Abstract
We have analyzed the Drosophila immune response in domino mutant larvae, which are devoid of blood cells. The domino mutants have a good larval viability, but they die as prepupae. We show that, on immune challenge, induction of the genes encoding antimicrobial peptides in the fat body is not affected significantly in the mutant larvae, indicating that hemocytes are not essential in this process. The hemocoele of domino larvae contains numerous live microorganisms, the presence of which induces a weak antimicrobial response in the fat body. A full response is observed only after septic injury. We propose that the fat body cells are activated both by the presence of microorganisms and by injury and that injury potentiates the effect of microorganisms. Survival experiments after an immune challenge showed that domino mutants devoid of blood cells maintain a wild-type resistance to septic injury. This resistance was also observed in mutant larvae in which the synthesis of antibacterial peptides is impaired (immune deficiency larvae) and in mutants that are deficient for humoral melanization (Black cells larvae). However, if domino was combined with either the immune deficiency or the Black cell mutation, the resistance to septic injury was reduced severely. These results establish the relevance of the three immune reactions: phagocytosis, synthesis of antibacterial peptides, and melanization. By working in synergy, they provide Drosophila a highly effective defense against injury and/or infection.
Insects are remarkably resistant to microbial infections. It is generally accepted that this resistance relies on both humoral and cellular reactions (reviewed in refs. 1–3). The humoral reactions involve the nearly immediate induction of proteolytic cascades, which lead to localized melanization and blood coagulation and to the rapid synthesis of potent antimicrobial peptides by the fat body. These molecules are secreted into the hemolymph where they contribute to the protection against invading microorganisms (systemic antimicrobial response; ref. 4). In Drosophila, at least seven distinct antimicrobial peptides (plus isoforms) are induced. Drosomycin, one such antimicrobial peptide, has exclusively antifungal activity (5). Others, such as cecropin (6), defensin (7), and drosocin (8), are antibacterial. Metchnikowin (9) has both antifungal and antibacterial activities, and it is assumed that diptericin (10) and attacin (11) are antibacterial in Drosophila as they are in other insects (12, 13), although this has not been proven to date. It was recently shown that, in adult Drosophila, the Toll signaling pathway controls the expression of the gene encoding the antifungal peptide drosomycin (14), whereas expression of the antibacterial peptides is mostly dependent on another pathway involving the immune deficiency gene (imd; ref. 15).
The cellular immune reactions of Drosophila are best illustrated by phagocytosis and, in the case of larger invading microorganisms, by capsule formation (reviewed in refs. 16–18). It has been proposed that in this species, blood cells originate from paired lymph glands associated with the anterior dorsal vessel (19). The predominant blood-cell type are the plasmatocytes, which are phagocytic. Crystal cells, which account for 5–10% of the blood cell population, are characterized by prominent crystalline inclusions in their cytoplasm, which reportedly contain the elements of the prophenoloxidase cascade required for defense-related melanization (20). Lamellocytes are large, flattened blood cells that participate in the encapsulation of large intruders or in the formation of melanotic tumors (reviewed in ref. 16).
Many questions remain unanswered in the evolving picture of the Drosophila host defense. In particular, it is unclear how the presence of microorganisms is signaled to the fat body, inducing it to synthesize large amounts of antimicrobial peptides. One working hypothesis is that blood cells recognize and bind microorganisms, thereafter releasing cytokine-like molecules. We have tested this hypothesis with the use of a recently isolated mutation, domino, which is characterized by the absence of circulating blood cells in third-instar larvae (21). In homozygous larvae, domino, a recessive second-chromosome mutation, results in the melanization of the lymph glands, which become observable as two dorsal black dots (hence the name domino). The differentiating hemocytes undergo massive cell death within the hematopoietic organ and are not released into the hemolymph. The domino mutation affects a gene involved in cell proliferation, because all diploid structures (i.e., the neuroblast region of the brain, imaginal discs, germ cells, and the lymph glands) are abnormal in mutant larvae. domino mutants exhibit a prolonged third larval instar and die as prepupae.
Here, we have analyzed the induction of antimicrobial peptides by immune challenge in domino larvae. Our study was extended to melanization reactions. We have examined further the survival rates of various infections of domino larvae and have compared these survival rates to those of mutants in which the synthesis of antimicrobial peptides or melanization reactions is compromised.
MATERIALS AND METHODS
Drosophila Stocks.
