Abstract
Cells of the monocyte/macrophage lineage play a central role in both innate and acquired immunity of the host. However, the acquisition of functional competence and the ability to respond to a variety of activating or modulating signals require maturation and differentiation of circulating monocytes and entail alterations in both biochemical and phenotypic profiles of the cells. The process of activation also confers survival signals essential for the functional integrity of monocytes enabling the cells to remain viable in microenvironments of immune or inflammatory lesions that are rich in cytotoxic inflammatory mediators and reactive free-radical species. However, the molecular mechanisms of activation-induced survival signals in monocytes remain obscure. To define the mechanistic basis of activation-induced resistance to apoptosis in human monocytes at the molecular level, we evaluated the modulation of expression profiles of genes associated with the cellular apoptotic pathways upon activation and demonstrate the following: (i) activation results in selective resistance to apoptosis particularly to that induced by signaling via death receptors and DNA damage; (ii) concurrent with activation, the most apical protease in the death receptor pathway, caspase-8/FLICE is rapidly down-regulated at the mRNA level representing a novel regulatory mechanism; and (iii) activation of monocytes also leads to dramatic induction of the Bfl-1 gene, an anti apoptotic member of the Bcl-2 family. Our findings thus provide a potential mechanistic basis for the activation-induced resistance to apoptosis in human monocytes.
Cells of the monocyte/macrophage lineage play a central role in both innate and acquired immunity of the host (1, 2). These cells are crucial in the defense against invading pathogens and in addition, exert a wide variety of functions that include regulation of the immune response, scavenging of senescent cells, lysis of infected or malignant cells, wound healing, repair, and remodeling of tissues (3, 4). However, the acquisition of functional competence and the ability to respond to a variety of activating or modulating signals require maturation and differentiation of circulating monocytes to macrophages that in turn entail alterations in both biochemical and phenotypic profiles of the cells (5, 6). Prominent manifestations of activation include the expression of adhesion molecules and secretion of potent proinflammatory cytokines such as tumor necrosis factor (TNF)-α, and IL-1β, enhanced metabolic activity with generation of free-radical metabolites, all of which enable these cells to converge, attack, and eliminate the noxious stimulus. Importantly, the process of activation also confers survival signals essential for the functional integrity of monocytes enabling the cells to remain viable in microenvironments of immune or inflammatory lesions that are rich in cytotoxic inflammatory mediators and reactive free-radical species. Although it is widely recognized that peripheral blood monocytes undergo apoptosis spontaneously upon culture unless supplemented with serum, growth factors, bacterial products, or inflammatory cytokines such as IL-1β or TNF-α (7–15), at present, the molecular mechanisms of activation-induced survival signals in monocytes remain obscure. In an attempt to define the mechanistic basis of activation-induced resistance to apoptosis in monocytes at the molecular level, we evaluated the modulation of expression profiles of genes associated with the cellular programmed cell death pathways after stimulation of human monocytes with bacterial products including lipopolysaccharide (LPS). We demonstrate that the activation-induced resistance to apoptosis is selective and is dependent on the triggering stimulus and involves rapid down-regulation of the most apical cysteine protease, caspase-8/FLICE that is critical for the Fas/TNF receptor death-inducing signaling pathway (16–19) and also results in dramatic induction of the Bfl-1 gene (20–22), an antiapoptotic member of the Bcl-2 family.
MATERIALS AND METHODS
Cell Culture.
Monocytes were isolated from the blood of normal healthy donors by a two-step procedure beginning with an automated leukopheresis followed by counterflow elutriation. Cells were cultured in RPMI medium 1640 supplemented with penicillin, streptomycin, 2 mM glutamine, and 20% heat-inactivated pooled AB human serum.
Reagents and Antibodies.
Staurosporine, C2-ceramide, etoposide, and the Dx2 mAb against the human Fas receptor were purchased from CLONTECH. Staurosporine was used at a final concentration of 1.1 μM. C2-ceramide was used at a final concentration of 50 μM. Etoposide was used at a final concentration of 10.5 μM. Dx2 antibody was used at a final concentration of 500 ng/ml. Phenol-extracted LPS from Escherichia coli serotype O128:B12 was purchased from Sigma. For UV irradiation experiments, cells were irradiated at room temperature after aspirating the culture medium with a Blak-Ray UV lamp Model XX-15S from Ultraviolet Products. Cells were exposed to a dose of 15 J/M2; growth medium was then replenished, and the cells were incubated at 37°C for the indicated time.
Determination of Apoptosis by DNA Electrophoresis.
Cells (2 × 107) were washed once with PBS and resuspended in 350 μl of lysis buffer containing 10 mM Tris, 10 mM EDTA, and 0.5% Triton X-100 and incubated on ice for 20 min before centrifugation (13,000 × g) at 4°C for 20 min to pellet chromosomal DNA. Supernatant was then collected and RNase A was added to a final concentration of 0.1 mg/ml before incubation at 37°C for 1 hr followed by the addition of proteinase K (1 mg/ml final concentration) and SDS (1% final concentration). Samples were then incubated at 50°C for 2 hr and extracted with phenol/chloroform twice before precipitating the DNA by adding 35 μl of 3 M sodium acetate. Precipitated DNA was resuspended in 25 μl of water, loaded on a 1.2% agarose gel containing ethidium bromide (100 ng/ml) and separated by electrophoresis.
