Abstract
Myasthenia gravis (MG) is a T cell-regulated, antibody-mediated autoimmune disease. Two peptides representing sequences of the human acetylcholine receptor α-subunit, p195–212 and p259–271, previously were shown to stimulate the proliferation of peripheral blood lymphocytes of patients with MG and were found to be immunodominant T cell epitopes in SJL and BALB/c mice, respectively. Single amino acid-substituted analogs of p195–212 and p259–271, as well as a dual analog composed of the tandemly arranged two single analogs, were shown to inhibit, in vitro and in vivo, MG-associated autoimmune responses. Stimulation of T cells through the antigen-specific T cell receptor activates tyrosine kinases and phospholipase C (PLC). Therefore, in attempts to understand the mechanism of action of the analogs, we first examined whether the myasthenogenic peptides trigger tyrosine phosphorylation and activation of phospholipase C. For that purpose, we measured generation of inositol phosphates and tyrosine phosphorylation of PLC after stimulation of the p195–212- and p259–271-specific T cell lines with these myasthenogenic peptides. Both myasthenogenic peptides stimulated generation of inositol phosphates as well as tyrosine phosphorylation of PLC. However, the single and dual analogs, although inducing tyrosine phosphorylation of PLC, could not induce PLC activity. Furthermore, the single and dual analogs inhibited the induced PLC activity whereas they could not inhibit tyrosine phosphorylation of PLC that was caused by the myasthenogenic peptides. Thus, the altered peptides and the dual analog act as partial agonists. The down-regulation of PLC activity by the analogs may account for their capacity to inhibit in vitro MG-associated T cell responses.
Keywords: T cell receptor/autoimmune myasthenia gravis/signal transduction/tyrosine phosphorylation/peptide analogs
Myasthenia gravis (MG) is a T cell-regulated, antibody-mediated autoimmune disease. The abnormality in MG is a deficiency of acetylcholine receptors at neuromuscular junctions caused by the antibody-mediated autoimmune attack (1–4). The current treatment of MG is nonspecific, and, although immunosuppressive drugs are usually beneficial in MG, they result in generalized suppression of the immune system. Ideally, treatment should inhibit specifically the immune response to acetylcholine receptors. Previous work in our laboratory has shown that two peptides representing sequences of the human acetylcholine receptor α-subunit, p195–212 and p259–271, were able to stimulate peripheral blood lymphocytes of patients with MG and to serve as immunodominant T cell epitopes of SJL and BALB/c mice, respectively (5, 6). Altered myasthenogenic peptides, which are single amino acid-substituted analogs of p195–212 (Ala-207) and p259–271 (Lys-262), as well as a dual analog composed of the tandemly arranged two single analogs (Lys-262-Ala-207), were synthesized and were shown to inhibit the proliferative responses of both p195–212- and p259–271-specific T cell lines in vitro as well as the in vivo priming to the myasthenogenic peptides (7–9). Furthermore, the dual analog could reverse myasthenogenic manifestations in mice with experimental autoimmune MG (9). In addition, the analogs differentially inhibited the induced secretion of some, but not all, cytokines by the specific cells of the lines (8). The single and dual analogs also were shown to be capable of inhibiting the proliferative responses of peripheral blood lymphocytes of MG patients to both myasthenogenic peptides p195–212 and p259–271 (10). Thus, the single and dual analogs are altered peptide ligands (APLs) that immunomodulate MG associated autoimmune responses by a mechanism that cannot be based on major histocompatibility complex (MHC) blockade only (7–9). A possible mechanism might be alteration of signal transduction by the peptide analogs that might act either as partial agonists or as T cell antigen receptor (TCR) antagonists.
Stimulation of a T cell through its antigen-specific receptor by antigen or antireceptor antibodies initiates a series of biochemical events that result in cellular proliferation and cytokine production. TCR engagement by ligand is a complex event that may activate multiple discrete pathways (reviewed in refs. 11 and 12). One of the earliest detectable events after stimulation with an antigen is the activation of a number of protein tyrosine kinases, which subsequently leads to receptor autophosphorylation and increased tyrosine phosphorylation of numerous cellular proteins including phospholipase C (PLC) (reviewed in refs. 11–14). The receptor autophosphorylation creates high affinity binding sites for several proteins containing SH2 domains, including PLCγ1, which is the main isoform of PLC in T cells. The phosphorylation and activation of PLCγ1 results in hydrolysis of phosphatidylinositol-4,5-biphosphate, which in turn yields the second messengers inositol-1,4,5-triphosphate and diacyl glycerol. These second messengers are respectively responsible for the TCR-induced rapid and sustained increase in the concentration of intracellular calcium ions and the activation of protein kinase C.