The mutant lines used in this study have been described elsewhere (domino, ref. 21; Black cells (Bc), ref. 20; imd, ref. 15; Toll10B, ref. 22; hopTum-l, ref. 23; cactusA2, ref. 24). The second-chromosome mutations (Bc, domino, and imd) were either homozygous or balanced with a CyO y+ chromosome in a y, w context on the X chromosome. Toll10B and hopTum-l are dominant mutations that result in abnormal proliferation of lamellocytes and a melanotic-tumor phenotype. The reporter transgenes used were diptericin-lacZ (25), for the gene encoding diptericin (antibacterial peptide), and drosomycin-green fluorescent protein (4), for the drosomycin (antifungal peptide) gene. In survival experiments where recombinants between second-chromosome mutations (Bc, imd, and domino) were analyzed, two or three recombined chromosomes were tested separately for all combinations, including domino, yielding similar results.
Injury and Survival Experiments.
Third-instar larvae were pricked with a sodium nitrite-sharpened tungsten needle that was either clean (rinsed in 70% ethanol before each use), dipped into a concentrated bacterial pellet, or coated with fungal spores.
Natural fungal infection was performed by placing larvae on spores of Beauveria bassiana (26) for 3 h, then on culture medium for 21 h. Bacterial mass infection was achieved by placing larvae on a 1:1 mixture of yeast paste and bacterial pellet (Escherichia coli strain DH5α) for 24 h, then on a filter paper soaked with a 5% glucose solution for 24 h. Larvae were then sterilized in 50% bleach and 70% ethanol and pricked to collect hemolymph. The hemolymph was added to 50 μl of PBS, then plated on Luria–Bertani agar (10 larvae per dish). All experiments were performed at 25°C, except for the survival experiments, which were done at 29°C.
Microorganisms present in mutant larvae were identified by the enzymatic API 20E test (BioMérieux, Charbonnier les Bains, France).
RNA Analysis.
Total RNA was extracted with the RNA Trizol (GIBCO/BRL) method and Northern blotting experiments were performed as described (15). The following probes were used: diptericin (10), drosomycin (5), cecropin A1 (6), metchnikowin (9), and rp49 cDNA (27).
Assay of Phenoloxidase Activity.
Filter paper was soaked with 10 mM phosphate buffer (pH 6.5) containing 2 mg/ml l-3,4-dihydroxyphenylalanine (Sigma), and phenoloxidase activity was detected in blood samples by dropping the hemolymph of a single larva on the paper. For quantification of phenoloxidase activity, 3 μl of hemolymph samples were added to 50 μl of 10 mM phosphate buffer (pH 5.9) containing 0.01 M l-3,4-dihydroxyphenylalanine, and the OD at 470 nm was recorded for 30 min.
RESULTS
The Number of Hemocytes Is Reduced Dramatically in domino Larvae.
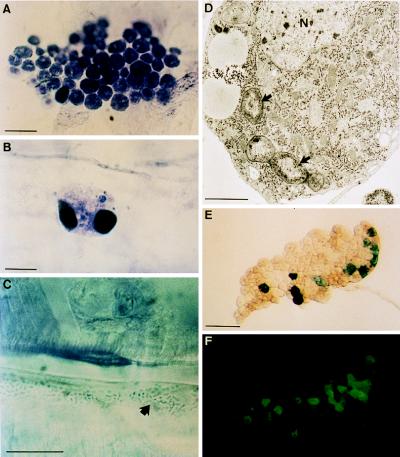
Analysis of hemolymph collected from homozygous third-instar domino larvae indicated a striking absence of circulating hemocytes. Only occasionally could we observe a few oversized cells, which often were filled with melanotic masses (Table 1). This situation is in sharp contrast to normal hemolymph, which contains 1,000–3,000 hemocytes per μl in larvae of approximately the same size (28). In addition to circulating hemocytes, Drosophila larvae contain a significant number of blood cells adherent to the integument and to muscles, particularly in the posterior region of the body (sessile hemocytes; Fig. 1A and Table 1). Homozygous domino larvae contain few sessile hemocytes, as evidenced by microscopic examinations of whole-mount preparations of dissected larvae that showed the presence of a few large abnormal cells containing melanotic masses (Fig. 1B).
Table 1.
Hemocyte counts in third-instar larvae (per individual; mean ± SD)
| Hemocytes | domino | Wild type wandering | Wild type before pupariation |
|---|---|---|---|
| Circulating | 0 to 10*† (n = 30) | 1,067 ± 381 (n = 10) | 1,883 ± 760 (n = 10) |
| Sessile | 39 ± 33* (n = 10) | 1,364 ± 503 (n = 10) | 2,677 ± 497 (n = 10) |
Oversized hemocytes with abnormal features.
Circulating hemocytes (<10 per animal) were observed in only a few cases.
Figure 1.
