Abstract
3.L2 T cell receptor transgenic T cells are activated by the 64–76 peptide of the mouse hemoglobin d β chain [Hb(64–76)], and their response is antagonized by the position 72 alanine substitution of this peptide (A72). To test the effect of this altered peptide ligand (APL) on 3.L2 T cell function in vivo, a transgene expressing A72 in major histocompatibility complex II positive cells (A72tg) has been introduced into mice. We demonstrate that 3.L2 T cells, when transferred to A72tg+ mice show a dramatically reduced proliferative response to Hb(64–76). Identical decreased responses were observed using T cells that developed in either A72tg+ or A72tg− hosts. This affect was not attributable to diminished precursor frequency, anergy, or competition for binding to I-Ek molecules. These results unequivocally demonstrate in vivo antagonism by an endogenous APL and characterize a class of self-peptides that, although inefficient in causing deletion in the thymus, effectively modulate T cell responses in the periphery.
The phenomenon of antagonism by altered peptide ligands (APLs) has been firmly established by a series of reports (reviewed in refs. 1–4). Such peptide antagonists are derived by making amino acid substitutions at critical T cell receptor (TCR) contact residues. They are a class of APLs that inhibit the response of T cells by a mechanism that is independent of their ability to compete with the stimulatory ligand for major histocompatibility complex (MHC) binding (5).
Despite extensive in vitro studies, it remains to be determined if endogenous peptides can antagonize T cell responses in vivo. Endogenous MHC-peptide complexes are critically involved in the development of T cells in the thymus. Their role in the periphery is less well described, although they have been shown to be crucial to maintaining T cells in the repertoire (6–8). Among the population of peptides presented in the periphery, there may exist ligands that could function as antagonists. Indeed, exogenously added peptides derived from self proteins have been shown to antagonize a cytotoxic T lymphocyte line (9) and a T cell hybrid (10) in vitro. It has not been established, however, if endogenous proteins containing antagonist peptides can modulate a T cell response to a foreign antigen in vivo.
The potential in vivo role of antagonism has been most apparent thus far with pathogens for which variants of T cell epitopes arise during the course of infection. Spontaneously arising mutants of multiple HIV proteins in infected patients have been shown to antagonize killing by cytotoxic T lymphocyte lines from the same individuals (11). Viral mutants with similar properties have arisen in chronic hepatitis B infection (12). Such effects are not restricted to viruses as cohabiting strains of Plasmodium falciparum have also been shown to use altered peptide ligand antagonism to down-regulate T cell responses reciprocally (13). Thus generation of TCR antagonists offers a mechanism by which variants of an infectious agent can simultaneously escape immune recognition and down-modulate the cellular response to their parent strains.
Despite these studies with pathogen-derived epitopes, the efficiency with which an endogenous antagonist peptide may modulate a T cell response remains to be addressed. Our group has previously reported an instance in which a peptide derived from murine hemoglobin was used as an endogenous antagonist (14). Here, exogenous addition of antagonist was required to achieve sufficient levels of complexes for a diminished response to the stimulatory ligand, making these studies inconclusive as to the in vivo relevance of TCR antagonism.
We sought to test if antagonism by endogenous ligands could occur, and, if so, to characterize their effect on T cell function. For that purpose, we have utilized the 3.L2 TCR transgenic mouse (3.L2tg), in which 3.L2 T cells are activated by a 13 amino acid peptide consisting of residues 64–76 of the mouse hemoglobin d β chain [Hb(64–76)]. These T cells are antagonized by an APL derived from Hb(64–76) that contains an alanine substituted for asparagine at position 72 (A72) (15). We have recently introduced a transgene comprised of the A72 peptide inserted into the sequence of a membrane-tethered form of hen egg lysozyme (mHEL) to generate the A72 transgenic mouse (A72tg). Transcription of the mHEL/A72 transgene is driven by the Eα promoter to produce expression on the cell surfaces of all MHC II positive thymic and peripheral antigen presenting cells (16). Notably, the added ligand induces only very modest negative selection of 3.L2 T cells in the thymus of a 3.L2tg×A72tg mouse. Furthermore, 3.L2 T cells populate the periphery and remain functional in this mouse. However, we have previously shown (17) that antigen presenting cells from A72tg mice can diminish the response of 3.L2 T cells to Hb(64–76) peptide in vitro, suggesting that they also may modulate a 3.L2 T cell response in vivo.