Studies of T cell responses to altered peptide ligands have provided functional evidence that a T cell receptor can respond to small changes in its ligand, resulting in different outcomes (reviewed in refs. 11, 15, and 16). Such small changes in the ligand can convert fully activating ligands into partially activating (partial agonists) or even inhibitory ones (antagonists). Stimulation by partial agonists of mouse T cells results in the following: (i) a predominance of the p21 phosphorylated form of the TCR ζ chain rather than the similar levels of both the p21 and p23 forms seen with an agonist; (ii) little or no CD3ɛ tyrosine phosphorylation; and (iii) association of the syk family kinase ZAP-70 with the phosphorylated ζ chains without the stable enzymatic activation of this key molecule.
In this study, we used T cell lines to examine some of the intracellular biochemical events induced by structurally related but functionally distinct TCR ligands. We specifically studied PLC activity and phosphorylation in T cells that express a TCR specific for the myasthenogenic peptides. We show here that the single and the dual analogs of the myasthenogenic peptides could not induce PLC activity but caused tyrosine phosphorylation of PLCγ1. Moreover, the analogs could inhibit PLC activity induced by the myasthenogenic peptides, but they could not inhibit the induced tyrosine phosphorylation.
MATERIALS AND METHODS
Synthetic Myasthenogenic Peptides and Their Peptide Analogs.
The synthetic peptides p195–212 (DTPYLDITYHFVMQRLPL) and p259–271 (VIVELIPSTSSAV) of the human acetylcholine receptor were synthesized and characterized as described (9). Single amino acid-substituted analogs of p259–271 (Lys-262; VIVKLIPSTSSAV) and p195–212 (Ala-207; DTPYLDITYHFVAQRLPL) were synthesized as described above. The dual peptide analog Lys-262-Ala-207 was constructed by sequential synthesis of the combined two single amino acid-substituted analogs starting with the p259–271 analog. A reversed form of the dual analog (LPLRQAVFHYTIDLYPTDVASSTSPILKVIV) was used as a control peptide.
Proliferative Responses of T Cell Lines.
T cell lines specific to p259–271 of BALB/c (H-2d) origin and to p195–212 of SJL (H-2s) origin were established as described (17). The capacity of the analogs to inhibit specific proliferative responses of the T cell lines was tested 7 days after antigenic stimulation as described (9). In brief, cells of the lines were cultured in microtiter plates with irradiated syngeneic spleen cells in the presence of different concentrations of the peptides, with or without the analogs. At the end of a 48-hr incubation period, [3H]thymidine was added for a period of 16 hr. Cells were harvested, and radioactivity was counted.
Measurement of Inositol Phosphate Accumulation.
Cells of the T cell lines were suspended (2 × 106/ml) in RPMI medium 1640 depleted of inositol, supernatant of concanavalin A-stimulated splenocytes, and serum and were incubated with myo-2-[3H]inositol (2 μCi/ml; New England Nuclear) overnight at 37°C. After labeling, cells were washed, were pretreated with lithium chloride (15 mM) for 30 min, and incubated (5 × 106 cells per sample) for the indicated length of time with 5 × 106 antigen-presenting cells (APCs) and the indicated peptides. Spleens of either normal BALB/c or SJL mice served as APCs. In cases in which the APLs and the dual analog were used, they were added concomitant with the myasthenogenic peptides and APCs. Reactions were terminated by the addition of chloroform and methanol (1/2, vol/vol), and inositol metabolites were extracted. After the addition of chloroform and water, aqueous phases were removed and applied to an anion exchange column (AG 1-X8 resin, 100–200 mesh formate form; Bio-Rad). After the removal of free inositol and glycerophosphoinositide by elution with 5 mM inositol and 60 mM sodium formate–5 mM sodium tetraborate, inositol monophosphate (IP1) was eluted with 0.2 M ammonium formate–0.1 M formic acid, and inositol (1, 4)-biphosphates (IP2) and inositol (1, 4, 5)-triphosphates (IP3) were eluted with 0.8 M ammonium formate–0.1 M formic acid. Radioactivity was assessed by liquid scintillation counting.
TCR-Induced Tyrosine Phosphorylation Assay.