Blood cells and fat body in domino larvae. Whole mounts of integument inner wall from wild-type (A) and domino (B and C) third-instar larvae. Groups of sessile plasmatocytes are frequently observed in wild-type larvae (A). However, in domino, a few abnormal hemocytes are occasionally seen (B), and microorganisms (arrow) accumulate in the hemocoele (C). Histochemical analysis showed the presence of toluidine and eosin. (For A–C, bars = 20 μm.) (D) Transmission electron micrograph of a plasmatocyte that has engulfed several bacteria (arrows). The experimental procedure was performed as described (21). N identifies the nucleus. (Bar = 1 μm.) Expression of antimicrobial reporter genes (E, diptericin-lacZ and F, drosomycin-green fluorescent protein) in a domino fat body lobe (in the absence of immune challenge). (Bar = 400 μm.)
Recently, we described two enhancer trap lines (l(3)03550 and l(3)03349; ref. 21) in which the reporter gene lacZ is expressed in the hemocytes, allowing for accurate detection of all hemocytes. We have placed these reporter genes into the domino context. The analysis of these strains confirmed that the hemocyte number was reduced dramatically in the domino mutant background and that the few hemocytes that were detectable were of abnormally large size (data not shown).
domino Larvae Are Often Filled with Live Microorganisms.
We were surprised to find that the majority of homozygous domino larvae contained large numbers of microorganisms, which were visible on the epithelium lining the integument and on muscles (Fig. 1C). For example, yeast cells, Staphylococci (Gram-positive) and Providencia sp. (Gram-negative) were present in the microorganisms recovered from the hemocoele of 3 of 11 domino mutant larvae. This condition contrasts with wild-type larvae where microorganisms are never observed within the body cavity.
We experimentally induced a massive infection of domino mutants by raising them in the presence of high numbers of E. coli (i.e., on a concentrated bacterial pellet complemented with yeast). In a pilot experiment, we collected the hemolymph of larvae grown with E. coli for 24 h. Collection was performed under sterile conditions that included sterilizing the cuticle in bleach. The hemolymph was plated on agar and the cultures were kept at 37°C overnight. Under these conditions, we observed the development of numerous colonies of E. coli (100–500 in four groups of 10 larvae). Importantly, however, no or few colonies developed when we tested hemolymph from wild-type larvae grown in the same conditions (0–50 in four groups of 10 larvae). The number of bacterial colonies obtained is certainly an underestimate, because most of the bacteria seen in domino larvae (see Fig. 1C) adhere to tissues. These results indicate that domino larvae, which are devoid of hemocytes, are infested readily by microorganisms which (or, at least, some of which) remain alive within the body cavity.
Injection of Bacteria into domino Larvae Induces a Wild-Type Systemic Antimicrobial Response.
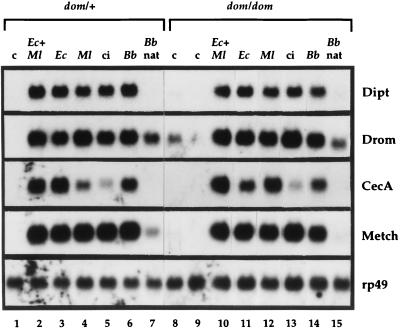
We wondered whether the quasiabsence of hemocytes in domino larvae has a detrimental effect on the inducible antimicrobial response. For this, we pricked homozygous and heterozygous domino larvae with needles dipped into culture pellets of either the Gram-positive Micrococcus luteus or the Gram-negative E. coli or spores of the entomopathogenic fungus Beauveria bassiana. We also infected larvae by mass-covering them with spores of this fungus, thus mimicking a natural route of infection (26). After appropriate intervals of time, we extracted the RNAs from the various types of larvae and probed them with cDNAs corresponding to diptericin, cecropin A1, drosomycin, and metchnikowin. The levels of transcripts of the different peptides were comparable in the mutant background to those of wild-type larvae, whatever the type of stimulus applied (Fig. 2). Similar results were obtained with fungal spores which generated a significant induction of the antifungal peptide drosomycin in domino mutants (26).
Figure 2.
Induction of genes encoding antimicrobial peptides in third-instar larvae of different genotypes. Total RNA was extracted from heterozygous (dom/+) or homozygous domino (dom/dom) larvae that were submitted to various treatments, and 20 μg samples were analyzed by Northern blotting. Blots were hybridized successively with the following random-primed cDNA probes: Dipt, diptericin; Drom, drosomycin; CecA, cecropin A1; Metch, metchnikowin, and rp49. c: control unchallenged larvae. ci: larvae collected 6 h after clean injury. Ec + Ml, Ec, Ml, Bb: larvae collected 6 h after pricking with a needle coated with E. coli and M. luteus (Ec + Ml), E. coli alone (Ec), M. luteus alone (Ml), or spores of B. bassiana strain 80.2 (Bb). Bb nat: larvae collected 24 h after natural infection by spores of B. bassiana. This experiment was repeated three times and yielded similar results.