To examine this issue, we have transferred 3.L2 receptor transgenic T cells into A72tg mice. Such adoptive transfer allows the separation of thymic and peripheral influences on transgenic T cell function and offers a well-defined system in which to characterize the response of a small lymphocyte population (18). Following immunization with Hb(64–76), it is shown that the endogenous APL can potently diminish the 3.L2 proliferative response to the stimulatory peptide. These results comprise the first in vivo demonstration of TCR antagonism by an endogenous ligand. They have significant implications for the ability of the particular subset of endogenous peptides that are too weak to negatively select efficiently in the thymus but can antagonize in the periphery to modulate T cell responses in vivo.
MATERIALS AND METHODS
Mice.
Three previously described transgenic mouse lines generated in the B6.AKR background were used. The 3.L2tg mouse (15) expresses a transgenic TCR specific for Hb(64–76)/I-Ek. The A72tg mouse (17) expresses the A72 peptide inserted between amino acids 43 and 44 of mHEL. E72tg mice (C.B.W., unpublished data) bear a construct that is identical with the exception of a glutamate substitution at position 72. Expression of the latter two transgenes is driven by the Eα promoter, which has been effective in restricting transgene expression to MHC II positive cells (16). A72tg and 3.L2tg mice were also intercrossed to generate 3.L2tg×A72tg double transgenic mice. All four lines were screened by PCR analysis of purified tail digest DNA. All A72tg, E72tg, and 3.L2tg×A72tg mice used in these studies were heterozygous for the transgenes. Nontransgenic B6.AKR mice were bred in our colony.
Peptides.
The synthetic Hb(64–76) peptide used for immunization in this study was generated, purified, and analyzed as described (19). The peptide sequence in single letter amino acid code is: GKKVITAFNEGLK.
Cell Transfer and Immunizations.
Several pooled 3.L2tg spleens were homogenized in Hanks’ balanced salt solution, and an aliquot was stained for fluorescence-activated cell sorter (FACS) to quantitate the percentage of splenocytes that were both CD4+ and expressed high levels of the 3.L2 TCR as stained by a clonotype-specific antibody (CAB) (typically 5–10%). Splenocytes were resuspended in a volume of Hanks’ balanced salt solution that would produce a CD4+CABhi cell concentration of 5 × 106/ml. A volume of 0.5 ml of this suspension was infused by tail vein into A72tg+ mice and A72tg− littermates to deliver a population of 2.5 × 106 CD4+CAbhi cells per recipient. Cell transfers to E72tg+ mice were performed similarly. When T cells purified from 3.L2tg×A72tg splenocytes were used as the donor population, they were analyzed and resuspended similarly before transfer. All donor mice and recipient mice were sex matched in each individual experiment, and recipient mice were approximately age matched. The following day an appropriate dose of peptide emulsified in either CFA or incomplete Freund’s adjuvant were injected s.c.
T Cell Purification.
T cells from A72tg mice into which 3.L2 T cells had been infused and from 3.L2tg×A72tg mice were purified by passing single cell suspensions of splenocytes over nylon wool columns as described (17). The purified T cells were analyzed by FACS for mHEL expression with the F10.6.6 antibody and MHC II expression with the 14-4-4S antibody. Depletion of mHEL and MHC II expressing cells was at least 85% (data not shown). If purified for tail vein infusion, cells were washed three times in Hanks’ balanced salt solution before transfer.
Antibodies.
Cells were stained for flow cytometry with the following antibodies: phycoerythrin anti-mouse CD4 (PharMingen); fluorescein isothiocyanate CAB (15); fluorescein isothiocyanate F10.6.6 (20); biotin 14-4-4S (21); and Tricolor streptavidin (Caltag, San Francisco, CA).
FACS Analysis.