Immunoblot analysis of tyrosine phosphorylation of PLCγ1 by using antiphosphotyrosine immunoprecipitates was performed as follows. Splenocytes from BALB/c mice were preadhered to six-well plates and were preloaded with the peptides for 2 hr. Cells of the lines (1.5 × 107) were added to the wells for 30 min at 37°C. The reaction was stopped by the addition of sodium orthovanadate and EDTA. T cells only (without the adherent splenocytes) were transferred to Eppendorf tubes and were lysed on ice for 30 min at 1.5 × 107 cells/ml in a lysis buffer containing 1% Triton X-100, 0.5% Nonidet P-40, 50 mM Tris (pH 7.5), 150 mM NaCl, 10 mM EDTA, 1 mM sodium orthovanadate, and protease inhibitor mixture (Sigma). Lysates were cleared by centrifugation (15,000 × g for 10 min at 4°C), and tyrosine phosphorylated proteins were immunoprecipitated at 4°C by using antiphosphotyrosine antibodies (PY20, Santa Cruz Biotechnology), followed by incubation with protein A. Proteins were eluted from the beads by boiling for 5 min in reducing Laemmli sample buffer. Samples were subjected to electrophoresis by using 8% acrylamide gel and were Western blotted onto nitrocellulose. The blot was probed with anti-PLCγ1 antibodies (Transduction Laboratories, Lexington, KY) and was developed by using chemiluminescence.
RESULTS
Activation of PLC in Peptide-Specific T Cell Lines by the Myasthenogenic Peptides and Their Analogs.
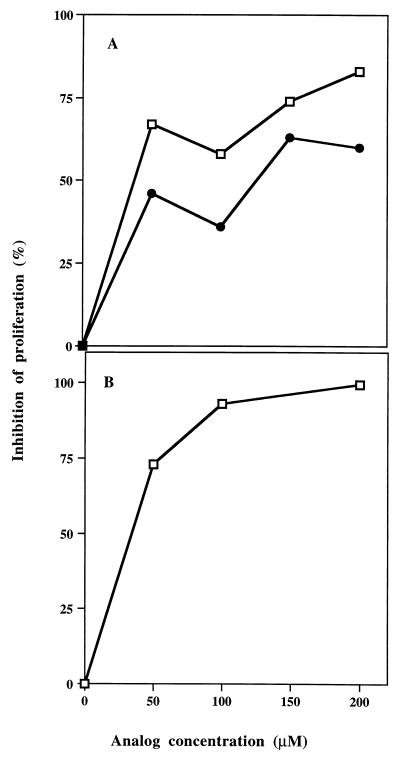
The single analog of p259–271 (Lys-262) as well as the dual analog Lys-262-Ala-207 were tested for their ability to inhibit the proliferative responses of both p195–212 and p259–271 specific T cell lines when cocultured with their stimulating peptides. Fig. 1 demonstrates a representative experiment of inhibition of the proliferative responses of both T cell lines by these analogs, as was shown (8, 9, 18). Thus, Fig. 1A shows that up to 63% and up to 87% of the proliferative responses induced by p259–271 were inhibited by the single analog 262-Lys and by the dual analog, respectively. Fig. 1B shows that the dual analog inhibited up to 93% of the proliferation induced by p195–212.
Figure 1.
Inhibition of the proliferative responses of T cell lines specific to myasthenogenic peptides. (A) Cultures of cells of the p259–271-specific T cell line of BALB/c origin were incubated with different concentrations of the single or dual analogs in the presence of 2.5 μM p259–271. (B) Cells of the p195–212-specific T cell line of SJL origin were incubated with different concentrations of the dual analog in the presence of 15 μM p195–212. Conditions of the assay are described in Materials and Methods. Results are expressed as percent of inhibition of p259–271- or p195–212-specific proliferative responses. □–□, dual analog; •–•, Lys-262.
In an attempt to get more insight into the mechanism(s) by which the analogs inhibit the proliferation induced by the myasthenogenic peptides, we decided to concentrate on PLC, which is one of the early enzymes participating in the signal transduction through the TCR. Therefore, we first examined whether the myasthenogenic peptides can activate PLC in the specific T cell lines. To this end, we prelabeled specifically the phosphoinositides of the cells of the line with [3H]inositol. Cells then were incubated with syngeneic APCs and with the indicated peptides, and, at the end of the reaction, the inositol phosphates were extracted and separated, and radioactivity was determined by a β counter.