The Microorganisms That Naturally Infest domino Larvae Induce a Weak Systemic Antimicrobial Response.
As reported above, the majority of homozygous domino mutants exhibited a natural infection by microorganisms. We extracted RNAs from these larvae but failed to detect the expression of the genes encoding diptericin, cecropin A1, and metchnikowin by Northern blots (Fig. 2, lanes 8 and 9). Only for drosomycin did we see more than a background signal in one of the control samples (Fig. 2, lane 8); this situation occasionally occurs in wild-type control larvae.
To analyze further a possible synthesis by the fat body of antimicrobial peptides resulting from the presence of microorganisms in the body cavity of domino larvae, we examined individual domino mutants carrying both a drosomycin-green fluorescent protein (4) and a diptericin-lacZ (25) reporter gene on the X chromosome. These reporter genes are induced strongly in all fat body cells by bacterial challenge in both wild-type and homozygous domino larvae. We did not observe reporter-gene expression in the absence of challenge in either wild-type or heterozygous domino larvae, neither of which contain microorganisms. However, when we scored 140 nonchallenged homozygous domino larvae from various culture vials, we found that one-third of the larvae had a weak mosaic expression of β-galactosidase or green fluorescent protein in the fat body (Fig. 1 E and F).
When we raised the domino larvae in the presence of a high number of E. coli, thus provoking a massive bacterial invasion of the body cavity, the expression of both reporter genes was induced strongly in most larvae (not shown). However, the level of induction showed some variability, and in a few larvae, no expression of the reporter genes occurred. The induction never reached levels equivalent to those after a septic injury. Raising wild-type larvae on concentrated bacteria never induced the reporter genes, because bacteria did not penetrate the hemocoele.
domino Mutants Fail to Mount a Wild-Type Melanization Response.
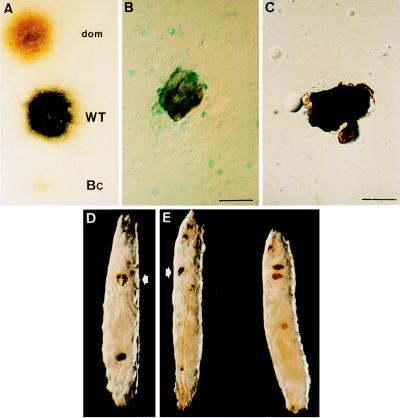
In pricked wild-type Drosophila larvae, melanization occurs within minutes at the site of the injury. When we pricked homozygous domino larvae, we noted that the melanization reaction was somewhat reduced in intensity compared with wild-type larvae. When domino hemolymph was deposited on filter paper in the presence of the prophenoloxidase substrate l-3,4-dihydroxyphenylalanine, a brownish coloration appeared, whereas wild-type hemolymph turned totally black within five minutes (Fig. 3A). To assess the validity of our assay, we also compared, in the same phenoloxidase test, the hemolymph from homozygous Bc mutants, which reportedly are devoid of blood phenoloxidase activity (20). No coloration was detected with Bc mutants. A quantitative assay indicated that Bc larvae exhibit 1.9 ± 1.6% (n = 10) of wild-type phenoloxidase activity, as compared with 45.8 ± 16.4% for domino larvae.
Figure 3.
Melanization and melanotic tumors in domino larvae. (A) Staining obtained 5 min after depositing a droplet of hemolymph from domino, OregonR (WT), and Bc larvae on a filter paper impregnated with l-3,4-dihydroxyphenylalanine, the substrate for phenoloxidase. Free-floating melanotic masses in Toll10B/+ single (B) and dom/dom; Toll10B/+ double mutants (C). In single mutants, the melanotic tumors are surrounded by layers of lamellocytes, as shown by the expression of the lacZ reporter of the l(3)03349 fly line (21), whereas no blood cells are associated in double mutants. (Bars = 40 μm.) (D) hopTum-l; dom/dom double-mutant larva exhibiting both the domino phenotype with black lymph glands (arrow) and a melanotic mass in the posterior region. (E) Natural infection of a domino larva by B. bassiana spores. On the left, the arrow indicates the lymph glands, and the larva on the right is an OregonR larva. Brown spots form at the penetration points of the fungi.