Single cell suspensions of splenocytes were stained in a solution of 0.5% BSA and 0.1% sodium azide in PBS. Cells (1.5 × 106/sample in 100 μl) were incubated for 30 min at 4°C with directly conjugated or biotinylated antibodies, washed, and incubated for 30 min at 4°C with streptavidin-tricolor conjugate when appropriate. Cells were again washed and then fixed in 1% paraformaldehyde in PBS. Analysis was performed on FACScan and FACScalibur flow cytometers (Becton Dickinson) using cellquest (Becton Dickinson) software. Samples were gated on live cells, and 50,000–200,000 events were collected per sample.
Proliferation Assay.
Proliferation assays were performed in RPMI 1640 medium supplemented with 10% fetal calf serum, 5 × 10−5 M 2-ME, 1 mM Glutamax (GIBCO), and 50 μg/ml gentamicin in 96-well tissue culture plates (Costar). Splenic T cells (4 × 105 per well), which had been depleted of antigen presenting cells as described, were added to irradiated B6.AKR splenocytes (5 × 105 per well) in the presence of the indicated concentrations of Hb(64–76) peptide. Culture wells were pulsed at 48 hr with 0.4 μCi [3H]thymidine (1 Ci = 37 GBq) and harvested 18–24 hr later as described (14). Proliferation was measured as counts incorporated (mean of triplicate wells).
RESULTS
The Expansion of 3.L2 T Cells is Reduced in Lymph Nodes of A72tg+ Mice but Not E72tg+ Mice.
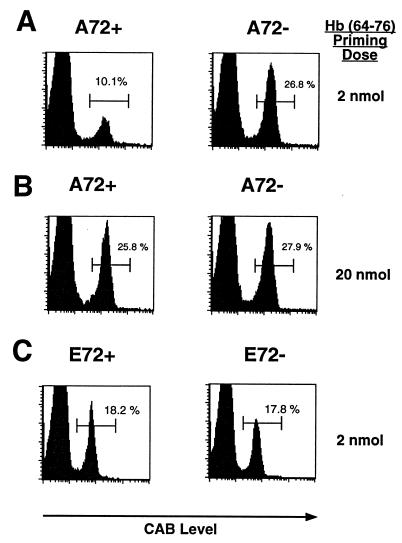
To test whether the A72 APL could modulate a 3.L2 T cell response in vivo, 3.L2tg splenocytes were adoptively transferred to A72tg+ mice and A72tg− littermates in equal numbers. Mice were immunized the following day with Hb(64–76) peptide in CFA. At a later time point, the number of 3.L2 T cells in draining lymph nodes of A72tg+ mice relative to A72tg− littermates was measured by FACS analysis by using a clonotypic antibody to the 3.L2 T cell receptor (CAB). Expansion of CAB+ cells in A72tg+ mice is drastically curtailed on day 5 at an immunizing peptide dose of 2 nmol (Fig. 1A), while not quite abrogated to the basal 2–4% of CD4+CAB+ cells seen in lymph nodes of mice immunized with CFA only (data not shown). This outcome suggested that the endogenous A72 ligand can reduce 3.L2 activation in vivo by antagonizing the 3.L2 receptor. At the same time, the endogenous ligand did not reduce 3.L2 proliferation at much higher doses of antigen. At a ten-fold higher dose of Hb(64–76) peptide (20 nmol), the reduced expansion of 3.L2 T cells in the A72tg+ lymph node was no longer observed (Fig. 1B). High doses of stimulatory ligand can therefore overcome the effect of the endogenous APL.
Figure 1.
Endogenous A72 decreases expansion of 3.L2 T cells in vivo after immunization. (A) 3.L2 splenocytes were transferred by tail vein into A72tg+ mice and A72tg− littermates as described in Materials and Methods. Mice were immunized s.c. with 2 nmol Hb(64–76) peptide emulsified in CFA the following day. Five days later, draining lymph nodes were analyzed by two-color FACS for the 3.L2 clonotypic TCR with CAB and for CD4. Histograms plot CD4 gated cells and measure CAB+ cells as a percentage of total CD4+ cells. (B) Mice were handled identically to those in A with the exception that a higher 20 nmol dose of Hb(64–76) peptide was given. (C) Experiment was performed identically to that in A with the exception that E72tg+ and E72tg− mice were used as recipients. Data in each panel are representative of four to eight pairs of mice.