Caspase-8 Assay.
Caspase-8 activity in whole cell lysates was determined by using the ApoAlert FLICE/caspase-8 Fluorescent Assay Kit from CLONTECH according to the manufacturer’s instructions. Briefly, cell lysates from 106 cells were incubated with IETD tetrapeptide conjugated to 7-amino-4-trifluoromethyl coumarin (AFC) for 1 hr at 37°C. Free AFC accumulation that resulted from cleavage of the aspartic-AFC bond was monitored by using a fluorometer (Wallac, Gaithersburg, MD) equipped with a 400-nm excitation filter and 505-nm emission filter.
Ribonuclease Protection Assay (RPA).
Total cellular RNA was isolated from monocytes by using TRIzol (Life Technologies, Gaithersburg, MD) according to the manufacturer’s instructions. The expression of various apoptosis-associated genes was measured by multi-probe RPAs (23). Template sets for the multi-probe RPA were purchased from PharMingen, and the assays were performed according to the manufacturer’s instructions. Briefly, 50 ng of DNA from each multi-probe set were used to generate 32P-labeled riboprobes of defined length with T7 RNA polymerase in the presence of 150 μCi of [32P]UTP. Template DNA was then eliminated by digestion with DNase free of RNase, followed by precipitation of labeled RNA. Fifteen micrograms of total cellular RNA were then mixed with 6 × 105 cpm of 32P-labeled riboprobe mixture in a hybridization buffer containing 40 mM Pipes, 1 mM EDTA, 0.4 M NaCl, and 80% formamide and incubated at 90°C for 5 min followed by 56°C for 12 hr. The hybridized RNA duplexes were then treated with an RNase mixture consisting of RNase A and RNase T1 followed by proteinase K digestion. RNase resistant duplex RNA was then extracted with phenol once and precipitated by the addition of an equal volume of 4 M ammonium acetate and 2 volumes of ethanol. Then, the RNA pellet was solubilized, resolved on a 6% sequencing gel, dried, and subjected to autoradiography or phosphorimage analysis.
RESULTS AND DISCUSSION
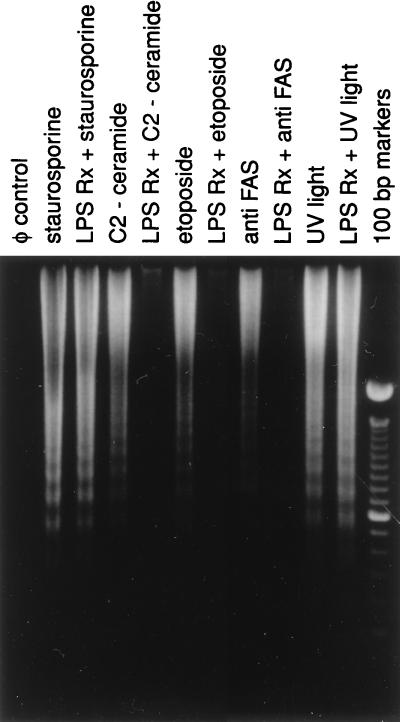
It is well established that freshly isolated monocytes from peripheral blood undergo apoptosis rapidly when cultured in the absence of serum (7). Nonetheless, in the presence of serum or other activating factors such as IL-1β, TNF-α, or bacterial products such as LPS, the cells not only resist spontaneous loss of viability, but also resist apoptosis induced by FAS receptor crosslinking, γ-irradiation, H2O2, or NO (12, 24–27). However, at present, it is not clear whether the activation-induced resistance to apoptosis is a global effect or, rather, is limited to specific death-inducing signals via a particular signaling pathway(s). To explore this issue, we cultured freshly isolated human monocytes in the presence of 20% pooled human serum, which as expected prevented spontaneous apoptosis as assessed by both cell morphology as well as the absence of oligonucleosomal DNA fragmentation. As shown in Fig. 1, the cells cultured in the presence of serum remained refractory to spontaneous apoptosis but were still fully susceptible to apoptosis induced by staurosporine (28), a broad spectrum protein kinase inhibitor; C2-ceramide (28), a water soluble ceramide analog able to penetrate cells; etoposide, a topoisomerase II inhibitor (29); ultraviolet light (UV); and crosslinking of surface Fas receptor (30). Intriguingly, when cells were activated by E. coli LPS, the activated cells displayed differential resistance to the same set of apoptotic inducers. Specifically, cells activated with LPS effectively suppressed C2-ceramide, etoposide, and Fas antibody-mediated apoptosis whereas no significant protection was evident in UV or staurosporine-induced apoptosis. The results shown in Fig. 1 thus suggest that the activation of monocytes imparts a selective desensitization to apoptotic signals mediated via specific signaling pathways but does not exert a global protective effect from programmed cell death. This phenomenon was not specific for LPS-mediated activation because another bacterial product, i.e., a soluble lysate of Mycobacterium leprae (kindly provided by P. Sieling, University of California School of Medicine, Los Angeles, CA) when used to activate the monocytes, yielded a similar pattern of selective resistance (data not shown). Our observation that monocytes once activated resist induction of apoptosis by crosslinking the cell surface death receptor Fas confirms previous similar observations (12, 24).