These data indicate that in the absence of hemocytes, the melanization process in the hemolymph of domino mutants is somewhat depressed but not abolished.
Finally, we tested the effect of natural infection by spores of B. bassiana on domino larvae. This entomopathogenic filamentous fungus penetrates the organism through the cuticle, probably by producing chitinases and proteases. This infection selectively induces the expression of the drosomycin gene (ref. 26; Fig. 2, lanes 7 and 15) and, to a lesser extent, the expression of the metchnikowin gene (26). Melanization plaques sometimes appear on the cuticle at the penetration spots in wild-type larvae. Such melanization plaques can also be seen on domino larvae after infection with the fungus (Fig. 3E), indicating that their formation does not require hemocytes.
Melanotic Tumor Formation Is Reduced in domino Mutants.
Melanotic tumors are noninvasive reactions involving capsule formation by hemocytes and concomitant melanization (29). It has been proposed that such tumors are autoimmune reactions of hemocytes against effete tissue fragments or result from the disregulated attack of hemocytes against normal self molecules (30). Various mutations have been reported to induce formation of melanotic tumors. The formation of these tumors occurs particularly with Toll10B, a dominant gain-of-function mutation of the Toll receptor (22), cactusA2, a strong allele of the gene encoding cactus (24), and hopTum-l, a dominant gain-of-function mutation of the JAK-kinase hopscotch (23). Under the microscope, the circulating melanotic masses appear surrounded by several layers of lamellocytes (Fig. 3B).
To study the effect of the domino mutation on the formation of melanotic tumors, we generated double mutants from each of the above strains. As expected, the double mutants hopTum-l; dom, dom; cactusA2, and dom; Toll10B were devoid of hemocytes as a consequence of the domino mutation. Unexpectedly, however, melanotic masses were present, although their frequency was markedly reduced (≈1% of the expected frequency), and they were smaller in size than in single mutants. These melanotic masses were devoid of hemocytes (Fig. 3 C and D). Thus, we conclude that melanotic masses can form in the absence of blood cells.
The domino Mutation Lowers the Survival Rates After Microbial Infections.
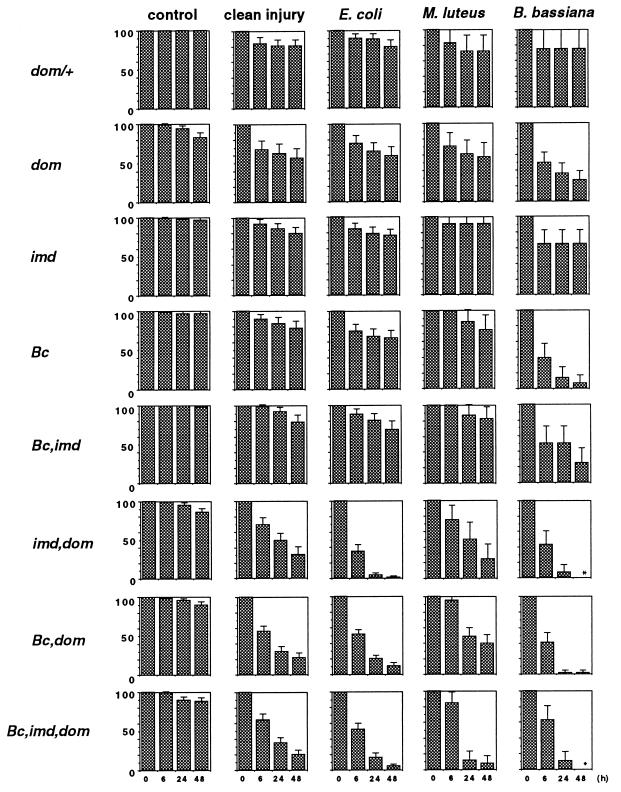
We asked whether the domino mutants, which lack hemocytes, are less resistant to experimental infections by various microorganisms than wild-type larvae of comparable age. We compared the effects of a simple injury performed with a sterile needle to injuries performed with needles contaminated with one of the following microorganisms: E. coli (Gram-negative), M. luteus (Gram-positive), and B. bassiana (fungal spores). We included imd mutants in these experimental series, because this mutation reduces the levels of induced antibacterial peptides and has been reported to lower the resistance to bacterial infections in adults (15). Because the domino mutation lowers the intensity of the melanization reaction somewhat, we also introduced Bc mutants, which are devoid of hemolymph phenoloxidase activity. Finally, we generated (i) imd, dom, (ii) Bc, imd, and (iii) Bc, dom double mutants and (iv) Bc, imd, dom triple mutants to study the effect of compromising the cellular and humoral defense reactions. Survival rates were scored in groups of 25–30 individuals over a 48-h period at a temperature of 29°C to allow for better growth of the microorganisms. After this period, wild-type, Bc, heterozygous domino, and imd larvae, but not domino mutants, had pupated. We also generated domino; spätzle and domino; Toll double mutants to test survival to fungal infections (14). However, the viability of these double-mutant larvae was compromised severely, ruling out survival experiments.
