Abstract
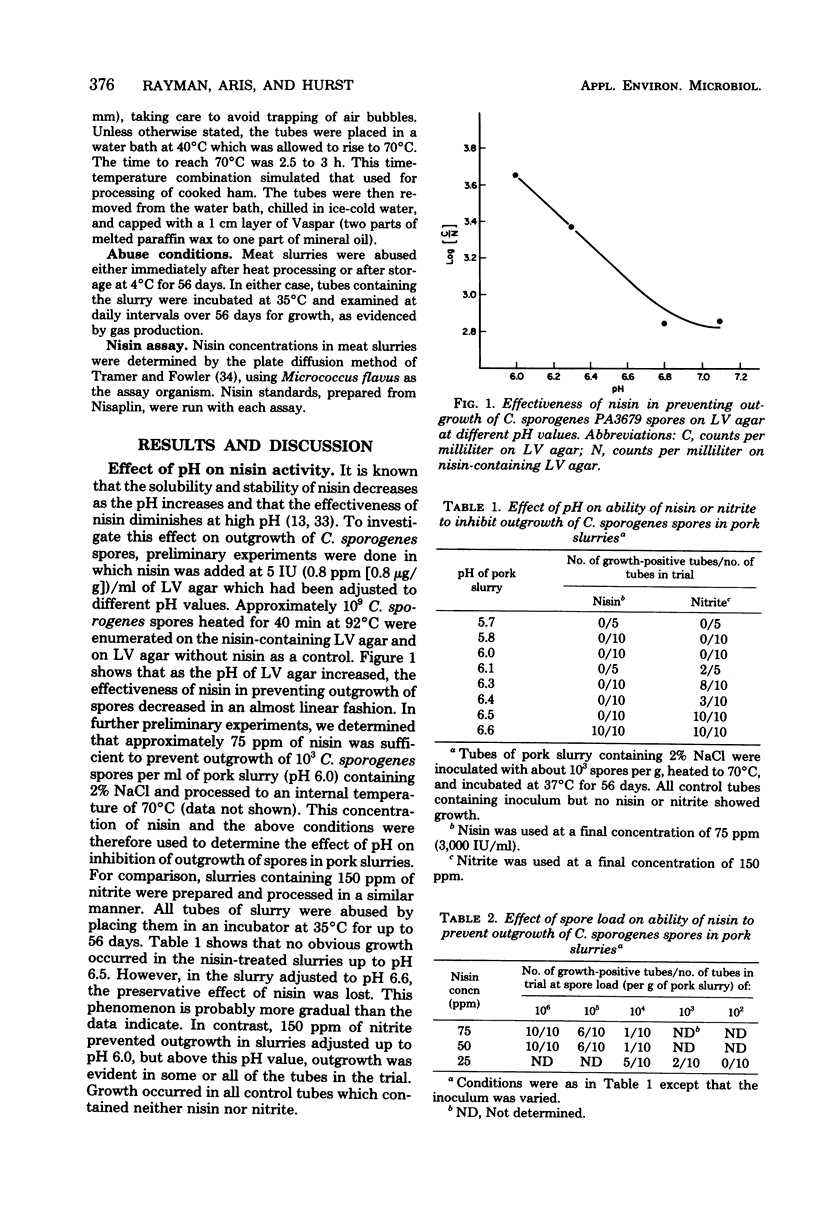
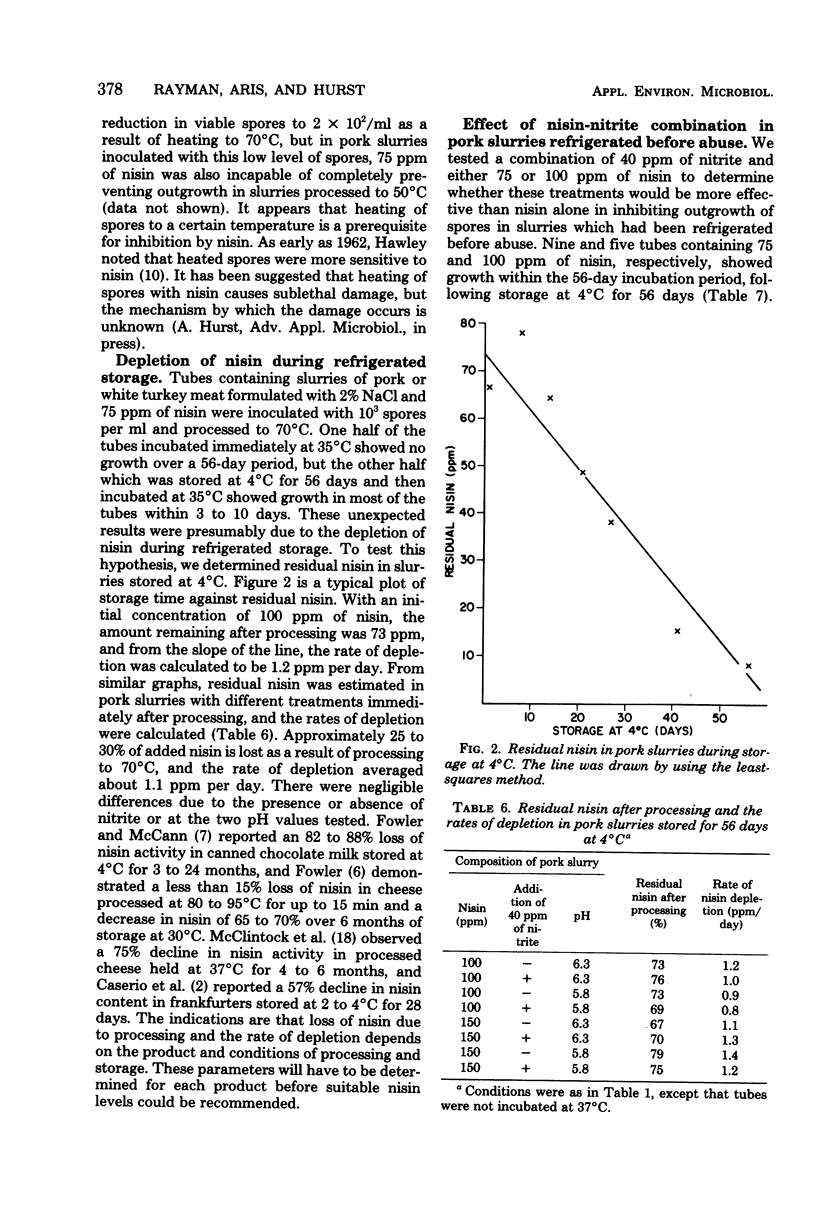
Nisin at 75 ppm (75 microgram/g) was superior to 150 ppm of nitrite in inhibiting outgrowth of Clostridium sporogenes PA3679 spores in meat slurries, which had been heated to simulate the process used for cooked ham. The inhibitory activity of nisin decreased as the spore load or pH of the slurries increased. Unlike nitrite, inhibition by nisin was unaffected by high levels of iron either as a constituent of meats or when added as an iron salt. In slurries treated with 75 ppm of nisin, refrigerated storage for 56 days resulted in depletion of nisin to a level low enough to allow outgrowth within 3 to 10 days if the slurries were subsequently abused at 35 degrees C. In contrast, a combination of 40 ppm of nitrite and either 75 or 100 ppm of nisin almost completely inhibited outgrowth in these slurries. The nisin-nitrite combination appeared to have a synergistic effect, and the low concentration of nitrite was sufficient to preserve the color in meats similar to that of products cured with 150 ppm of nitrite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hurst A. Nisin: its preservative effect and function in the growth cycle of the producer organism. Soc Appl Bacteriol Symp Ser. 1978;7:297–314. [PubMed] [Google Scholar]
- Mirvish S. S. N-nitroso compounds: their chemical and in vivo formation and possible importance as environmental carcinogens. J Toxicol Environ Health. 1977 Jul;2(6):1267–1277. doi: 10.1080/15287397709529529. [DOI] [PubMed] [Google Scholar]
- Newberne P. M. Nitrite promotes lymphoma incidence in rats. Science. 1979 Jun 8;204(4397):1079–1081. doi: 10.1126/science.451551. [DOI] [PubMed] [Google Scholar]