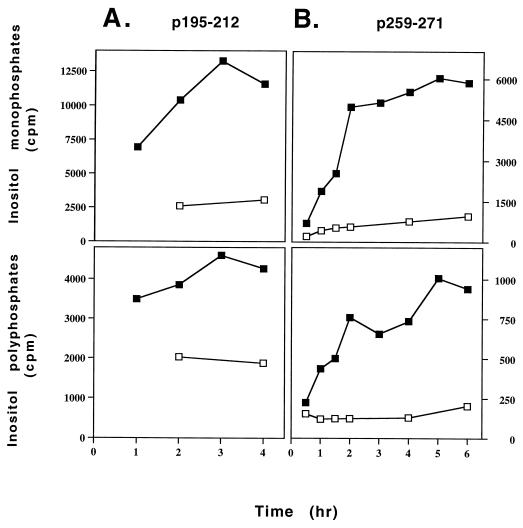
As can be seen in Fig. 2, p259–271 and p195–212 stimulated generation of inositol phosphates in a time-dependent manner. Most of the inositol phosphates generated were IP1 (six folds of the amount of inositol polyphosphates in the case of p259–271-specific T cell line and three folds in the case of p195–212). According to the kinetic experiments, the reactions reach a plateau after 2 hr of stimulation with p259–271 or after 3 hr of stimulation with p195–212. In the absence of peptide, no elevation in inositol phosphate levels could be observed. Table 1 demonstrates that the generation of inositol phosphates by the p259–271-specific T cell line of BALB/c origin resulted from the specific stimulation by the appropriate myasthenogenic peptide whereas other peptides (p195–212 and a reversed p259–271) restricted to the same MHC class II molecules of BALB/c (H-2d) mice did not cause the production of inositol phosphates. The single amino acid-substituted peptide Lys-262 and the dual analog Lys-262-Ala-207, which is composed of the tandemly arranged two single analogs, could not induce generation of inositol phosphates (Table 1).
Figure 2.
Kinetics of induction of inositol phospholipid hydrolysis by the myasthenogenic peptides in the specific T cell lines. Cells of the peptide specific lines were prelabeled with myo-[2-3H]inositol and were incubated with APCs in the presence or absence of either p195–212 (15 μM) (A) or p259–271 (8 μM) (B) for the indicated time points. The levels of inositol phosphates were measured as described in Materials and Methods. The experiment is a representative of two independent experiments. □–□, without peptide; ■–■, in the presence of peptide.
Table 1.
p259–271, but not other peptides, induce inositol phospholipid hydrolysis in a specific T cell line
| Peptide | IP1, cpm | IP2 + IP3, cpm |
|---|---|---|
| No peptide | 1105 ± 43 | 239 ± 22 |
| p259–271 | 26642 ± 224 | 2100 ± 308 |
| Reversed p259–271 | 921 ± 38 | 225 ± 15 |
| p195–212 | 1008 ± 70 | 224 ± 25 |
| Lys-262 | 1574 ± 520 | 396 ± 98 |
| Lys-262–Ala-207 | 1105 ± 108 | 444 ± 59 |
Cells of the peptide-specific line were prelabeled with myo-[2-3H]inositol and were incubated for 1.5 hr with the different peptides (8 μM), in the presence of APCs. The concentration of the dual analog was 125 μM. The levels of the inositol phosphates generated were measured as described in Materials and Methods. The results represent three similar experiments.
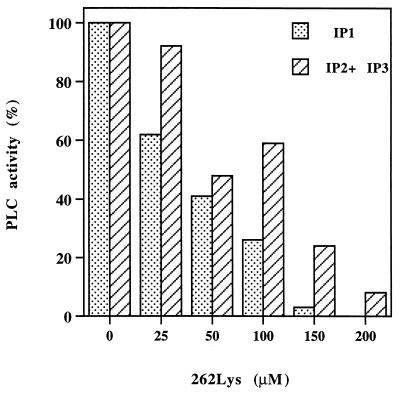
After demonstrating that the single and dual analogs do not induce generation of inositol phosphates in both lines, their ability to inhibit the PLC activity induced by each of the myasthenogenic peptides p259–271 and p195–212 was tested. As can be seen in Fig. 3, the concomitant addition of 262-Lys to the assay mixture, which included the p259–271-specific T cell line, APC and the myasthenogenic peptide could inhibit almost completely (100% inhibition in the case of IP1 and 92% inhibition of inositol polyphosphates) the generation of inositol phosphates induced by p259–271.
Figure 3.