The results of our study are presented in Fig. 4. The first interesting observation was that all strains, including the Bc, imd, dom triple mutants, were perfectly viable without challenge in the conditions of the experiments, with, at most, 1 of 10 larvae dying over the 48-h period of observation. A second result, which was somewhat unexpected, was that in heterozygous domino mutants and in all larvae carrying a single mutation (dom, imd, or Bc), the survival rates (ranging from 60 to 90%) did not differ significantly between larvae pricked with a sterile needle and a needle coated with Gram-positive or Gram-negative bacteria. However, infection with fungal spores induced over 60% lethality in domino or Bc mutants. The third result was that the association of the domino mutation with either Bc or imd, or with both, dramatically compromised the survival of the larvae after injury (whether it was a clean injury or an injury combined with infection). Associating the Bc and imd mutations alone did not result in survival rates significantly different from those of single Bc and imd mutants (with the possible exception of B. bassiana-infected Bc; imd double mutants). Although we do not know the precise function of the three mutated genes, we can be confident that the observed survival rates after septic injury are linked to altered immune mechanisms, because nonchallenged larvae survived perfectly at 29°C.
Figure 4.
Survival of third-instar larvae of various genotypes to immune challenge. The survival rates (in percentages) of dom/+ (equivalent to wild-type rates, not shown), dom/dom (dom), Bc/Bc (Bc), imd/imd (imd), Bc, imd/Bc, imd (Bc,imd), Bc, dom/Bc, dom (Bc,dom), imd, dom/imd, dom (imd,dom) and Bc, imd, dom/Bc, imd, dom (Bc,imd,dom) larvae were scored over a 48-h period at 29°C after immune challenge and are presented with their confidence interval (P < 0.05). The larvae were either untreated (control), pricked with a clean needle (clean injury), or pricked with a needle coated with E. coli, M. luteus, or B. bassiana spores. For each experiment, 20–70 animals (M. luteus and B. bassiana) or 70–300 animals (control, clean injury, and E. coli) were used. Asterisks indicate 0% survival. For Bc, dom, and imd/dom as well as Bc, imd, dom combinations, two or three different recombinant chromosomes were tested, and all values were cumulated.
DISCUSSION
The data presented in this study show that domino mutants lack both circulating and sessile hemocytes. The few blood cells that can be observed in these mutants are obviously abnormal. Nonetheless, the larvae are perfectly viable and exhibit a prolonged third instar before pupation and subsequent death. The lethality is caused by a defect in cell proliferation as this mutant is devoid of imaginal discs (21). The good larval viability is somewhat surprising, because it implies that blood cells are not necessary for survival in normal rearing conditions during larval development.
Our data also show that the systemic antimicrobial response to bacterial or fungal challenge, as illustrated by the induction of expression of the antimicrobial peptide genes, is not impaired in the absence of blood cells. In a preliminary report, E. Gateff (31) mentioned that in 1(3)hem (hematopoiesis missing) third-instar larvae, which are also devoid of hemocytes, a normal immune response was detectable with an inhibition zone assay. Our present results extend this observation to a mutation in a second locus. They indicate that blood cells do not play a mandatory role in the induction of the fat body response. Alternatively, other cells could substitute for the blood cells in domino larvae (e.g., injured cells at the level of the wound). We were surprised to observe that homozygous domino larvae contained numerous microorganisms, in sharp contrast to wild-type larvae. This observation is a strong indication that microorganisms from the surrounding medium invade the larvae and normally are scavenged by the blood cells (Fig. 1D). As illustrated by our data, the process of invasion of domino larvae by microorganisms induces only a weak expression of antimicrobial peptides. This weak expression is obviously not sufficient to kill the microorganisms, because live microbes can be recovered from infested larvae. We could not determine whether the presence of these microorganisms would eventually lead to septicemia, because the larvae died from the intrinsic lethality of the domino mutation. How the bacteria gain access to the body cavity is still an open question. The gut and tracheal system are probable routes.
