Abstract
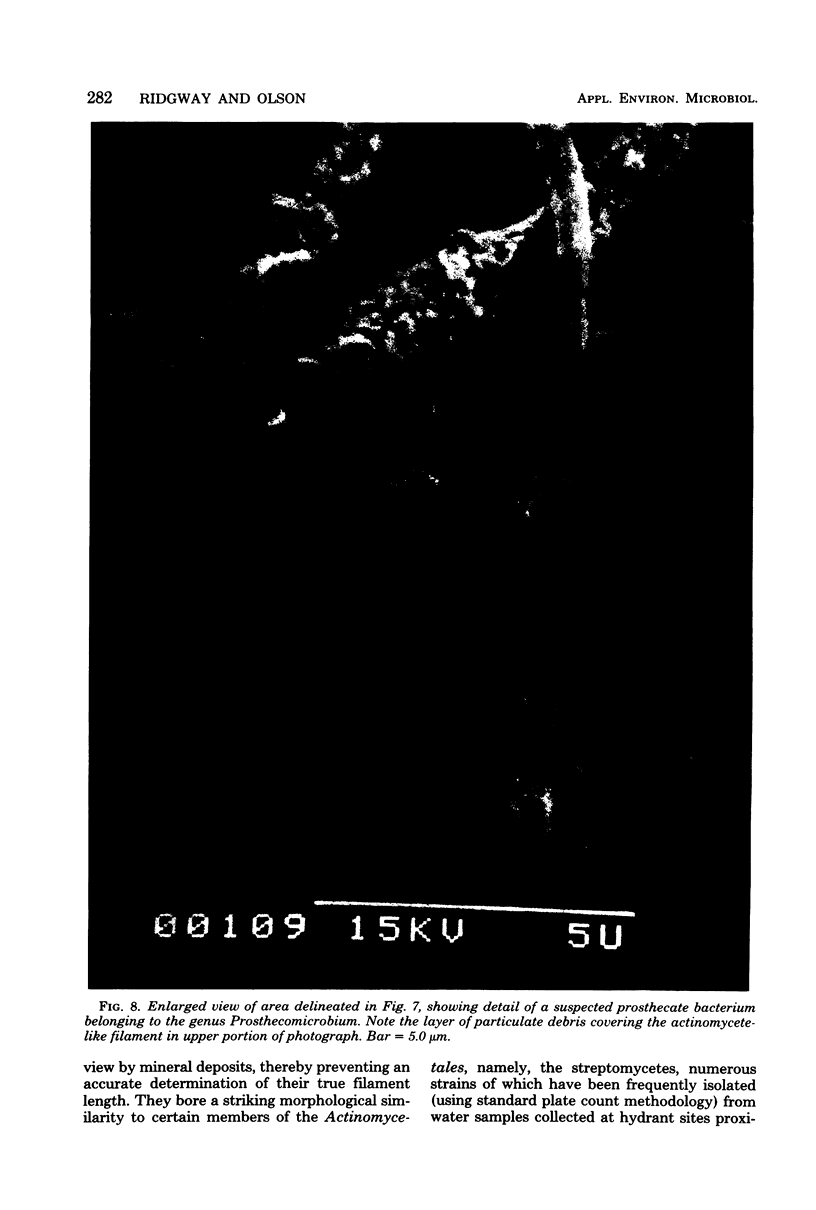
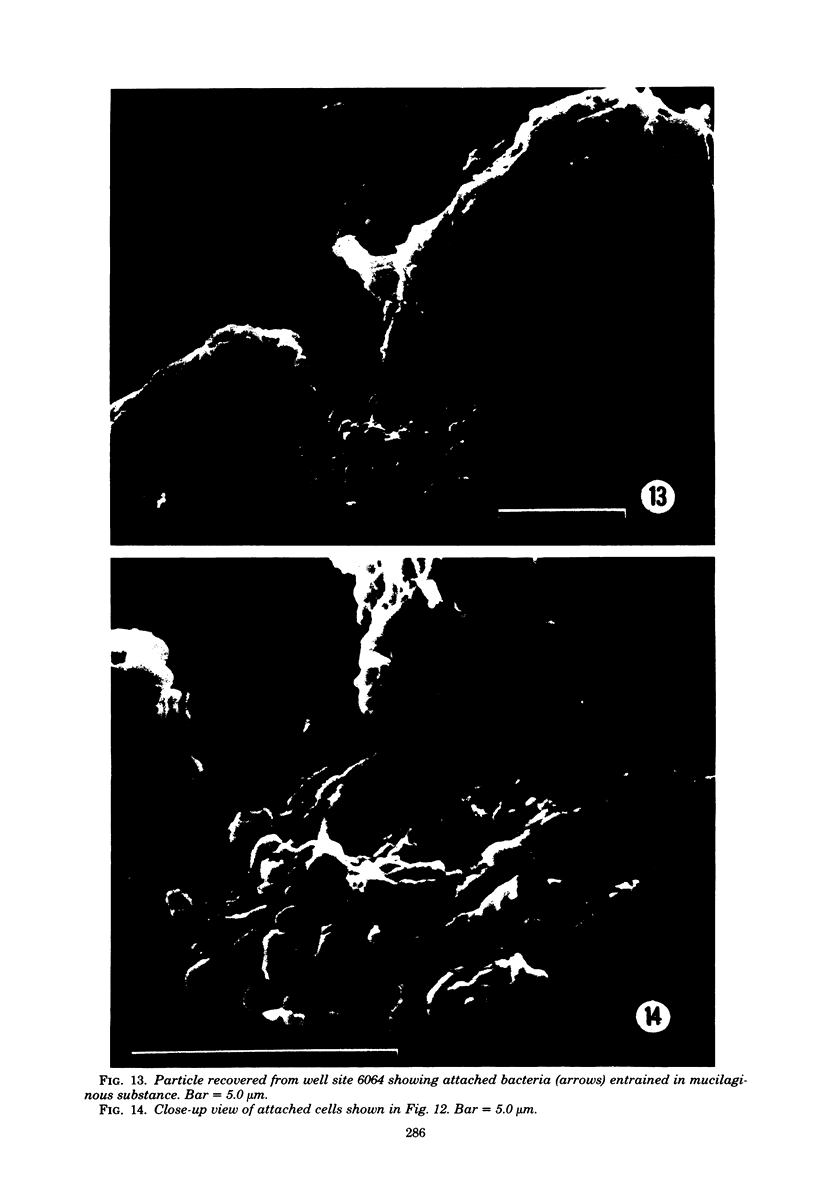
The surfaces of water distribution mains and suspended particulate matter from drinking water were examined by using scanning electron microscopy to investigate the nature and extent of association of microorganisms with these surfaces. In addition, X-ray energy-dispersive microanalysis was used to determine the elemental constitution of the pipe surface. Though distributed sparsely and randomly along the pipe surface, a variety of morphologically distinguishable bacteria-like structures and microcolonies were observed. The morphologies of the individual cells varied form chain-forming cocci to filamentous and prosthecate cell types. The iron-oxidizing bacterium Gallionella, recognized by its characteristic helical stalks, was observed both in water samples and attached to pipe surfaces. Attachment of some microbes to the pipe surface was apparently mediated by extracellular fibrillar appendages. Large numbers of rod-shaped bacteria were also evident adhering to the surfaces of suspended detritus or silt particles recovered from water samples by filtration. X-ray energy scans of the pipe surface revealed the presence of five major elemental constituents including silicon, phosphorous, sulfur, calcium, and