The single analog of p259–271 (Lys-262) inhibits inositol phospholipid hydrolysis induced by p259–271. Cells of the peptide specific line were prelabeled with myo-[2-3H]inositol and were incubated for 1.5 hr with different concentrations of the single analog (Lys-262) and the myasthenogenic peptide (10 μM) in the presence of APCs. The levels of the generated inositol phosphates were measured as described in Materials and Methods. The value (cpm) of inositol phosphates generated spontaneously (in the absence of the myasthenogenic peptides) by the cells was subtracted from all of the other values. The levels of inositol phosphates generated by cells that were activated by p259–271 in the absence of the analog were considered as 100% activation. The experiment is a representative of three independent experiments.
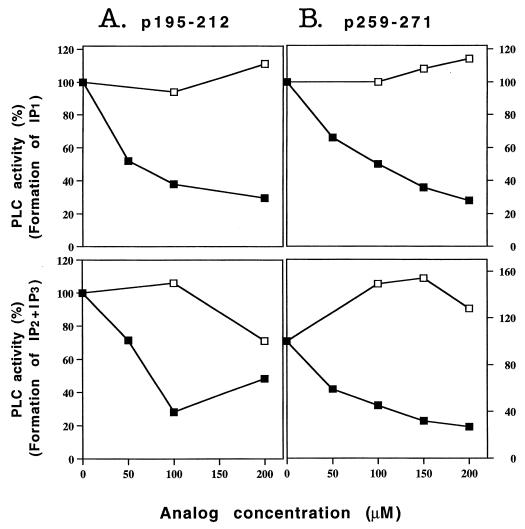
Fig. 4 demonstrates the capacity of the dual analog to inhibit the PLC activity induced by both myasthenogenic peptides. As can be seen above, 70% of the generation of IP1 and IP2 + IP3 induced by p259–271 or p195–212 were inhibited by the dual analog. To verify that the inhibition was not a mere result of the MHC blockade, we performed similar assays in which the reversed dual analog or other peptides (that are compatible with the MHC of BALB/c mice) at the same concentration were added to the cells concomitant with the myasthenogenic peptides. Although the dual analog inhibited the stimulation by the myasthenogenic peptides, the reversed dual analog (Fig. 4) as well as other peptides (data not shown) at the same concentration did not prevent the activation of PLC by the myasthenogenic peptides. These results indicate that the inhibition by either the single or the dual analogs is not a result of MHC blockade.
Figure 4.
The dual analog inhibits inositol phospholipid hydrolysis induced by the myasthenogenic peptides. Cells of the peptide specific line were prelabeled with myo-[2-3H]inositol and were incubated with different concentrations of the dual analog or its reversed form, together with the appropriate peptide [15 μM for p195–212 (A) or 10 μM for p259–271 (B)], and with APCs. The reaction was terminated after 2 hr for p259–271 or after 3.5 hr for p195–212. The levels of the generated inositol phosphates were measured as described in Materials and Methods. Results were calculated and expressed as described in legend to Fig. 3. ■–■, dual analog; □–□, reversed dual analog. The experiment is a representative of three independent experiments.
Induction of PLCγ1 Phosphorylation in Peptide-Specific T Cell Lines by the Myasthenogenic Peptides and Their Analogs.
Because PLC activity is not the first event after TCR ligation, it was of interest to examine whether the differences between the signals transduced by the myasthenogenic peptide p259–271 and its dual analog are expressed even earlier in the signaling cascade. One of the earliest events of TCR-mediated signaling is tyrosine phosphorylation of PLCγ1, which occurs before phospholipid hydrolysis (19). The relevant isoform of PLC for TCR-mediated activation in Th1 type T cells and CD8+ cytotoxic T lymphocytes appears to be PLCγ1 (20). Therefore, in attempts to investigate further the mechanism of action of the dual analog, we studied tyrosine phosphorylation of PLCγ1 after exposure of the cells of the line to the myasthenogenic peptide p259–271 and/or its analog. To this end, we incubated the cells with preadhered APCs in the presence of the myasthenogenic peptide p259–271 alone, the dual analog alone, or their combination. After the incubation period, cells were collected, and their proteins were extracted and immunoprecipitated with antiphosphotyrosine antibodies. Fig. 5 shows that both p259–271 and the dual analog and their combination could induce tyrosine phosphorylation of PLCγ1. However, the extent of PLCγ1 phosphorylation by the myasthenogenic peptide p259–271 was higher than that by the dual analog. Of interest, the dual analog, which was shown to inhibit PLC activity (Fig. 4) when added concomitant with the stimulating peptide (p259–271), did not inhibit PLCγ1 phosphorylation induced by the myasthenogenic peptide p259–271.