Figure 1.
Activation of human monocytes leads to selective resistance to apoptosis. Elutriated monocytes were cultured in RPMI medium 1640 supplemented with 20% pooled human serum and activated with E. coli LPS (25 ng/ml) for 6 hr before the introduction of indicated apoptotic agents. Staurosporine, C2-ceramide, and etoposide were added at 1.1 μM, 50 μM, and 10.5 μM, respectively. Anti-Fas antibody was used at a concentration of 500 ng/ml. A dose of 15 J/m2 was used in UV irradiation experiments. The cells were then cultured for an additional 12 hr before harvesting to assess the extent of oligonucleosomal DNA fragmentation as described in Materials and Methods. Similar results were obtained from monocytes derived from three other donors.
Death receptor-induced apoptosis is an important event in tissue homeostasis. In addition to the Fas receptor (31), other members of the family of cell surface death receptors include TNF R1 (32), DR3 [also known as TRAMP (33–36)], DR4 (also known as TRAIL-R), and DR5 (37–40). The accumulating evidence indicates that the signal transduction after the engagement of various cell surface death receptors may proceed via a common pathway requiring efficient recruitment and activation of the caspase-8/FLICE enzyme, which is the most apical caspase. The resistance of activated but not resting monocytes to induction of apoptosis by surface crosslinking of Fas receptors taken together with the observation that the activated monocytes synthesize copious quantities of TNF-α without any obvious self injury suggest that with activation the assembly of the death-inducing signaling complex (DISC) is interrupted (41, 42). To gain insights as to how activation of human monocytes leads to disruption of the death receptor-mediated apoptosis, the effects of activation on the expression of a panel of cellular genes associated with death receptor pathway signaling were assessed by a sensitive RPA after exposure of monocytes to either E. coli LPS or a soluble lysate of M. leprae.
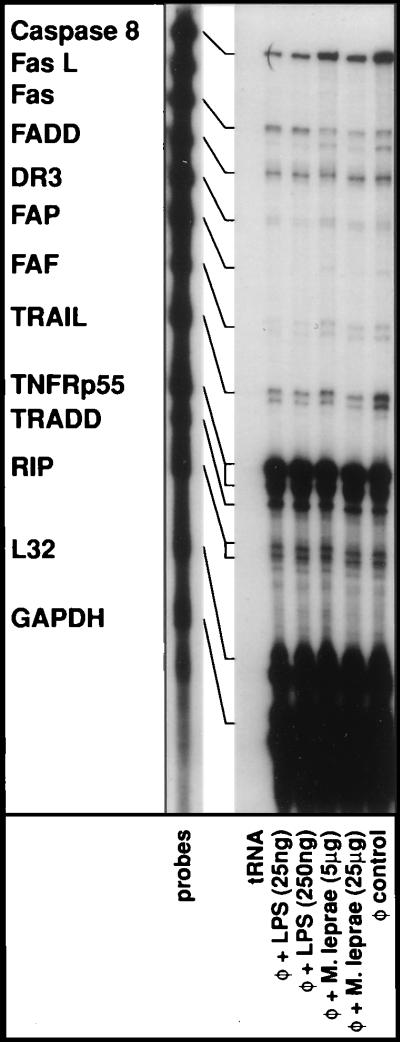
As shown in Fig. 2, when we examined the steady–state mRNA levels of Fas receptor after stimulation of monocytes with LPS for 6 hr, no significant quantitative differences were apparent in comparison to unstimulated cells. Our results are consistent with previous observations that the expression of Fas was not modulated during activation of monocytes (12, 24). The activation-induced resistance to apoptosis in the presence of an agonist anti-Fas antibody is then likely to operate downstream of receptor engagement. Moreover, the mRNA expression of TNF-R1 (p55), which was abundant in monocytes, remained unmodulated after stimulation with bacterial products. Similarly, activation of monocytes failed to modulate the mRNA expression of adaptor molecules TRADD (43), RIP (44), or FADD (45) that facilitate the recruitment of caspase-8/FLICE to assemble the DISC. However, the expression of caspase-8/FLICE diminished sharply upon activation (see Figs. 2 and 3). We also noted a slight reduction in TRAIL mRNA expression upon activation. The expression levels of FasL (46), DR3 death receptor (34–36), FAP (47), and FAF-1 (48) mRNA in human monocytes were minimal and required lengthy exposures of the autoradiograms to visualize signals from these genes and were not altered upon activation. To further explore whether the reduction in the steady–state mRNA levels of caspase-8/FLICE as detected by the RPA actually results in loss of intracellular caspase-8/FLICE activity, we first attempted to determine the presence of any detectable caspase-8/FLICE activity in human monocytes. Concordant with the abundant levels of steady–state mRNA as evident in Fig. 2, detectable levels of specific caspase-8/FLICE activity were present in lysates of freshly isolated human monocytes. In the absence of any apoptotic manifestations in these cells, the caspase-8 activity is presumably due to unprocessed proprotein, which has been shown to possess inherent protease activity. Nonetheless, as shown in Table 1, there was ≈90% reduction in the cellular caspase-8/FLICE activity after LPS-mediated activation. The reduction in caspase-8/FLICE activity was maintained up to 30 hr after LPS treatment and then gradually returned to normal levels over a period of 48 hr (data not shown).