The reduced expansion of 3.L2 T cells in A72tg+ mice could have been explained by competition for MHC binding by endogenous mHEL/A72 derived peptides, causing a lower density of Hb(64–76) peptide to be presented. This possibility was eliminated by examining mice that expressed a construct that is identical to the A72 transgene with the exception of a glutamate substitution in place of alanine at position 72 (E72tg). E72tg mice express comparable levels of the E72 ligand (C.B.W., unpublished data), which is a null ligand for the 3.L2 receptor and is substituted at a position known not to affect I-Ek binding (19). When used as recipients of 3.L2 T cells in an identical adoptive transfer experiment, E72tg+ mice showed no reduction in 3.L2 T cell expansion relative to E72tg− littermates (Fig. 1C) at the 2 nmol dose of Hb(64–76) peptide.
Upon Adoptive Transfer, 3.L2 T cells Populate A72tg+ and A72tg− Lymphoid Organs in Identical Numbers.
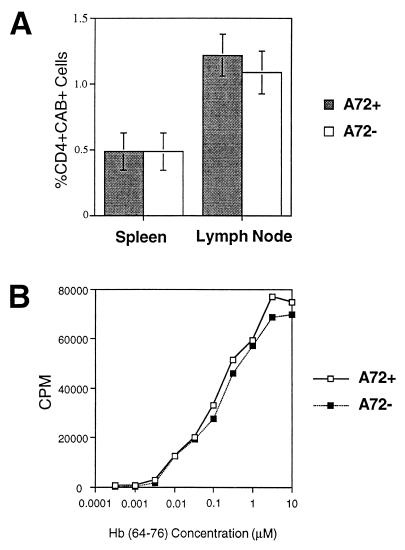
Another explanation for the observed effect in A72tg+ hosts was that 3.L2 T cells may have less efficiently populated lymphoid organs in which A72 is presented. It may therefore have been a reduced starting precursor frequency and not antagonism that accounted for the diminished expansion. However, when equal numbers of 3.L2tg spleen cells are transferred, identical numbers of CD4+CAB+ positive T cells are found 4 days later in A72tg+ and A72tg− recipients (Fig. 2A). Therefore, the reduced percentage of 3.L2 T cells in A72tg+ mice after immunization cannot be explained by a lower precursor frequency in lymphoid organs of these mice.
Figure 2.
3.L2 T cells establish in similar numbers and maintain equal proliferative ability in A72tg+ and A72tg− recipients. (A) Equal numbers of unpurified 3.L2tg splenocytes were transferred to A72tg+ and A72tg− recipients as described. The percentage of CD4+CAB+ T cells was quantitated by FACS in spleen and popliteal plus superficial inguinal lymph nodes 4 days later and is shown as the mean of values from three mice in each group. (B) Three spleens in each group from A were pooled and depleted of antigen presenting cells over nylon wool columns. Purified T cells (4 × 105 per well) were added to irradiated B6.AKR splenocytes (5 × 105/well) in the presence of the indicated concentrations of Hb(64–76) peptide. Proliferation was measured as 3H thymidine incorporation after pulsing at 48 hr and harvesting at 72 hr. Data are representative of two identical experiments.
3.L2 T Cells Retain the Same Intrinsic Proliferative Capacity in the Presence of Endogenous A72 Expression.
T cells clones have previously been reported to be anergized by particular APLs (22). For that reason, it was also plausible that 3.L2 T cells, when exposed to the A72 ligand in vivo, may become anergized. Thus the diminished 3.L2 expansion upon immunization could be attributed to an anergizing effect of A72 and not to its role as an antagonist. To address this possibility, 3.L2 T cells that had been previously transferred to A72tg+ mice were purified away from endogenous antigen presenting cells. When presented with increasing concentrations of Hb(64–76) in vitro, they proliferated identically to 3.L2 cells transferred into A72tg− littermates (Fig. 2B). 3.L2 T cells thus are not anergized or otherwise altered in their intrinsic functional properties when exposed to endogenous A72. This outcome confirms previous ex vivo studies with 3.L2 T cells from 3.L2tg×A72tg mice (17) and further establishes that A72 is antagonizing the 3.L2 T cell response in vivo.