When we increased the level of invasion of the domino larvae by mass-introducing E. coli into the rearing medium, we observed a marked expression of antimicrobial peptide reporter genes in the fat body as opposed to the weak expression under normal conditions. This result relates the level of expression of the genes to the degree of invasion by microorganisms in the domino larvae. It also shows that bacteria within the body cavity are capable of inducing the antimicrobial genes in the absence of an experimental injury. However, we wish to stress that the level of induction under these conditions always remained significantly lower than that induced by an experimental injury. It is highly probable therefore that the fat body can be activated by several mechanisms to produce antimicrobial peptides and that these mechanisms are cumulative.
The origin of the prophenoloxidase system in Drosophila remains somewhat conjectural. Bc mutants that exhibit blackened crystal cells resulting from intracellular melanization are reportedly devoid of activatable prophenoloxidase in the cell-free hemolymph (20), an observation that we confirm in the present study. This phenotype has often been interpreted by assuming that hemolymph prophenoloxidase originates from crystal cells in Drosophila. However domino mutants, which lack hemocytes altogether, still exhibit a significant phenoloxidase activity that has to be present in cell-free hemolymph. The existence of a humoral phenoloxidase has been documented already by Shrestha and Gateff (19) in melanization experiments with different larval hemolymph components. The cDNA encoding a Drosophila prophenoloxidase has been cloned recently (32), and the tissue-specific analysis of its expression will clarify this problem. In the case of domino larvae, it is possible that some enzyme is produced in the abnormal lymph-gland cells. Finally, we observe an effect of the Bc mutation in the survival rates against infection. Although we do not know at which level of the phenoloxidase cascade the Bc product is required, we may infer from the above experiments that melanization plays a significant role in the host defense of Drosophila larvae.
The survival experiments are instructive in several respects. First of all, we find that larvae are slightly more sensitive to pricking than adults (14). We attribute this difference to the turgidity of the larvae that leads to a significant blood loss after wounding and to the subsequent crawling of the larvae in the medium containing microorganisms. Therefore, it is probably not surprising that the survival rates did not differ significantly whether the needle was sterile (clean injury) or dipped into bacterial cultures. It is interesting to note that survival rates were similar in heterozygous domino larvae (60–90% survival at 48 h) and in the three mutant backgrounds where the larvae either lacked blood cells (domino), had undetectable phenoloxidase activity (Bc), or had reduced rates of antibacterial peptide synthesis (imd). In regard to surviving bacterial infections, the effect of the imd mutation is less marked in larvae than in adults, as noted in a parallel study (P. Manfruelli, personal communication). Our results show that the majority of these larvae could cope with injury or bacterial infection. Thus, when a mutation inactivates one of the facets of the antibacterial response, the others ensure an efficient defense. Strikingly however, the combination of domino with either the Bc or the imd mutation had, in all cases, a dramatic effect on survival, particularly from infection by E. coli or B. bassiana. Associating the Bc and imd mutations did not, however, result in increased mortality compared with each of these mutations taken alone. These results underline the functional relevance of blood cells in these experiments.
Our data show that the synthesis of antimicrobial peptides in larvae can be induced in the absence of blood cells. Such larvae exhibit relatively good survival rates after septic injury, probably as a result of the synthesis of the antimicrobial peptides. Combining mutations that affect production of both blood cells and antimicrobial peptides, however, severely affects survival rates. Our results suggest that blood cells function under normal conditions to scavenge microorganisms that gain access to the body cavity.
Acknowledgments