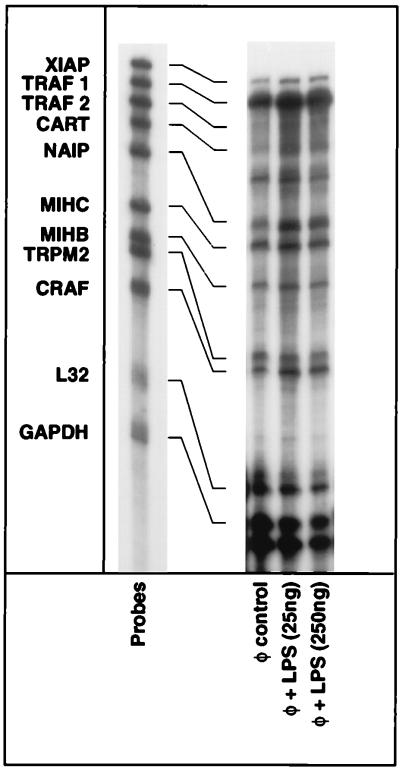
Figure 2.
Effects of monocyte activation on the expression of cell surface death receptors and death receptor-associated genes. Elutriated monocytes were treated with E. coli LPS or a soluble lysate of M. leprae for 6 hr. Total cellular RNA was extracted, and RPAs were performed as described in Materials and Methods. The expression levels of ribosomal L32 and cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serve as internal controls as well as RPA performed with yeast tRNA and with RNA derived from monocytes cultured in medium alone (φ, control); similar results were obtained from cells derived from two other donors.
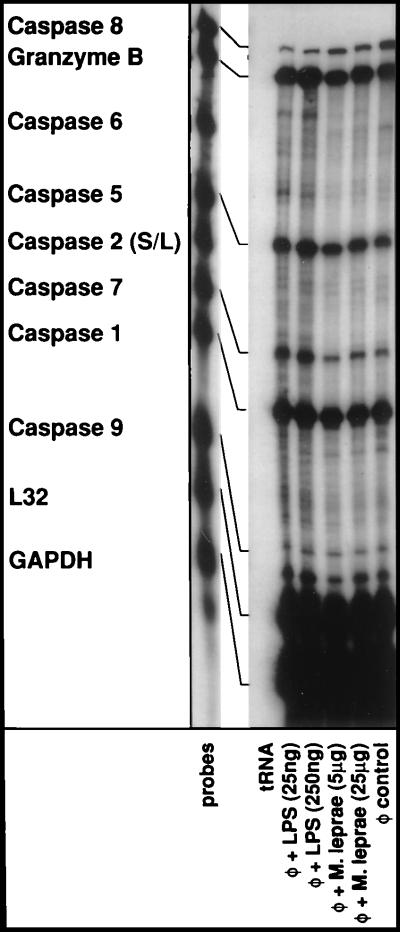
Figure 3.
Effects of monocyte activation on the expression of cellular caspases. Elutriated monocytes were treated with E. coli LPS or a soluble lysate of M. leprae for 6 hr. Total cellular RNA was extracted and RPAs were performed essentially as described in the Fig. 2 legend. Granzyme B was included because of its ability to cleave and activate multiple caspase members.
Table 1.
Activation of monocytes leads to down-regulation of caspase-8/FLICE activity
| Treatment | % Inhibition of fluorescence* |
|---|---|
| LPS 25 ng/ml | 86.0 ± 0.4 |
| LPS 250 ng/ml | 86.6 ± 0.5 |
*Cell lysates from LPS-treated cells were incubated with IETD-AFC. Free AFC accumulation was monitored by using a fluorometer with 400-nm excitation filter and 505-nm emmision filter. Percent inhibition of fluorescence was calculated in comparison to untreated control samples and represents the mean ± SD of samples done in triplicate.
Having established that, upon activation of human monocytes, there was rapid down regulation of the most apical protease in the Fas death pathway, we next assessed the effects of monocyte activation, if any, on the expression of other cellular caspases (reviewed in refs. 49 and 50). As shown in Fig. 3, caspase-1 was most abundantly expressed although no modulation of its expression was evident upon activation. Similarly, there was detectable caspase-9 expression although that too was refractory to any alterations upon activation. Expression of caspase-2 and caspase-6 were minimal in human monocytes. Importantly, unlike caspase-8, the steady–state mRNA levels of caspase-5 and caspase-7 were consistently elevated although slightly upon activation. Granzyme B is an aspartate-directed serine protease that plays a dominant effector function in cytotoxic T cell-mediated killing of target cells (51). It is the only non-caspase enzyme known to cleave and activate multiple caspase members involved in apoptosis including caspase-2, -3, -6, -7, -8, -9, and -10, but not the ICE subfamily proteases (ref. 52 and references therein). As can be seen in Fig. 3, there were relatively high levels of constitutively expressed Granzyme B in monocytes, which up-regulated modestly upon activation.