3.L2 T Cells from 3.L2tg×A72tg Mice Remain Sensitive to Antagonism following Thymic and Peripheral Exposure to Endogenous A72.
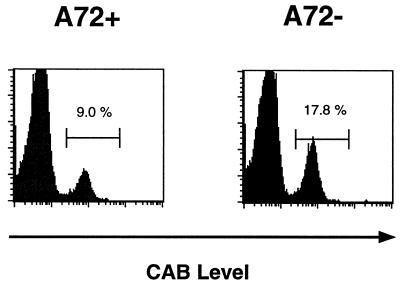
The 3.L2 T cells we had used in the experiments described above had never been exposed to A72 during selection in the thymus. To examine if T cells that developed in the presence of an antagonist would be selected to ignore it in the periphery, we examined 3.L2 T cells that developed in a A72tg+ mouse. T cells purified from 3.L2tg×A72tg mouse spleens were used as the donor population for transfer to A72tg+ and A72tg− mice. Upon immunization with Hb(64–76), a pattern of expansion of clonotype positive cells in A72tg+ versus A72tg− mice identical to that seen in Fig. 1 is detected. Reduced expansion is demonstrable at a 2 nmol antigen dose (Fig. 3) but not at high doses (data not shown) of immunizing ligand. This result indicates that 3.L2 T cells that were selected in the presence of the A72 ligand in the thymus retain the potential to be antagonized by the same APL in the periphery. The physiologic relevance of our observations may then be extended to circumstances in which an antagonist ligand for a given TCR is expressed both in the thymus and the periphery.
Figure 3.
3.L2 T cells from 3.L2tg×A72tg mice are still sensitive to antagonism after thymic and peripheral exposure to endogenous A72. Donor 3.L2 transgenic T cells from 3L2×A72 splenocytes were depleted of endogenous antigen presenting cells by passage over nylon wool columns and transferred to A72tg+ or A72tg− recipient mice at a dose of 2.5 × 106 CD4+CAB+ cells per mouse. Mice were immunized with 2 nmol Hb(64–76) peptide emulsified in CFA, and draining lymph nodes analyzed 5 days subsequently as described in Fig. 1. Results are representative of two experiments containing four total pairs of mice.
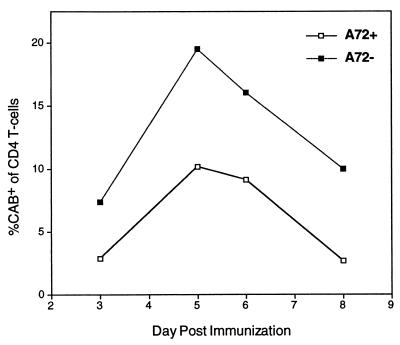
The Kinetics of the 3.L2 Response in the Presence of Antagonist is Unaltered.
The reduced 3.L2 T cell expansion in A72tg+ mice observed upon immunization could be influenced by a change in the kinetics of the response and thus may not represent a reduction in its overall magnitude. To assess this possibility, we tracked the expansion of 3.L2 T cells in A72tg+ and A72tg− littermates after immunization with Hb(64–76) peptide over 6 days. It was shown that the percentage of CD4+CAB+ T cells is reduced in A72tg+ mice at every time point whereas the peak of expansion in each coincides (Fig. 4). Thus the endogenous APL truly reduces the overall magnitude of the 3.L2 response, while leaving the kinetics of its induction and regression unaltered.
Figure 4.
The kinetics of the 3.L2 response is unaltered in the presence of endogenous A72. 3.L2tg splenocytes were adoptively transferred to A72tg+ mice and A72tg− littermates as described. All mice were immunized on the following day with 2 nmol of Hb(64–76) peptide emulsified in CFA, and pairs of mice were sacrificed on days 3–8 postimmunization. The percentage of CD4+ T cells that are CAB+ in draining lymph nodes was quantitated by FACS on the indicated days and plotted as a function of time. The data shown are representative of three similar time course experiments.