Figure 5.
The dual analog does not inhibit tyrosine phosphorylation of PLCγ1 induced by the myasthenogenic peptide p259–271. Cells of the peptide-specific line (1.5 × 107 cells/well) were incubated with preadhered APCs alone or in the presence of p259–271 (1 μM) with or without the dual analog (150 μM) for 30 min at 37°C. The proteins were extracted and immunoprecipitated by antiphosphotyrosine antibodies followed by immunoblotting by using anti-PLCγ1 antibodies. The experiment is a representative of three independent experiments.
DISCUSSION
Previous results from our laboratory have shown that a dual analog of two myasthenogenic T cell epitopes could inhibit autoimmune-related myasthenogenic peptide-specific T cell responses in SJL and BALB/c mice both in vitro and in vivo (9). The aim of the present study has been to gain more insight into signaling events that may be responsible for the activation of the specific T cell lines by the myasthenogenic peptides and for the inhibition of this activation by the APL.
The main findings of the present report are that the dual analog, which is composed of two altered myasthenogenic peptides, is a partial agonist. Thus, it did not inhibit tyrosine phosphorylation of PLC but inhibited PLC activation induced by the myasthenogenic peptides. Both myasthenogenic peptides induced PLC activity in their specific T cell lines, and the dual analog could inhibit both responses specifically. These effects were observed for the generation of both IP1 and inositol polyphosphates. The inhibition of PLC activity was shown to correlate with the inhibition of T cell proliferation triggered by the myasthenogenic peptides.
It is noteworthy that most of the studies reported by other researchers used anti-CD3 antibodies to activate the cells and to measure PLC activity (11, 14, 19, 20). Further studies performed using peptides and their analogs (21) were focused on the inability of the altered peptides to activate PLC and not on the potential use of the analogs to inhibit the signal transduced by the original peptide and thus to inhibit autoreactive T cells.
One mechanism by which altered peptides antagonize the T cell agonist is MHC blockade. Alternatively, the APL might deliver a partial and altered signal that independently predominates over the agonist response. Our results support the latter mechanism. The possibility of MHC blockade was ruled out because both the reversed dual analog as well as other peptides (peptide p195–212 and the reversed p259–271), which are restricted to the MHC of BALB/c (H-2d), did not inhibit the PLC activity induced by p259–271. Moreover, MHC blockade cannot explain the previous findings that the dual analog is capable of inhibiting in vivo priming of lymph node cells 1 week after the primary immunization with the myasthenogenic peptides, when the T cells already are activated to the latter. Further, the ability of the dual analog to reverse the manifestations of an established experimental autoimmune MG (9) support the notion that, at least in vivo, the dual analog does not operate via MHC blockade (9). The possibility of being a full antagonist also is ruled out because the dual analog differentially inhibits the PLC activity but not its tyrosine phosphorylation induced by p259–271. Furthermore, as previously reported, analog Lys-262 inhibited completely the secretion of interleukin 2 and interleukin 4 whereas it did not inhibit interferon γ secretion and could induce by itself the secretion of low but significant levels of the latter (8). As for the dual analog, it has been shown that it could induce by itself the secretion of several, but not all, cytokines triggered by p195–212 and could inhibit differentially cytokine secretion by p195–212 (M.P.-R., E.M., and M.S., unpublished results). The results presented in this work show that the dual analog can transduce a signal leading to the phosphorylation of PLCγ1, although to a lesser extent than p259–271. All of these results lead to the conclusion that it acts as a partial agonist of the myasthenogenic peptides.