iron. Smaller quantities of the elements zinc, magnesium, aluminum, potassium, and manganese were also detected. The public health significance of sessile microbial communities in drinking-water distribution systems is discussed.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Geesey G. G., Costerton J. W. Microbiology of a northern river: bacterial distribution and relationship to suspended sediment and organic carbon. Can J Microbiol. 1979 Sep;25(9):1058–1062. doi: 10.1139/m79-162. [DOI] [PubMed] [Google Scholar]
- Marshall K. C., Cruickshank R. H. Cell surface hydrophobicity and the orientation of certain bacteria at interfaces. Arch Mikrobiol. 1973 Apr 8;91(1):29–40. doi: 10.1007/BF00409536. [DOI] [PubMed] [Google Scholar]
- Paerl H. W. Detritus in lake tahoe: structural modification by attached microflora. Science. 1973 May 4;180(4085):496–498. doi: 10.1126/science.180.4085.496. [DOI] [PubMed] [Google Scholar]
- Parsons T. R., Strickland J. D. Oceanic Detritus. Science. 1962 Apr 27;136(3513):313–314. doi: 10.1126/science.136.3513.313. [DOI] [PubMed] [Google Scholar]
- Staley J. T. Prosthecomicrobium and Ancalomicrobium: new prosthecate freshwater bacteria. J Bacteriol. 1968 May;95(5):1921–1942. doi: 10.1128/jb.95.5.1921-1942.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]