DISCUSSION
Many of the endogenous peptides presented in vivo may have functionally significant interactions with the mature T cell repertoire. Previous ex vivo experiments have suggested that an endogenous peptide that is ineffective in negatively selecting a T cell clone in the thymus may antagonize its activation in the periphery (17). Here the A72 ligand provides a definitive in vivo example of an endogenous peptide antagonizing a T cell response. The reduction in T cell expansion in the presence of the antagonist may reflect either diminished proliferation or decreased survival after cell division, and further studies will be required to distinguish these two potential mechanisms. Though the overall magnitude of the response in the presence of the endogenous ligand is significantly reduced, the kinetics of the response remains largely unaffected.
Endogenous ligands that fall in this newly defined class of peptides do not anergize T-cells during their peripheral life spans. Moreover, T cells that have been selected in the endogenous ligand’s presence in the thymus remain fully susceptible to antagonism by it in the periphery. The described effect therefore functions not only for T cells exposed to tissue-specific antagonists but also for those encountering ubiquitously expressed APLs. The number of peptides eligible to modulate T cell responses in this manner is thus quite vast.
These results compel us to reinterpret our previous report in which a peptide from a natural endogenous protein, mouse Hbd, is presented in vivo at insufficient levels to antagonize a T cell response (14). It is proposed in that instance that T cells may be selected to ignore the constitutive levels of endogenous complexes they encounter in the periphery. However, no evidence of such selection is apparent when T cells from a 3.L2tgxA72tg mouse are examined in our system. The discrepancy likely arises from the fact that membrane expression on MHC II+ cells produces more available antagonist complexes in an A72tg+ mouse, whereas the soluble antigen in the previous report likely does not produce sufficient MHC II presentation to observe an antagonist effect.
We show that the efficacy of an endogenous antagonist in decreasing expansion of a given T cell clone is influenced by the dose of stimulatory ligand seen in a given antigenic encounter. One consequence of this dose dependence of antagonism may be that certain T cells possess thresholds of antigen exposure that must be surpassed for them to participate in an immune response. In addition, the spectrum of different clones that participate in a response to a particular epitope may influenced by endogenous peptides that preferentially antagonize a subset of the TCRs with recognition capacity for the stimulatory ligand. Thus a given clone’s affinity for a particular peptide-MHC complex relative to its competitors may not alone be sufficient to predict the degree to which it will participate in the response.
In addition to determining which clones predominate in a cellular response, an antagonist may further function to down-modulate the response’s overall magnitude. Although the effects of antagonists are limited by their receptor specificity, peptides have been generated that successfully antagonize the majority of in vitro clones responsive to a given ligand (23, 24). The appropriate endogenous ligands may thus inhibit enough clones to attenuate the total polyclonal response to an epitope even in the presence of a diverse TCR repertoire.
On a broader level, our results contribute to the rapidly emerging concept of endogenous TCR ligands as essential modulators of T cell function. This notion has been introduced by several reports demonstrating the requirement for MHC expression to maintain αβ T cells in the periphery (6–8). Clearly TCR engagement by endogenous complexes participates in maintenance of the repertoire, though this phenomenon remains to be studied at the level of specific contributions of particular endogenous peptide ligands.
The ultimate importance of our observations here will be dictated by the percentage of the T cell repertoire that exists under the influence of such endogenous antagonists. Peptides with antagonist properties for a given TCR certainly do not seem to be rare discreet entities. They have been attainable with numerous different single substitutions at different TCR contact residues of a given stimulatory ligand (25). For that reason it seems inevitable that at least a handful of T cells in the repertoire will fall under the influence of endogenous antagonists in the manner described here. However, whether endogenous antagonists influence a small subset of T cells or are broad sculpting forces that carve out the landscapes of all T cell responses remains to be seen.
Acknowledgments
We would like to thank Jonathan Katz, Gilbert Kersh, Robert Schreiber, and Brian Wipke for their critical review of the manuscript. We also would like to recognize the assistance of Darren Kreamalmeyer, Kathy Frederick, and Donna Thompson for their work in maintaining the mouse colony. Additionally, we appreciate the technical assistance of Stephen Horvath in synthesis and purification of peptides, and Jerri Smith for secretarial support in the preparation of this manuscript. The work was supported by grants from the National Institutes of Health and the American Cancer Society. D.B. is supported by National Institutes of Health Training Grant AI07163.