If a certain threshold of tyrosine phosphorylation is needed for PLC to become active, then the lower PLCγ1 phosphorylation caused by the dual analog alone may account for the inability of the dual analog to activate PLC. However, it seems that tyrosine phosphorylation of PLCγ is not sufficient for activation of this enzyme because the dual analog, both in the presence or absence of p259–271, causes tyrosine phosphorylation of PLC, but the enzyme remains inactive. Moreover, although the combination of the peptide and the dual analog did not inhibit PLCγ1 phosphorylation, it did inhibit its activity. Jensen et al. (22) presented data to indicate that TCRζ was capable of transducing signals resulting in tyrosine phosphorylation of PLCγ1 but was incapable of transducing signals resulting in IP3 generation and calcium mobilization. Involvement of other proteins in the signal leading to PLC activity was reported. For example, Motto et al. (23) have reported tyrosine phosphorylation of PLCγ1 in the absence of inositol phosphate production, and their results suggested that a tyrosine-phosphorylated Grb2-binding protein(s) is necessary in addition to PLCγ1 phosphorylation for IP3 generation. Other phosphoproteins, like SLP-76 (24), might be necessary for translocation of phosphorylated PLCγ1 to the plasma membrane. Taken together, these data suggest that PLC activation is influenced by signaling pathways additional to PLCγ phosphorylation, and it is possible that these pathways are not activated by the dual analog. Alternatively, it is also possible that the dual analog has the potential to signal for PLC activation but also may transduce a signal of itself, which is not transduced by p259–271, and this signal may inhibit PLC activity. Elevated cAMP levels have been associated with the down-regulation of several T cell responses, including lymphokine secretion and cell proliferation (ref. 25, and for review, see ref. 26). It also has been shown that increased intracellular cAMP inhibits inositol phospholipid hydrolysis as well as PLCγ tyrosine phosphorylation (27–29). It is possible that the altered peptides and the dual analog (but not p259–271) transduce a signal that leads to elevation in cAMP levels and thus inhibits the PLC activity induced by the myasthenogenic peptides.
TCR partial agonists have been shown to induce anergy in several systems (21, 30–33). Results that show that the inhibition of proliferation by the dual analog was reversed by the addition of interleukin 2 to the culture suggest that the dual analog induces T cell anergy (M.P.-R., E.M., and M.S., unpublished results). Signaling mechanisms directly associated with analog-induced anergy have not been unraveled completely. However, the altered pattern of tyrosine phosphorylation events induced by partial agonists has led to the hypothesis that this altered phosphorylation is directly responsible for the induction of anergy (30, 32). Other studies, however, have shown that anergy induction and reversal are not correlated with the changed pattern of TCR-induced phosphorylation, suggesting that the partial signaling manifested by the altered tyrosine phosphorylation is not necessary for anergy induction (31). This may be in line with our results that show that the altered peptides, although inducing anergy in the cells, did not change the phosphorylation pattern induced by p259–271. Little is known about PLC activity in anergic cells. In agreement with our results, Sloan-Lancaster et al. (21) have shown that APLs that induce anergy and can stimulate the tyrosine phosphorylation pathway on TCR engagement do not activate the phospholipid hydrolysis pathway.
Activated PLC exerts its effect not only by generating messenger molecules, diacyl-glycerol and IP3, but also by reducing the levels of phosphatidylinositol-4,5-biphosphate. By reducing the levels of phosphatidylinositol-4,5-biphosphate in the membrane, the peptide also affects other cellular activities (reviewed in ref. 34). Phosphatidylinositol-4,5-biphosphate serves as a cofactor for one of the isoforms of phospholipase D, as a membrane attachment site for proteins containing pleckstrin homology domains, as a regulator of actin-binding proteins, and as a substrate for phosphatidylinositol (PI) 3-kinase. Thus, the inhibition of PLC activity may affect all of these signaling pathways.
The discovery that APLs have antagonistic properties for specific T cells enables their use as therapeutic means in disease states such as autoimmune diseases. The inhibitory capacity using antagonistic APLs should be more efficient than that using MHC-blockers alone because it is more specific and involves interactions with individual TCRs. For a successful treatment with MHC blockers, a prolonged administration of large doses of the blocker is needed whereas, in the case of the dual analog, a single administration (concomitant with immunization or 1 week after immunization) of 5- to 10-fold excess of the dual analog led to successful inhibition of autoimmune-associated responses (9). Because the dual analog is capable of inhibiting in vivo cell proliferation and was shown to reverse an already existing experimental MG (9), it will be of interest to find out whether the down-regulation of in vivo autoimmune responses caused by the dual analog is a result of its inhibitory capacity of PLC activation triggered by pathogenic epitopes.
Acknowledgments
This research was supported by Teva Pharmaceutical Industries Limited., Israel (M.S. and E.M.).
