Abstract
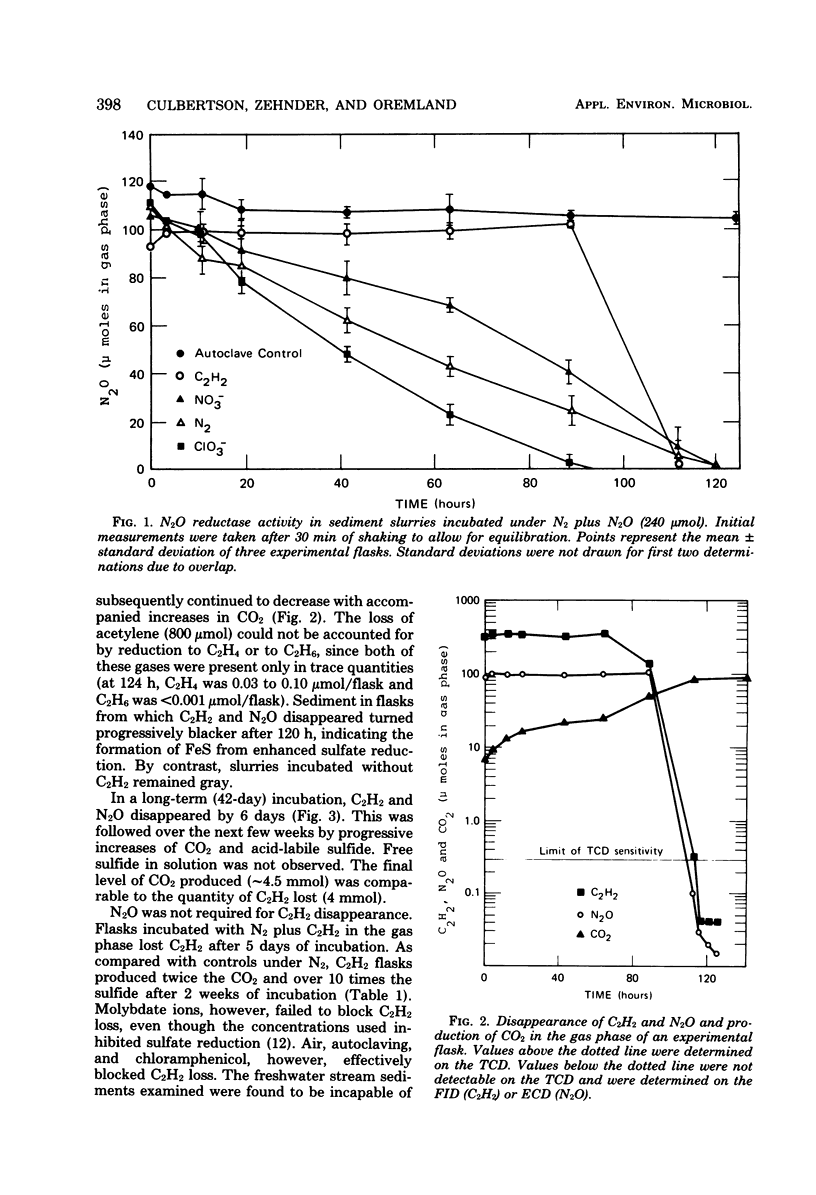
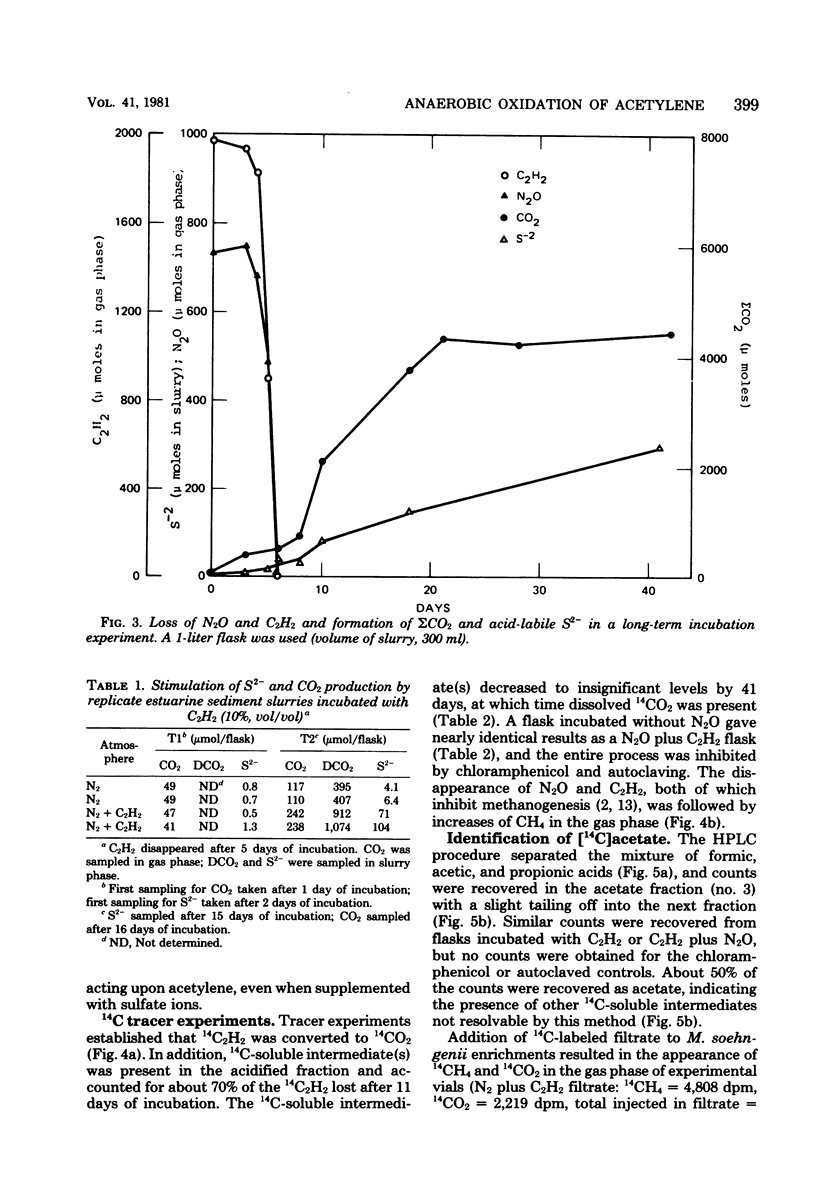
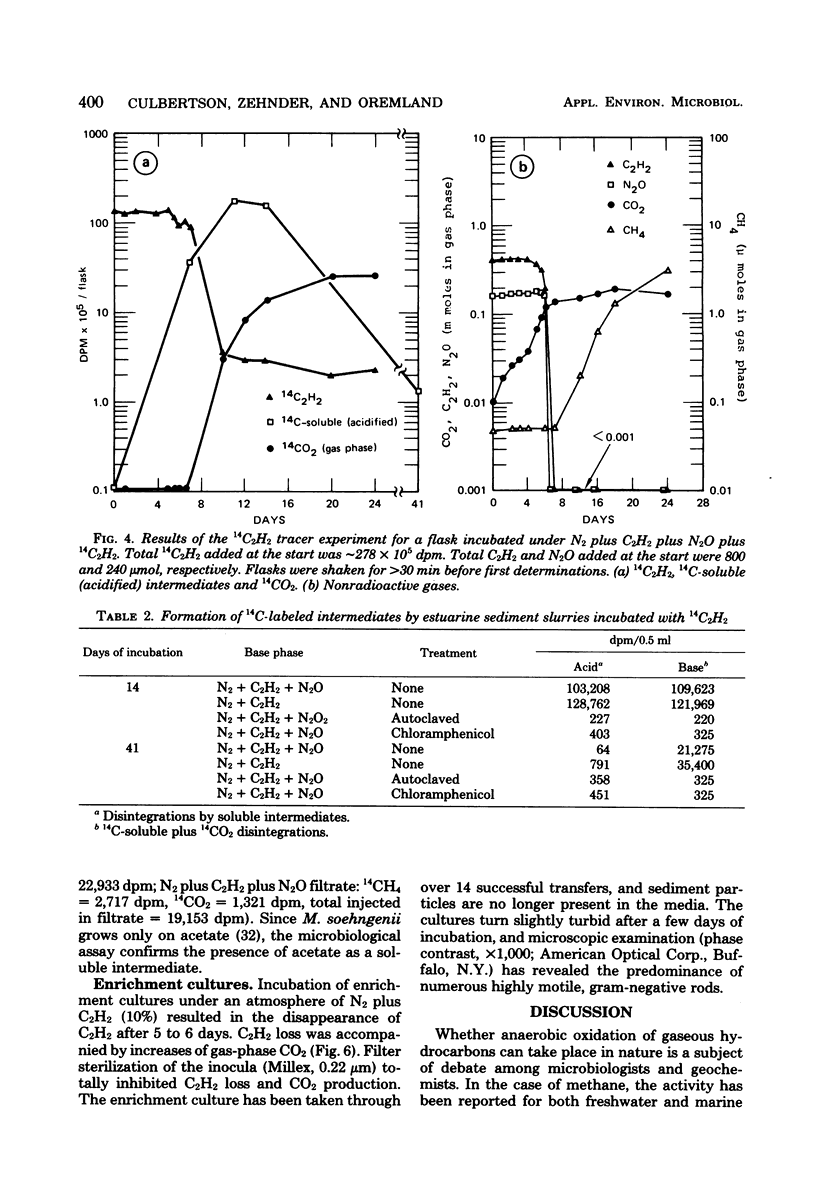
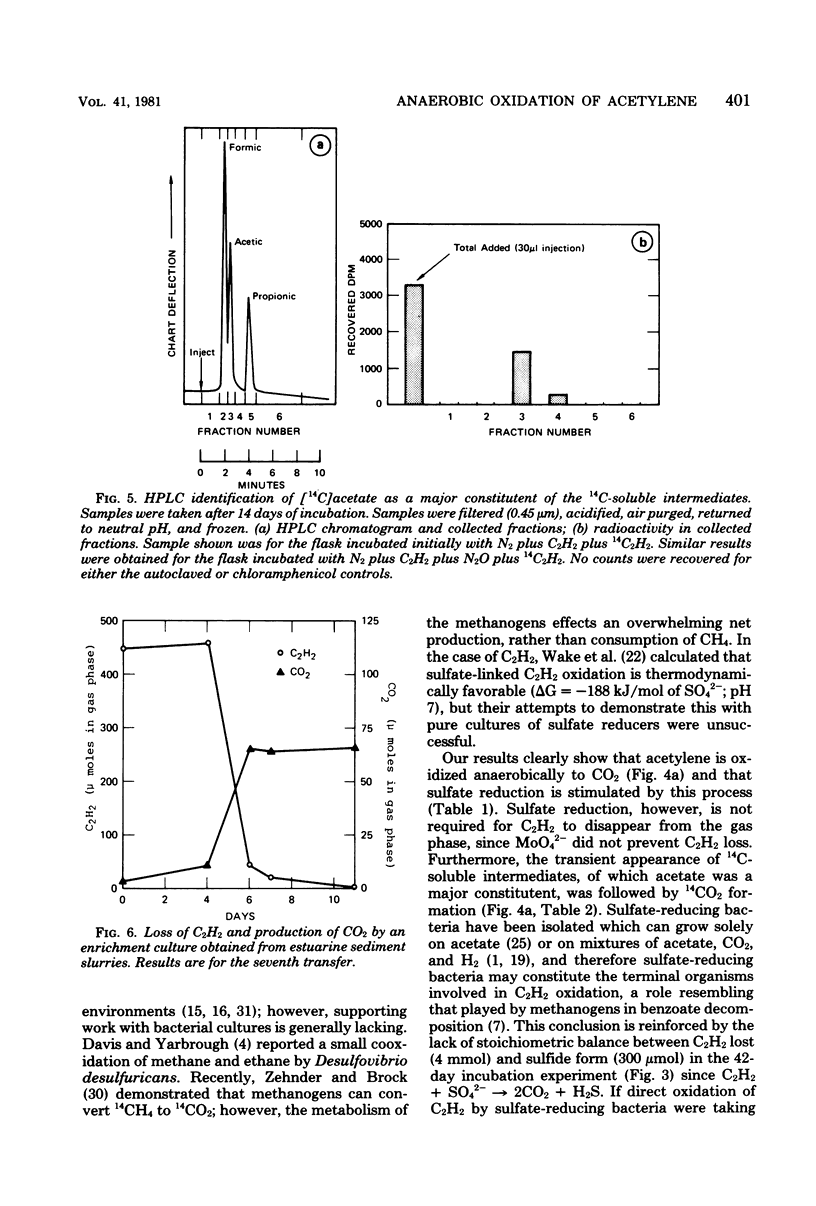
Acetylene disappeared from the gas phase of anaerobically incubated estuarine sediment slurries, and loss was accompanied by increased levels of carbon dioxide. Acetylene loss was inhibited by chloramphenicol, air, and autoclaving. Addition of 14C2H2 to slurries resulted in the formation of 14CO2 and the transient appearance of 14C-soluble intermediates, of which acetate was a major component. Acetylene oxidation stimulated sulfate reduction; however, sulfate reduction was not required for the loss of C2H2 to occur. Enrichment cultures were obtained which grew anaerobically at the expense of C2H2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badziong W., Thauer R. K., Zeikus J. G. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol. 1978 Jan 23;116(1):41–49. doi: 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- Balderston W. L., Payne W. J. Inhibition of methanogenesis in salt marsh sediments and whole-cell suspensions of methanogenic bacteria by nitrogen oxides. Appl Environ Microbiol. 1976 Aug;32(2):264–269. doi: 10.1128/aem.32.2.264-269.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bont J. A., Mulder E. G. Invalidity of the acetylene reduction assay in alkane-utilizing, nitrogen-fixing bacteria. Appl Environ Microbiol. 1976 May;31(5):640–647. doi: 10.1128/aem.31.5.640-647.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry J. G., Wolfe R. S. Anaerobic degradation of benzoate to methane by a microbial consortium. Arch Microbiol. 1976 Feb;107(1):33–40. doi: 10.1007/BF00427864. [DOI] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner D., Bartha R. Growth of Nocardia rhodochrous on acetylene gas. J Bacteriol. 1979 Jul;139(1):225–230. doi: 10.1128/jb.139.1.225-230.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Taylor B. F. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol. 1975 Oct;30(4):707–709. doi: 10.1128/am.30.4.707-709.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T., Jr, Patt T. E., Hart W., Hanson R. S. Oxidation of methane in the absence of oxygen in lake water samples. Appl Environ Microbiol. 1979 Feb;37(2):303–309. doi: 10.1128/aem.37.2.303-309.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin Y. I. Role of carbon dioxide and acetate in biosynthesis by sulphate-reducing bacteria. Nature. 1966 Apr 30;210(5035):551–552. doi: 10.1038/210551a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Tiedje J. M., Firestone R. B. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980 Jan;39(1):105–108. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam T. Y., Knowles R. Effects of sulfide and acetylene on nitrous oxide reduction by soil and by Pseudomonas aeruginosa. Can J Microbiol. 1979 Oct;25(10):1133–1138. doi: 10.1139/m79-176. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Wake L. V., Christopher R. K., Rickard P. A., Andersen J. E., Ralph B. J. A thermodynamic assessment of possible substrates for sulphate-reducing bacteria. Aust J Biol Sci. 1977 Apr;30(1-2):155–172. [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Anaerobic methane oxidation: occurrence and ecology. Appl Environ Microbiol. 1980 Jan;39(1):194–204. doi: 10.1128/aem.39.1.194-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol. 1979 Jan;137(1):420–432. doi: 10.1128/jb.137.1.420-432.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B. A., Brock T. D., Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980 Jan;124(1):1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]