- Niskanen A., Nurmi E. Effect of starter culture on staphylococcal enterotoxin and thermonuclease production in dry sausage. Appl Environ Microbiol. 1976 Jan;31(1):11–20. doi: 10.1128/aem.31.1.11-20.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Palumbo S. A. Injury to Staphylococcus aureus during sausage fermentation. Appl Environ Microbiol. 1978 Dec;36(6):857–860. doi: 10.1128/aem.36.6.857-860.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofos J. N., Busta F. F., Allen C. E. Sodium nitrite and sorbic acid effects on Clostridium botulinum spore germination and total microbial growth in chicken frankfurter emulsions during temperature abuse. Appl Environ Microbiol. 1979 Jun;37(6):1103–1109. doi: 10.1128/aem.37.6.1103-1109.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkin R. B., Christiansen L. N., Shaparis A. B. Antibotulinal efficacy of sulfur dioxide in meat. Appl Environ Microbiol. 1980 Jun;39(6):1096–1099. doi: 10.1128/aem.39.6.1096-1099.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkin R. B., Christiansen L. N., Shaparis A. B., Bolin H. Effect of potassium sorbate on salmonellae, Staphylococcus aureus, Clostridium perfringens, and Clostridium botulinum in cooked, uncured sausage. Appl Microbiol. 1974 Aug;28(2):262–264. doi: 10.1128/am.28.2.262-264.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkin R. B., Christiansen L. N., Shaparis A. B. Iron and the antibotulinal efficacy of nitrite. Appl Environ Microbiol. 1979 Feb;37(2):351–353. doi: 10.1128/aem.37.2.351-353.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEHARA M., FUJIOKA R. S., FRANK H. A. METHOD FOR OBTAINING CLEANED PUTREFACTIVE ANAEROBE 3679 SPORES. J Bacteriol. 1965 Mar;89:929–930. doi: 10.1128/jb.89.3.929-930.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]