ABBREVIATIONS
References
- 1.Fuchs S, Nevo D, Tarrab-Hazdai R. Nature (London) 1976;263:329–330. doi: 10.1038/263329a0. [DOI] [PubMed] [Google Scholar]
- 2.Conti Tronconi B M, McLane K E, Raftery M A, Grando S A, Protti M P. Crit Rev Biochem Mol Biol. 1994;29:69–123. doi: 10.3109/10409239409086798. [DOI] [PubMed] [Google Scholar]
- 3.Drachman D B. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 4.Lindstrom J, Shelton D, Fuji Y. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 5.Brocke S, Brautbar C, Steinman L, Abramsky O, Rothbard J, Neumann D, Fuchs S, Mozes E. J Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brocke S, Dayan M, Rothbard J, Fuchs S, Mozes E. Immunology. 1990;69:495–500. [PMC free article] [PubMed] [Google Scholar]
- 7.Kirshner S L, Zisman E, Fridkin M, Sela M, Mozes E. Scand J Immunol. 1996;44:512–521. doi: 10.1046/j.1365-3083.1996.d01-330.x. [DOI] [PubMed] [Google Scholar]
- 8.Katz-Levy Y, Dayan M, Wirguin I, Fridkin M, Sela M, Mozes E. J Neuroimmunol. 1998;85:78–86. doi: 10.1016/s0165-5728(97)00265-8. [DOI] [PubMed] [Google Scholar]
- 9.Katz-Levy Y, Paas-Rosner M, Kirshner S, Dayan M, Zisman E, Fridkin M, Wirguin I, Sela M, Mozes E. Proc Natl Acad Sci USA. 1997;94:3200–3205. doi: 10.1073/pnas.94.7.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zisman E, Katz-Levy Y, Dayan M, Kirshner S L, Paas-Rosner M, Karni A, Abramsky O, Brautbar H, Fridkin M, Sela M, et al. Proc Natl Acad Sci USA. 1996;93:4492–4497. doi: 10.1073/pnas.93.9.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberola-Ila J, Takaki S, Kerner J D, Perlmutter R M. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- 12.Cantrell D. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 13.Chan A C, Shaw A S. Curr Opin Immunol. 1995;8:394–401. doi: 10.1016/s0952-7915(96)80130-0. [DOI] [PubMed] [Google Scholar]
- 14.Chan A C, Desai D M, Weiss A. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 15.Evavold B D, Sloan-Lancaster J, Allen P M. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 16.Sloan-Lancaster J, Allen P M. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Kirshner S L, Katz-Levy Y, Wirguin Y, Argov Z, Mozes E. Cell Immunol. 1994;157:11–28. doi: 10.1006/cimm.1994.1201. [DOI] [PubMed] [Google Scholar]
- 18.Katz-Levy Y, Kirshner S L, Sela M, Mozes E. Proc Natl Acad Sci USA. 1993;90:7000–7004. doi: 10.1073/pnas.90.15.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.June C H, Fletcher M C, Ledbetter J A, Samelson L E. J Immunol. 1990;144:1591–1599. [PubMed] [Google Scholar]
- 20.Park D J, Rho H W, Rhee S G. Proc Natl Acad Sci USA. 1991;88:5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sloan-Lancaster J, Evavold B D, Allen P M. Nature (London) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 22.Jensen W A, Pleiman C M, Beaufils P, Wegener A-M, Malissen B, Cambier J C. Eur J Immunol. 1997;27:707–716. doi: 10.1002/eji.1830270320. [DOI] [PubMed] [Google Scholar]
- 23.Motto D G, Musci M A, Ross S E, Koretzky G A. Mol Cell Biol. 1996;16:2823–2829. doi: 10.1128/mcb.16.6.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackman J K, Motto D G, Sun Q, Tanemoto M, Turck C W, Peltz G A, Koretzky G A, Findell P R. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 25.Johnson K W, Davis B H, Smith K A. Proc Natl Acad Sci USA. 1988;85:6072–6076. doi: 10.1073/pnas.85.16.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kammer G M. Immunol Today. 1988;9:222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- 27.Tamir A, Isakov N. J Immunol. 1994;152:3391–3399. [PubMed] [Google Scholar]
- 28.Park D J, Min H K, Rhee S G. J Biol Chem. 1992;267:1496–1501. [PubMed] [Google Scholar]
- 29.Alava M A, DeBell K E, Conti A, Hoffman T, Bonvini E. Biochem J. 1992;284:189–199. doi: 10.1042/bj2840189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan-Lancaster J, Shaw A S, Rothbard J B, Allen P M. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 31.Madrenas Q, Schwartz R H, Germain R N. Proc Natl Acad Sci USA. 1996;93:9736–9741. doi: 10.1073/pnas.93.18.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madrenas J, Wange R L, Wang J L, Isakov N, Samelson L E, Germain R N. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz R H. J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S B, Rhee S G. Curr Opin Cell Biol. 1995;7:183–189. doi: 10.1016/0955-0674(95)80026-3. [DOI] [PubMed] [Google Scholar]