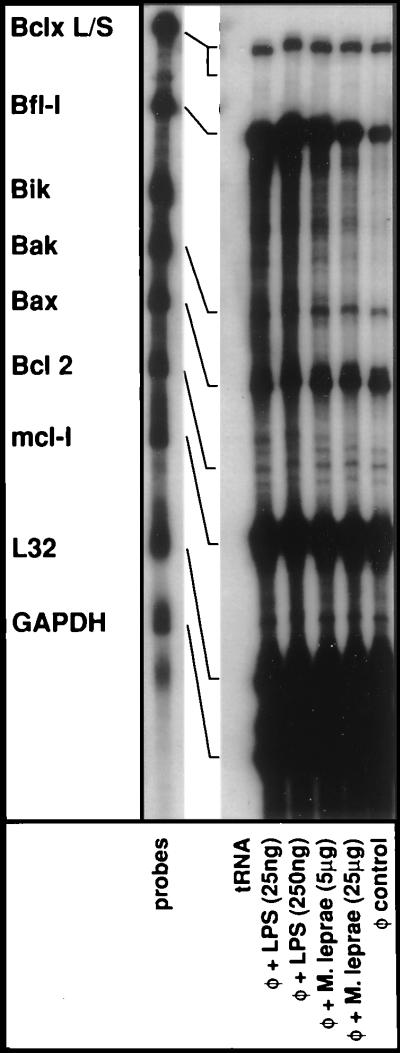
Considering the pivotal role of Bcl-2 family of proteins in modulating the cellular death program, we next assessed whether during the activation of monocytes the mRNA expression profiles of Bcl-2 family members were altered (reviewed in refs. 49, 53, 54). In Fig. 4, anti-apoptotic Bcl-2 and BclXL were constitutively expressed in monocytes although levels were consistently higher for the BclXL gene. Upon activation, however, no significant enhancement of expression was noted for either of these genes. In addition, the expression profiles of the proapoptotic members namely, Bik, Bak, and Bax remained unchanged upon activation of monocytes. Nonetheless, it should be noted that the basal constitutive expression levels of proapototic Bak and particularly Bax were quite high in human monocytes. Mcl-1 is also a member of the Bcl-2 family and encodes a 37 kDa polypeptide, which forms heterodimers with Bax to promote cell survival under various conditions that cause apoptotic death (49). As evident from Fig. 4, Mcl-1 was the most abundantly expressed member in human monocytes. However, its expression was not affected by activation and thus, is less likely to be relevant for activation induced resistance to apoptosis. Bfl-1 is another member of the Bcl-2 family and encodes a 175-amino acid, early response polypeptide predominantly expressed in hematopoietic tissues (20–22). Bfl-1 has been shown to effectively suppress apoptosis induced by p53 tumor suppressor polypeptide, TNF-α, and IL-3 deprivation (49). Consistent with previous reports, there was a dramatic increase in the expression of Bfl-1 gene when monocytes were activated. In fact, Bfl-1 was the most inducible Bcl-2 family member in human monocytes.
Figure 4.
Effects of monocyte activation on the expression of genes belonging to Bcl-2 family. Elutriated monocytes were treated with E. coli LPS or a soluble lysate of M. leprae for 6 hr. Total cellular RNA was extracted, and RPAs were performed essentially as described in the Fig. 2 legend.
Proteins that can specifically inhibit the apoptotic death pathway were first identified in viruses, and the subsequent discovery of their cellular counterparts continues to expand our understanding of the cellular suicide machinery (55). To assess whether any of these inhibitors of apoptosis (IAP) could dictate the anti-apoptotic state seen in activated monocytes, we examined the mRNA expression profiles of an array of IAP genes as well as TRAF-1 and TRAF-2, which recruit many of the IAP to the TNF receptors in monocytes (59). As shown in Fig. 5, XIAP (56), NAIP (57), MIHB (c-IAP1), MIHC (c-IAP2) (58, 59), TRPM2 (60), and CRAF (61), all showed detectable levels of expression in human monocytes. However, upon activation of monocytes, the expression of these genes did not change meaningfully (less than 2-fold). The expression of TRAF2 was minimal whereas abundant expression of TRAF1 was evident in human monocytes although its responsiveness to activation was minimal.
Figure 5.
Effects of monocyte activation on the expression of cellular genes encoding IAP. Elutriated monocytes were treated with E. coli LPS for 6 hr. Total cellular RNA was extracted, and RPAs were performed essentially as described in the Fig. 2 legend. TRAF1 and TRAF2 also were included because of their involvement in the recruitment of IAP.