We thank Bruno Lemaitre for helpful discussions, Elena Levashina for statistical data analysis, Daniel Zachary for electron microscopy, and René Lanot for hemocyte counts. We also thank Istvan Kiss and Charles Dewolf of the Indiana Drosophila and the Tübingen Stock Centers for fly stocks. This work was funded by the Centre National de la Recherche Scientifique, the Association pour la Recherche sur le Cancer, and the Ligue Nationale contre le Cancer. A.B. was supported by a fellowship from the Ligue Nationale contre le Cancer.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Boman H G. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann J A, Reichhart J M, Hetru C. Curr Opin Immunol. 1996;8:8–13. doi: 10.1016/s0952-7915(96)80098-7. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann J A, Reichhart J M. Trends Cell Biol. 1997;7:309–316. doi: 10.1016/S0962-8924(97)01087-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferrandon D, Jung A, Criqui M C, Lemaitre B, Uttenweiler-Joseph S, Michaut L, Reichhart J M, Hoffmann J A. EMBO J. 1998;17:1217–1227. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert W F, Hetru C, Hoffmann J A. J Biol Chem. 1994;269:33159–33163. [PubMed] [Google Scholar]
- 6.Kylsten P, Samakovlis C, Hultmark D. EMBO J. 1990;9:217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimarcq J L, Hoffmann D, Meister M, Bulet P, Lanot R, Reichhart J M, Hoffmann J A. Eur J Biochem. 1994;221:201–209. doi: 10.1111/j.1432-1033.1994.tb18730.x. [DOI] [PubMed] [Google Scholar]
- 8.Bulet P, Dimarcq J L, Hetru C, Lagueux M, Charlet M, Hégy G, Van Dorsselaer A, Hoffmann J A. J Biol Chem. 1993;268:14893–14897. [PubMed] [Google Scholar]
- 9.Levashina E A, Ohresser S, Bulet P, Reichhart J M, Hetru C, Hoffmann J A. Eur J Biochem. 1995;233:694–700. doi: 10.1111/j.1432-1033.1995.694_2.x. [DOI] [PubMed] [Google Scholar]
- 10.Wicker C, Reichhart J M, Hoffmann D, Hultmark D, Samakovlis C, Hoffmann J A. J Biol Chem. 1990;265:22493–22498. [PubMed] [Google Scholar]
- 11.Åsling B, Dushay M S, Hultmark D. Insect Biochem Mol Biol. 1995;25:511–518. doi: 10.1016/0965-1748(94)00091-c. [DOI] [PubMed] [Google Scholar]
- 12.Dimarcq J L, Keppi E, Dunbar B, Lambert J, Reichhart J M, Hoffmann D, Rankine S M, Fothergill J E, Hoffmann J A. Eur J Biochem. 1988;171:17–22. doi: 10.1111/j.1432-1033.1988.tb13752.x. [DOI] [PubMed] [Google Scholar]
- 13.Hultmark D, Engström A, Andersson K, Steiner H, Bennich H, Boman H G. EMBO J. 1983;2:571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffmann J A. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 15.Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart J M, Hoffmann J A. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizki T M, Rizki R M. In: Insect Ultrastructure. King R C, Akai H, editors. Vol. 2. New York: Plenum; 1984. pp. 579–604. [Google Scholar]
- 17.Nappi A J, Vass E. Pigm Cell Res. 1993;6:117–126. doi: 10.1111/j.1600-0749.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 18.Ratcliffe N A. In: Parasites and Pathogens of Insects. Beckage N E, Thompson S N, Federici B A, editors. Vol. 1. San Diego: Academic; 1993. pp. 267–304. [Google Scholar]
- 19.Shrestha R, Gateff E. Dev Growth Differ. 1982;24:65–82. doi: 10.1111/j.1440-169X.1982.00065.x. [DOI] [PubMed] [Google Scholar]
- 20.Rizki T M, Rizki R M, Grell E H. Roux’s Arch Dev Biol. 1980;188:91–99. doi: 10.1007/BF00848799. [DOI] [PubMed] [Google Scholar]
- 21.Braun A, Lemaitre B, Lanot R, Zachary D, Meister M. Genetics. 1997;147:623–634. doi: 10.1093/genetics/147.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gertulla S, Jin Y, Anderson K V. Genetics. 1988;119:123–133. doi: 10.1093/genetics/119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo H, Hanratty W P, Dearolf C R. EMBO J. 1995;14:1412–1420. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth S, Hiromi Y, Godt D, Nüsslein-Volhard C. Development (Cambridge, UK) 1991;112:371–388. doi: 10.1242/dev.112.2.371. [DOI] [PubMed] [Google Scholar]
- 25.Reichhart J M, Meister M, Dimarcq J L, Zachary D, Hoffmann D, Ruiz C, Richards G, Hoffmann J A. EMBO J. 1992;11:1469–1477. doi: 10.1002/j.1460-2075.1992.tb05191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaitre B, Reichhart J M, Hoffmann J A. Proc Natl Acad Sci USA. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connel P, Rosbach M. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brehélin M. Cell Tissue Res. 1982;221:607–615. doi: 10.1007/BF00215704. [DOI] [PubMed] [Google Scholar]
- 29.Sparrow J C. In: The Genetics and Biology of Drosophila. Ashburner M, Wright T R F, editors. 2B. New York: Academic; 1978. pp. 277–313. [Google Scholar]
- 30.Watson K, Johnson T K, Denell R E. Dev Genet (Amsterdam) 1991;12:173–187. doi: 10.1002/dvg.1020120302. [DOI] [PubMed] [Google Scholar]
- 31.Gateff E. Ann NY Acad Sci. 1994;712:260–279. doi: 10.1111/j.1749-6632.1994.tb33578.x. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto K, Okino N, Kawabata S, Iwanaga S, Ohnishi E. Proc Natl Acad Sci USA. 1995;92:7769–7773. doi: 10.1073/pnas.92.17.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]