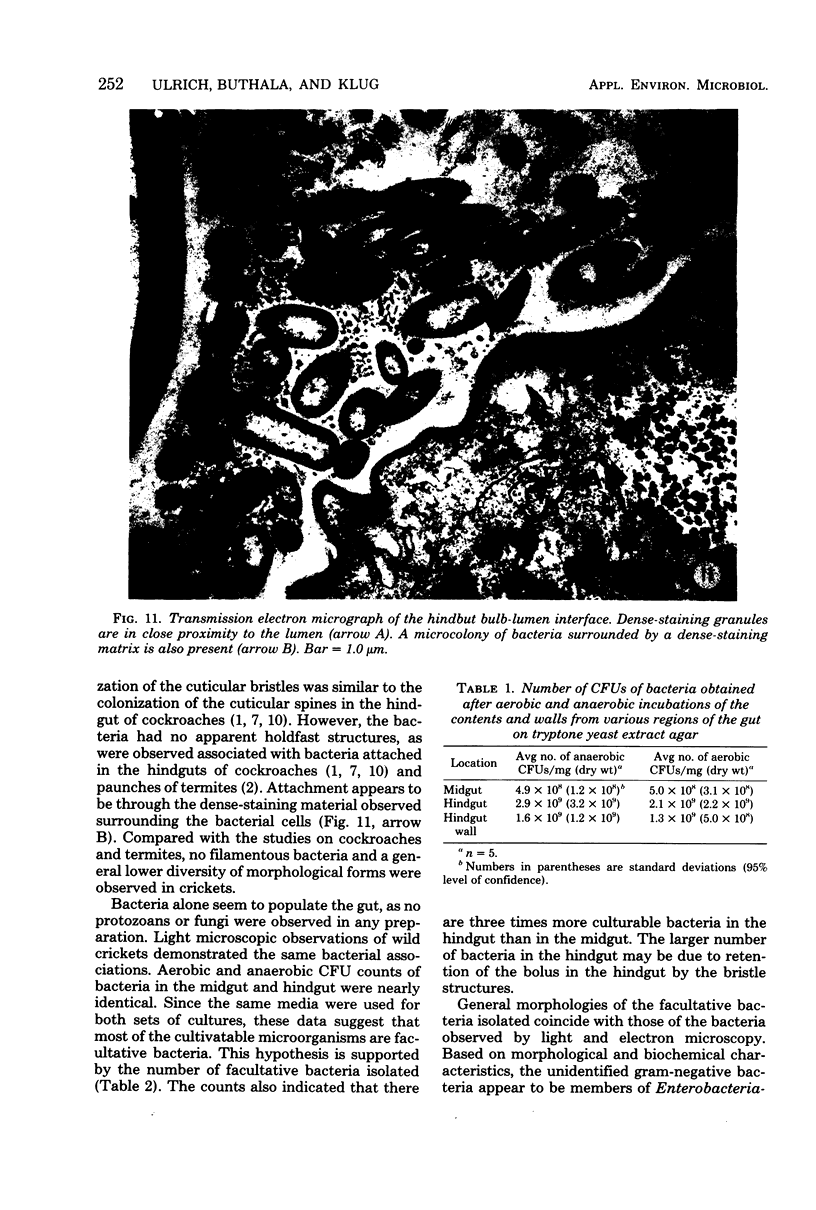
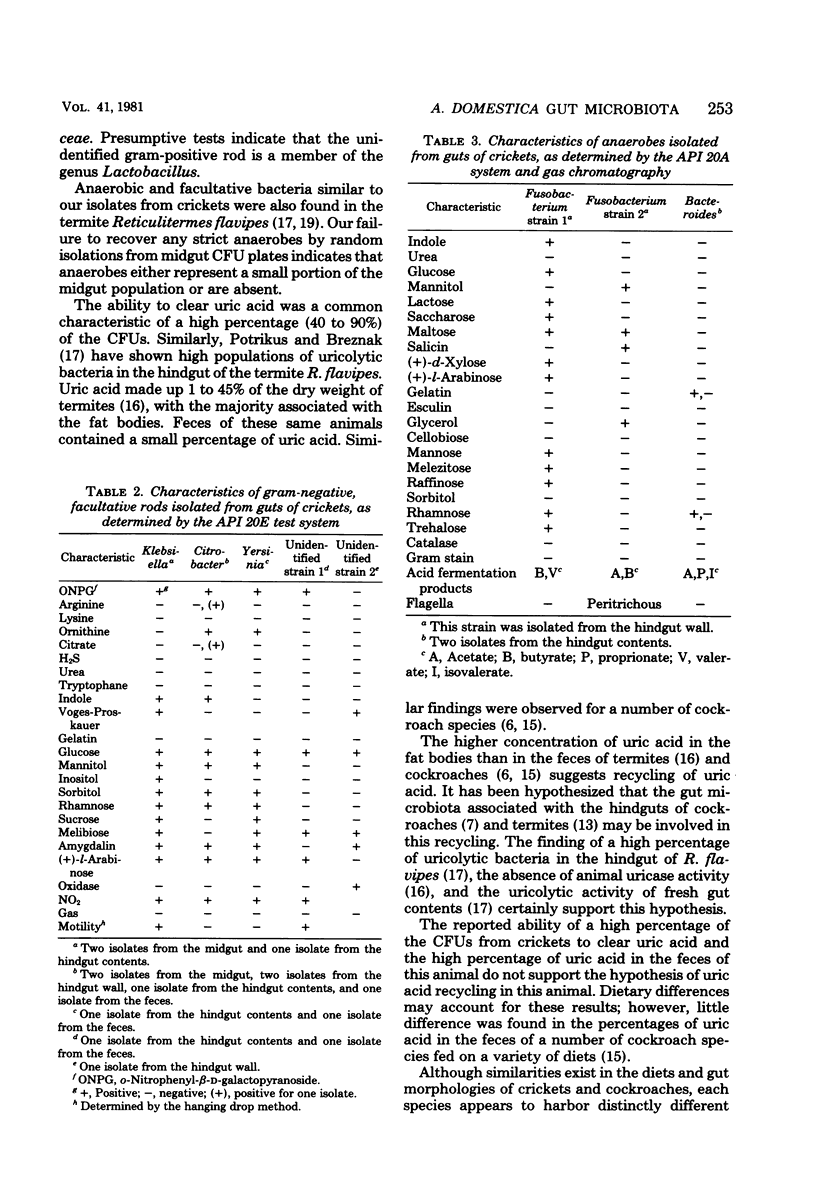

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracke J. W., Cruden D. L., Markovetz A. J. Intestinal microbial flora of the of the American cockroach, Periplaneta americana L. Appl Environ Microbiol. 1979 Nov;38(5):945–955. doi: 10.1128/aem.38.5.945-955.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A., Pankratz H. S. In situ morphology of the gut microbiota of wood-eating termites [Reticulitermes flavipes (Kollar) and Coptotermes formosanus Shiraki]. Appl Environ Microbiol. 1977 Feb;33(2):406–426. doi: 10.1128/aem.33.2.406-426.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden D. L., Gorrell T. E., Markovetz A. J. Novel microbial and chemical components of a specific black-band region in the cockroach hindgut. J Bacteriol. 1979 Nov;140(2):687–698. doi: 10.1128/jb.140.2.687-698.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglesong M. A., Walker D. H., Jr, Puffer J. S., Markovetz A. J. Ultrastructal morphology of some prokaryotic microorganisms associated with the hindgut of cockroaches. J Bacteriol. 1975 Jul;123(1):336–345. doi: 10.1128/jb.123.1.336-345.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. G., Granovsky A. A. NITROGEN IN THE NUTRITION OF TERMITES. Science. 1938 Jan 21;87(2247):66–67. doi: 10.1126/science.87.2247.66-a. [DOI] [PubMed] [Google Scholar]
- Mullins D. E., Cochran D. G. A comparative study of nitrogen excretion in twenty-three cockroach species. Comp Biochem Physiol A Comp Physiol. 1976;53(4):393–399. doi: 10.1016/s0300-9629(76)80162-4. [DOI] [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Uric Acid-Degrading Bacteria in Guts of Termites [Reticulitermes flavipes (Kollar)]. Appl Environ Microbiol. 1980 Jul;40(1):117–124. doi: 10.1128/aem.40.1.117-124.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Breznak J. A. Heterotrophic bacteria present in hindguts of wood-eating termites [Reticulitermes flavipes (Kollar)]. Appl Environ Microbiol. 1978 May;35(5):930–936. doi: 10.1128/aem.35.5.930-936.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]