Abstract
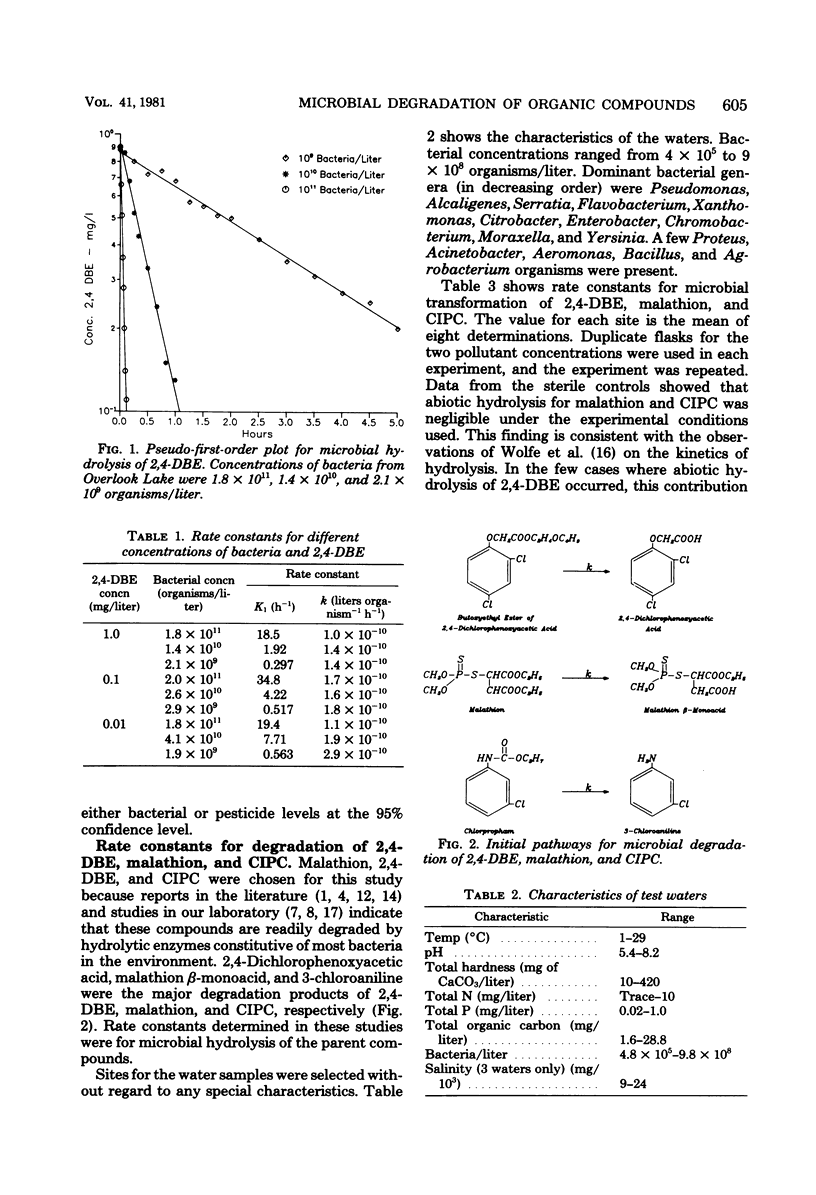
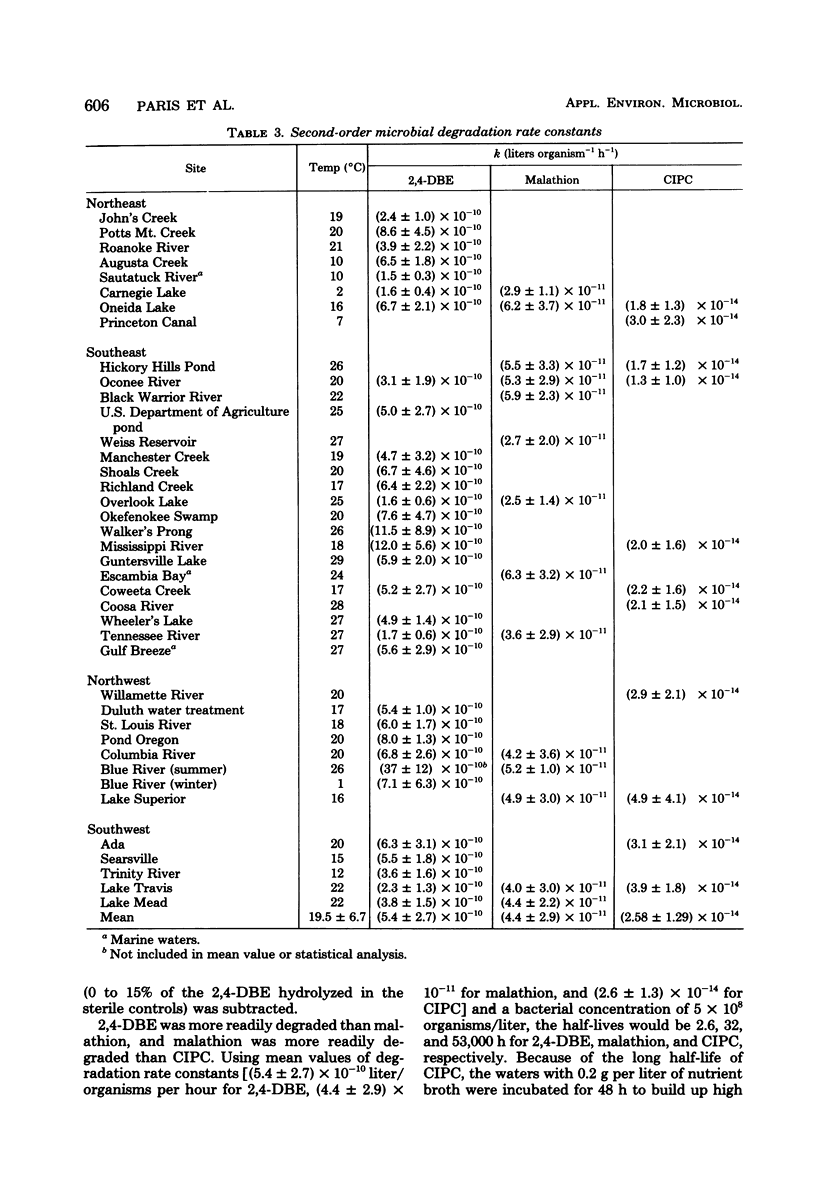
The reliability of second-order rate constants for assessing microbial degradation kinetics in natural waters was examined by using three compounds that undergo hydrolytic degradation. The butoxyethyl ester of 2,4-dichlorophenoxyacetic acid was studied in water samples from 31 sites, malathion was examined in water from 14 sites, and chlorpropham was studied in samples from 11 sites. The coefficient of variation for rate constants for each compound was less than 65% over all sites. Additional studies indicated that the rate conformed to second-order kinetics; that is, the rate was proportional to both bacterial and xenobiotic concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- KAUFMAN D. D., KEARNEY P. C. MICROBIAL DEGRADATION OF ISOPROPYL-N-3 -CHLOROPHENYLCARBAMATE AND 2-CHLOROETHYL-N-3-CHLOROPHENYLCARBAMATE. Appl Microbiol. 1965 May;13:443–446. doi: 10.1128/am.13.3.443-446.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoum A. M. Thin-layer chromatographic separation and colorimetric detection of malathion and some of its metabolites from stored grains. J Agric Food Chem. 1970 May-Jun;18(3):542–543. doi: 10.1021/jf60169a013. [DOI] [PubMed] [Google Scholar]