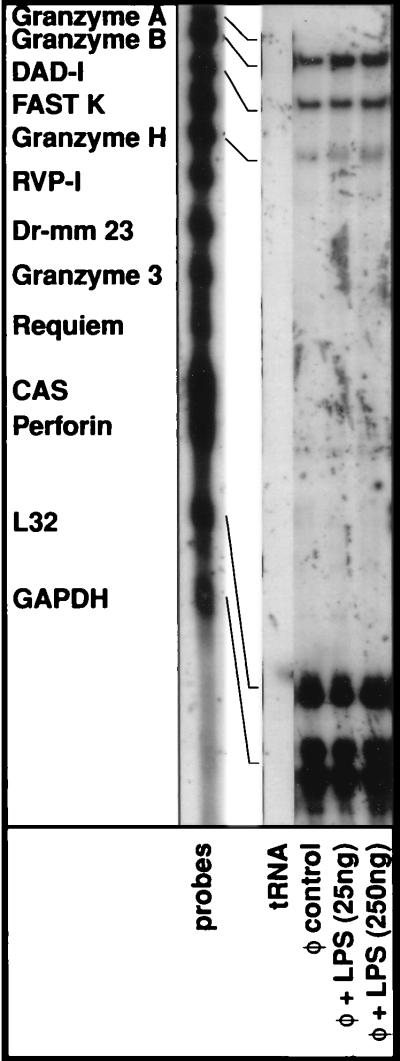
In Fig. 6, we examined the mRNA expression profile of an additional set of genes including Granzyme A (62), Granzyme B (63), DAD-1 (64), FASTK (65), Granzyme H (66), RVP-1 (67), Dr mm 23 (68), Granzyme 3 (69), Requiem (70), CAS (71), and perforin (72). The expression of each one of these genes has been shown to influence the cellular death pathway in various systems. In addition to Granzyme B, only Granzyme H and DAD-1 displayed any detectable levels of expression in human monocytes although their expression remained unchanged upon activation. Nonetheless, reaffirming the data previously shown in Fig. 3, Granzyme B expression levels were elevated modestly upon activation.
Figure 6.
Effects of monocyte activation on the expression of apoptotic modulators. Elutriated monocytes were treated with E. coli LPS for 6 hr. Total cellular RNA was extracted, and RPAs were performed essentially as described in the Fig. 2 legend.
After assessing the expression of an array of genes associated with the cellular death pathway, two potential mechanisms that could confer an antiapoptotic state in activated monocytes seem to emerge from the present work: (i) rapid down regulation of caspase-8/FLICE expression upon activation of human monocytes, and (ii) dramatic induction of the antiapoptotic Bfl-1 gene that belongs to the Bcl-2 family.
Cell surface death receptors constitute a steadily growing group of cell surface molecules belonging to the TNF receptor superfamily and include CD95 (Fas receptor), TNF-R1, DR3, DR4, and DR5. The death receptors share a cytoplasmic “death domain” responsible for recruiting adaptor molecules such as FADD and/or TRADD proteins to the plasma membrane through homotypic interactions. Receptor bound adaptor molecules then couple the death protease caspase-8/FLICE to the death receptor leading to its proteolytic processing and activation. Thus FLICE represents the most apical caspase in the death receptor pathway, and activation of FLICE initiates the apoptotic cascade presumably by cleaving relevant downstream substrates (reviewed in ref. 49). Our data suggest that concurrent with monocyte activation there is rapid down regulation of caspase-8/FLICE expression at the level of mRNA thereby effectively preventing the formation of the DISC that could be triggered by the engagement of cell surface death receptors.
Control of the death receptor-induced suicide program at the level of FLICE appears to be evolutionarily favored. For instance, complex large DNA viruses such as gamma herpesviruses and molluscipoxviruses encode proteins, known as viral FLICE inhibitory proteins (v-FLIPS) that efficiently interfere with the recruitment of FLICE by interacting with the adaptor molecule FADD thereby preventing the assembly of a receptor associated DISC (55, 73). More recently, existence of a cellular homologue of viral FLIPs has been established and its temporal expression during the early phase of T cell activation appear to rescue T cells from premature activation-induced cell death and allow activated T cells to mediate help or cytotoxicity (74). However, because of the transient nature of c-FLIP expression, with its disappearance, Fas-mediated signaling is reestablished and T cells undergo activation-induced cell death to maintain homeostasis.
Our demonstration that, in monocytes during activation, there is down regulation of FLICE both at the level of mRNA as well as protein represents yet another unique mechanism nature has exploited to control the death receptor-signaling pathway. Because FLICE is central in linking signals originating from various cell surface death receptors to the cellular death pathway, rapid repression of its synthesis during the early phase of activation ensures the survival of monocytes to execute their functions especially in a micro-environment rich in cytotoxic proinflammatory cytokines such as TNF-α as well as activated cells bearing FasL. It is important to note that the subsequent return of FLICE activity may still permit death receptor-mediated programmed cell death in elimination of functionally redundant activated macrophages that could be detrimental to the host (27, 75).
Activated monocytes in addition to being resistant to death receptor mediated apoptosis are also resistant to apoptosis triggered by γ-irradiation as well as DNA damaging agents, for example, etoposide as illustrated in Fig. 1. Considering the rapid generation of large quantities of free radical reactive species intracellularly upon activation of monocytes, it is conceivable that these cells have evolved effective strategies to prevent DNA damage and/or to protect from DNA-damage induced cell death. The expression profiles of the bcl-2 family are relevant in this regard because of their importance in reactive oxygen/DNA damage induced apoptosis (49, 53, 54). The ratio of death antagonists (Bcl-2, Bcl-XL, Mcl-1, and Bfl-1) to agonists (Bax, Bak, and Bik) regulates the competitive dimerization between selective pairs of antagonists and agonists of Bcl-2 family proteins. Induced expression of antagonists favor heterodimerization, which in turn leads to cell survival. Dramatic induction of Bfl-1 during activation of monocytes (see Fig. 4) thus could very well favor equilibrium toward heterodimerization. Thus, the ability of Bfl-1 to suppress DNA-damage induced apoptosis largely regulated by p53 tumor suppressor protein and also TNF-α mediated apoptosis taken together with its rapid inductive profile makes a compelling argument for Bfl-1 to be important in activation-induced resistance to apoptosis in human monocytes.
