Abstract
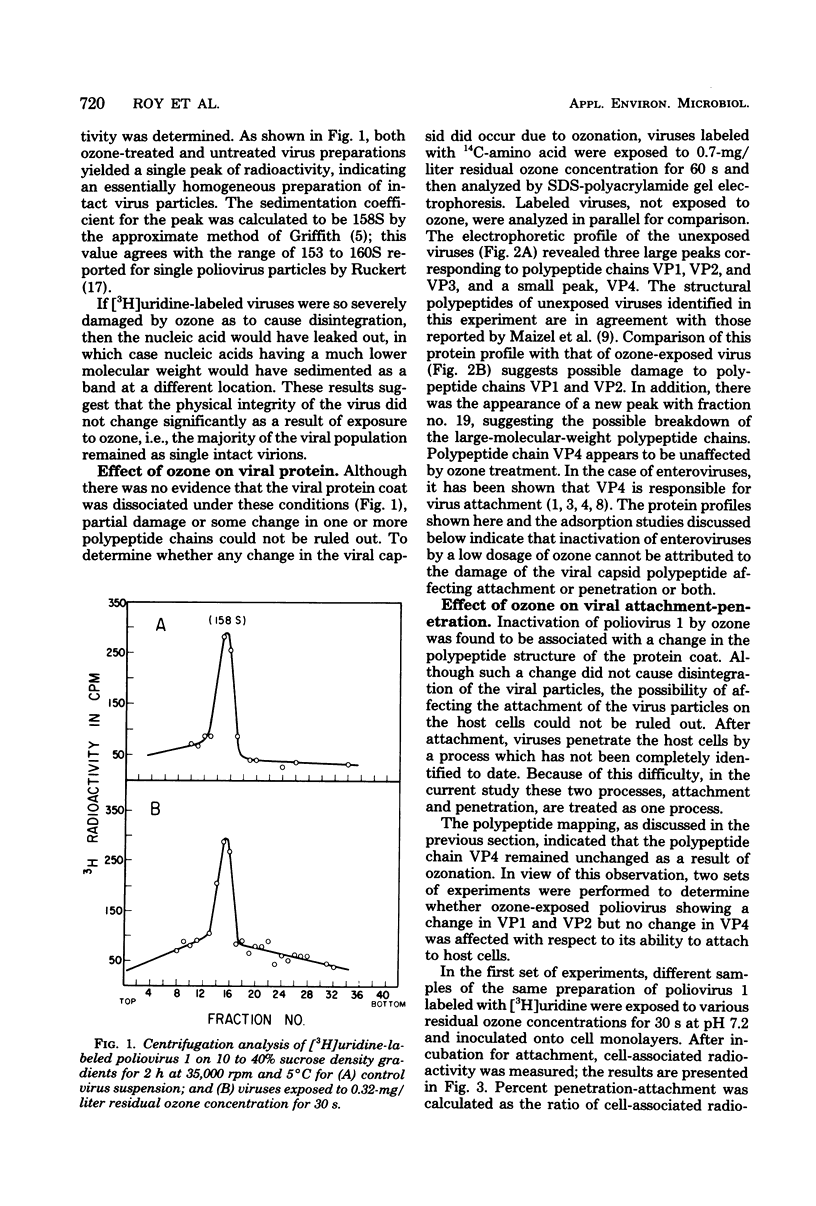
The mechanism of enteroviral inactivation by ozone was investigated with poliovirus 1 (Mahoney) as the model virus. Ozone was observed to alter two of the four polypeptide chains present in the viral protein coat of poliovirus 1. However, the alteration of the protein coat did not significantly impair virus adsorption or alter the integrity of the virus particle. Damage to the viral RNA after exposure to ozone was demonstrated by velocity sedimentation analysis. It was concluded that the damage to the viral nucleic acid is the major cause of poliovirus 1 inactivation by ozone.