ABBREVIATIONS
- Hb(64–76)
64–76 peptide of the β chain of the mouse hemoglobin d allele
- A72
position 72 alanine-substituted Hb(64–76) peptide
- E72
position 72 glutamate-substituted Hb(64–76) peptide
- TCR
T cell receptor
- mHEL
membrane hen egg lysozyme
- A72tg
mouse with transgene comprised of mHEL plus A72
- 3.L2tg
3.L2 TCR transgenic mouse
- MHC
major histocompatibility complex
- CAB
3.L2 TCR clonotype specific antibody
- CFA
complete Freund’s adjuvant
- FACS
fluorescence-activated cell sorter
Footnotes
A Commentary on this article begins on page 14001.
References
- 1.Sette A, Alexander J, Ruppert J, Snoke D, Franco A, Ishioka G, Grey H M. Annu Rev Immunol. 1994;12:413–431. doi: 10.1146/annurev.iy.12.040194.002213. [DOI] [PubMed] [Google Scholar]
- 2.Jameson S C, Bevan M J. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 3.Germain R N, Levine E H, Madrenas J. Immunologist. 1995;3:113–121. [Google Scholar]
- 4.Sloan-Lancaster J, Allen P M. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 5.De Magistris M T, Alexander J, Coggeshall M, Altman A, Gaeta F C A, Grey H M, Sette A. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 6.Takeda S, Rodewald H-R, Arakawa H, Bluethmann H, Shimizu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 7.Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 8.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hlavac F, Choppin J, Guillet J-G. Hum Immunol. 1997;54:48–53. doi: 10.1016/s0198-8859(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 10.Schountz T, Kasselman J P, Ford S R, Murray J S. Cell Immunol. 1996;168:193–200. doi: 10.1006/cimm.1996.0066. [DOI] [PubMed] [Google Scholar]
- 11.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Köppe B, Rosenberg W, Boyd D, Edwards A, et al. Nature (London) 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 12.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Nature (London) 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert S C, Plebanski M, Gupta S, Morris J, Cox M, Aidoo M, Kwiatkowski D, Greenwood B M, Whittle H C, Hill A V S. Science. 1998;279:1173–1177. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 14.Vidal K, Hsu B L, Williams C B, Allen P M. J Exp Med. 1996;183:1311–1321. doi: 10.1084/jem.183.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kersh G J, Donermeyer D L, Frederick K E, White J M, Hsu B L, Allen P M. J Immunol. 1998;161:585–593. [PubMed] [Google Scholar]
- 16.Kouskoff V, Fehling H-J, Lemeur M, Benoist C, Mathis D. J Immunol Methods. 1993;166:287–291. doi: 10.1016/0022-1759(93)90370-m. [DOI] [PubMed] [Google Scholar]
- 17.Williams C B, Vidal K, Donermeyer D L, Peterson D A, White J M, Allen P M. J Immunol. 1998;161:128–137. [PubMed] [Google Scholar]
- 18.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 19.Kersh G J, Allen P M. J Exp Med. 1996;184:1259–1268. doi: 10.1084/jem.184.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischmann T, Souchon H, Riottot M-M, Tello D, Poljak R J. J Mol Biol. 1988;203:527–529. doi: 10.1016/0022-2836(88)90022-8. [DOI] [PubMed] [Google Scholar]
- 21.Ozato K, Mayer N, Sachs D H. J Immunol. 1980;124:533–540. [PubMed] [Google Scholar]
- 22.Sloan-Lancaster J, Shaw A S, Rothbard J B, Allen P M. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 23.Snoke K, Alexander J, Franco A, Smith L, Brawley J V, Concannon P, Grey H M, Sette A, Wentworth P. J Immunol. 1993;151:6815–6821. [PubMed] [Google Scholar]
- 24.Franco A, Southwood S, Arrhenius T, Kuchroo V K, Grey H M, Sette A, Ishioka G Y. Eur J Immunol. 1994;24:940–946. doi: 10.1002/eji.1830240424. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y-Z, Matsushita S, Nishimura Y. J Immunol. 1996;157:3783–3790. [PubMed] [Google Scholar]