In conclusion, our data suggest that activation of monocytes results in selective resistance to apoptosis particularly to signaling via death receptors and DNA damage. Moreover, rapid induction of Bfl-1, an anti apoptotic member of the bcl-2 family and a marked reduction in the expression of the most apical caspase, i.e., caspase-8/FLICE provide a potential mechanistic basis for the activation-induced resistance to apoptosis in human monocytes.
Acknowledgments
We thank Dr. Yutaka Tagaya for critical reading of the manuscript, Dr. Pin-Yu Perera for helpful suggestions, and Dr. Peter Seiling for providing M. leprae lysates.
ABBREVIATIONS
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- DISC
death-inducing signaling complex
- RPA
ribonuclease protection assay
- IAP
inhibitors of apoptosis
- AFC
7-amino-4-trifluoromethyl coumarin
References
- 1.Van Furth R. In: Hematopoietic Growth Factors and Mononuclear Phagocytes. Van Furth R, editor. Basel: Karger; 1993. pp. 14–38. [Google Scholar]
- 2.Solbach W, Moll H, Rollinghoff M. Immunol Today. 1991;12:4–6. doi: 10.1016/0167-5699(91)90103-Z. [DOI] [PubMed] [Google Scholar]
- 3.Unanue E R, Allen P M. Science. 1987;236:551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- 4.Evans R, Alexander P. Immunology. 1972;23:615–626. [PMC free article] [PubMed] [Google Scholar]
- 5.Mayernik D G, Ul-Haq A, Rinehart J J. J Immunol. 1983;130:2156–2160. [PubMed] [Google Scholar]
- 6.Karnovsky M L, Lazdins J K. J Immunol. 1978;121:809–813. [PubMed] [Google Scholar]
- 7.Musson R A. Am J Pathol. 1983;111:331–340. [PMC free article] [PubMed] [Google Scholar]
- 8.Becker S, Warren M K, Haskill S. J Immunol. 1987;139:3703–3709. [PubMed] [Google Scholar]
- 9.Pabst M J, Hedegaard H B, Johnston R B. J Immunol. 1982;128:123–128. [PubMed] [Google Scholar]
- 10.Mangan D F, Wahl S M. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 11.Mangan D F, Welch G R, Wahl S M. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 12.Kiener P A, Davis P M, Starling G C, Mehlin C, Klebanoff S J, Ledbetter J A, Liles W C. J Exp Med. 1997;185:1511–1516. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiener P A, Moran-Davis P, Rankin B M, Wahl A F, Aruffo A, Hollenbaugh D. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 14.Heidenreich S, Schmidt M, August C, Cullen P, Rademaekers A, Pauels H G. J Immunol. 1997;159:3178–3188. [PubMed] [Google Scholar]
- 15.Okada S, Zhang H, Hatano M, Tokuhisa T. J Immunol. 1998;160:2590–2596. [PubMed] [Google Scholar]
- 16.Chinnaiyan A M, Dixit V M. Semin Immunol. 1997;9:69–76. doi: 10.1006/smim.1996.0055. [DOI] [PubMed] [Google Scholar]
- 17.Muzio M, Salvesen G S, Dixit V M. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi S S, Park I C, Yun J W, Sung Y C, Hong S I, Shin H S. Oncogene. 1995;9:1693–1698. [PubMed] [Google Scholar]
- 21.Karsan A, Yee E, Kaushansky K, Harlan J M. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- 22.Kenny J J, Knobloch T J, Augustus M, Carter K C, Rosen C A, Lang J C. Oncogene. 1997;14:997–1001. doi: 10.1038/sj.onc.1200898. [DOI] [PubMed] [Google Scholar]
- 23.Naylor M S, Relf M, Balkwill F R. In: Cytokines: A Practical Approach. Balkwill F R, editor. Oxford: Oxford Univ. Press; 1995. pp. 35–56. [Google Scholar]
- 24.Um H-D, Orenstein J M, Wahl S M. J Immunol. 1996;156:3469–3477. [PubMed] [Google Scholar]
- 25.Kikuchi H, Iizuka R, Sugiyama S, Gon G, Mori H, Arai M, Mizumoto K, Imajoh-Ohmi S. J Leukocyte Biol. 1996;60:778–783. doi: 10.1002/jlb.60.6.778. [DOI] [PubMed] [Google Scholar]
- 26.Estaquier J, Ameisen J C. Blood. 1997;90:1618–1625. [PubMed] [Google Scholar]
- 27.Munn D H, Beall A C, Song D, Wrenn R W, Throckmorton D C. J Exp Med. 1995;181:127–136. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onishi Y, Azuma Y, Sato Y, Mizuno Y, Tadakuma T, Kizaki H. Biochim Biophys Acta. 1993;1175:147–154. doi: 10.1016/0167-4889(93)90017-j. [DOI] [PubMed] [Google Scholar]
- 30.Yonehara S, Ishii A, Yonehara M. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia L A, Ayres T M, Wong G H, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 33.Bodmer J L, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, Bornand T, Hahne M, Schroter M, Becker K, et al. Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 34.Kitson J, Raven T, Jiang Y P, Goeddel D V, Giles K M, Pun K T, Grinham C J, Brown R, Farrow S N. Nature (London) 1996;384:372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 35.Marsters S A, Sheridan J P, Donahue C J, Pitti R M, Gray C L, Goddard A D, Bauer K D, Ashkenazi A. Curr Biol. 1996;6:1669–1676. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 36.Yu G L, Lyons R H, Garg M, Duan D R, Xing L, Gentz R, Ni J, Dixit V M. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 37.Pan G, O’Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 38.Pan G, Ni J, Wei Y F, Yu G, Gentz R, Dixit V M. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 39.Schneider P, Bodmer J L, Thome M, Hofmann K, Holler N, Tschopp J. FEBS Lett. 1997;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- 40.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, et al. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 41.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, et al. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 43.Hsu H, Xiong J, Goeddel D V. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 44.Stanger B Z, Leder P, Lee T H, Kim E, Seed B. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 45.Chinnaiyan A M, O’Rourke K, Tewari M, Dixit V M. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 46.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D H. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato T, Irie S, Kitada S, Reed J C. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 48.Chu K, Niu X, Williams L T. Proc Natl Acad Sci USA. 1995;92:11894–11898. doi: 10.1073/pnas.92.25.11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen R T, Cluck M W, Agrawal D K. Cell Mol Life Sci. 1998;54:427–445. doi: 10.1007/s000180050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen G M. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berke G. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 52.Humke E W, Ni J, Dixit V M. J Biol Chem. 1998;273:15702–15707. doi: 10.1074/jbc.273.25.15702. [DOI] [PubMed] [Google Scholar]
- 53.Chao D T, Korsmeyer S J. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 54.Kroemer G. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 55.Tschopp J, Thome M, Hofmann K, Meinl E. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 56.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda J E, MacKenzie A, et al. Nature (London) 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 57.Roy N, Mahadevan M S, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, et al. Cell. 1995;81:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 58.Uren A G, Pakusch M, Hawkins C J, Puls K L, Vaux D L. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rothe M, Pan M G, Henzel W J, Ayres T M, Goeddel D V. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 60.O’Bryan M K, Baker H W, Saunders J R, Kirszbaum L, Walker I D, Hudson P, Liu D Y, Glew M D, d’Apice A J, Murphy B F. J Clin Invest. 1990;85:1477–1486. doi: 10.1172/JCI114594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng G, Cleary A M, Ye Z S, Hong D I, Lederman S, Baltimore D. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 62.Gershenfeld H K, Hershberger R J, Shows T B, Weissman I L. Proc Natl Acad Sci USA. 1988;85:1184–1188. doi: 10.1073/pnas.85.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haddad P, Clement M V, Bernard O, Larsen C J, Degos L, Sasportes M, Mathieu-Mahul D. Gene. 1990;87:265–271. doi: 10.1016/0378-1119(90)90311-e. [DOI] [PubMed] [Google Scholar]
- 64.Nakashima T, Sekiguchi T, Kuraoka A, Fukushima K, Shibata Y, Komiyama S, Nishimoto T. Mol Cell Biol. 1993;13:6367–6374. doi: 10.1128/mcb.13.10.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian Q, Taupin J, Elledge S, Robertson M, Anderson P. J Exp Med. 1995;182:865–874. doi: 10.1084/jem.182.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haddad P, Jenne D, Tschopp J, Clement M V, Mathieu-Mahul D, Sasportes M. Int Immunol. 1991;3:57–66. doi: 10.1093/intimm/3.1.57. [DOI] [PubMed] [Google Scholar]
- 67.Briehl M M, Miesfeld R L. Mol Endocrinol. 1991;5:1381–1388. doi: 10.1210/mend-5-10-1381. [DOI] [PubMed] [Google Scholar]
- 68.Venturelli D, Martinez R, Melotti P, Casella I, Peschle C, Cucco C, Spampinato G, Darzynkiewicz Z, Calabretta B. Proc Natl Acad Sci USA. 1995;92:7435–7439. doi: 10.1073/pnas.92.16.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Przetak M M, Yoast S, Schmidt B F. FEBS Lett. 1995;364:268–271. doi: 10.1016/0014-5793(95)00407-z. [DOI] [PubMed] [Google Scholar]
- 70.Gabig T G, Mantel P L, Rosli R, Crean C D. J Biol Chem. 1994;269:29515–29519. [PubMed] [Google Scholar]
- 71.Brinkmann U, Brinkmann E, Gallo M, Pastan I. Proc Natl Acad Sci USA. 1995;92:10427–10431. doi: 10.1073/pnas.92.22.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shinkai Y, Yoshida M C, Maeda K, Kobata T, Maruyama K, Yodoi J, Yagita H, Okumura K. Immunogenet. 1989;30:452–457. doi: 10.1007/BF02421177. [DOI] [PubMed] [Google Scholar]
- 73.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, et al. Nature (London) 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 74.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, et al. Nature (London) 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 75.Ashany D, Song X, Lacy E, Nikolic-Zugic J, Friedman S M, Elkon K B. Proc Natl Acad Sci USA. 1995;92:11225–11229. doi: 10.1073/pnas.92.24.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]