Full text
PDF

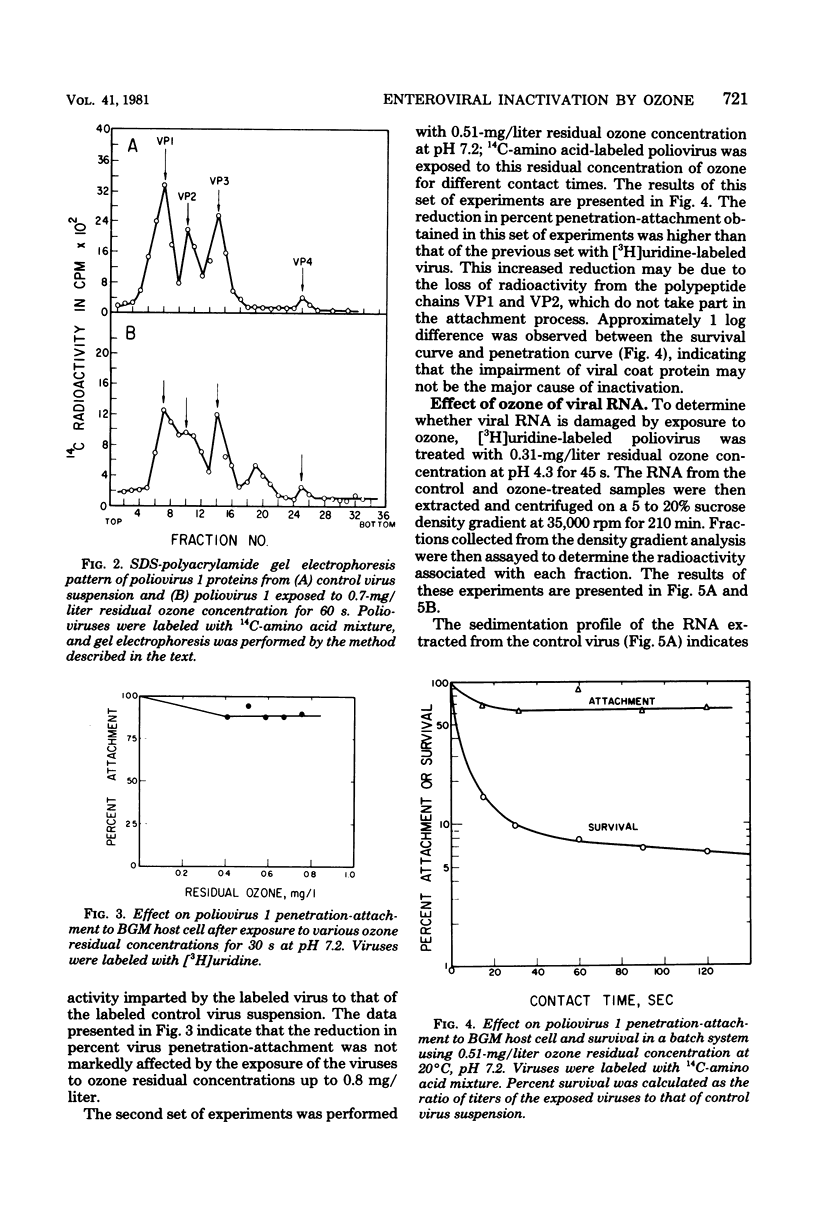

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breindl M., Koch G. Competence of suspended HeLa cells for infection by inactivated poliovirus particles and by isolated viral RNA. Virology. 1972 Apr;48(1):136–144. doi: 10.1016/0042-6822(72)90121-3. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN E., GIESE A. C. Changes in absorption spectra of nucleic acids and their derivatives following exposure to ozone and ultraviolet radiations. Arch Biochem Biophys. 1954 Jul;51(1):208–216. doi: 10.1016/0003-9861(54)90468-3. [DOI] [PubMed] [Google Scholar]
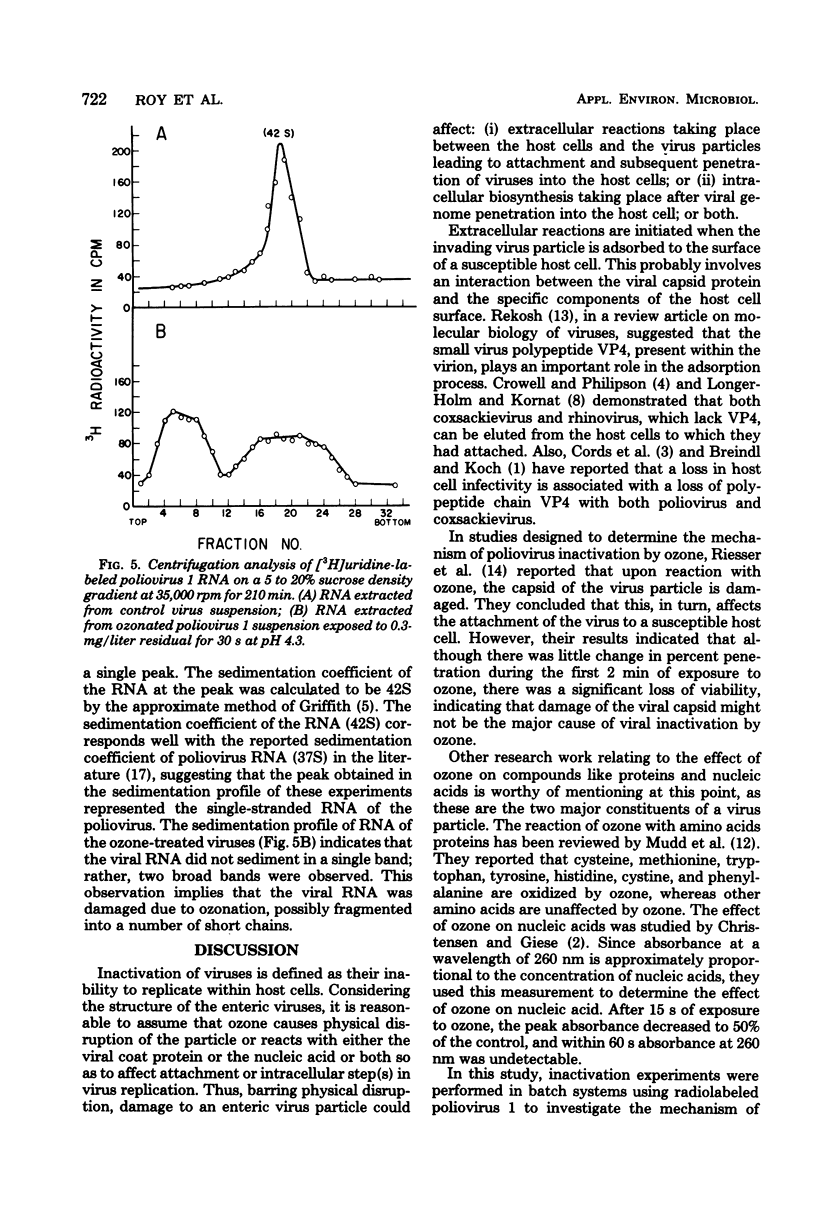
- Cords C. E., James C. G., McLaren L. C. Alteration of capsid proteins of coxsackievirus A13 by low ionic concentrations. J Virol. 1975 Feb;15(2):244–252. doi: 10.1128/jvi.15.2.244-252.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDEL B. THE EXTRACTION OF RIBONUCLEIC ACID FROM POLIOVIRUS BY TREATMENT WITH SODIUM DODECYL SULFATE. Virology. 1964 Mar;22:360–367. doi: 10.1016/0042-6822(64)90026-1. [DOI] [PubMed] [Google Scholar]
- MARTIN E. M., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 5. The kinetics of synthesis of virus protein and of virus ribonucleic acid in Krebs II mouse-ascites-tumour cells infected with encephalomyocarditis virus. Biochem J. 1962 Jun;83:574–582. doi: 10.1042/bj0830574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Summers D. F., Scharff M. D. SDS-acrylamide gel electrophoresis and its application to the proteins of poliovirus- and adenovirus-infected human cells. J Cell Physiol. 1970 Dec;76(3):273–287. doi: 10.1002/jcp.1040760307. [DOI] [PubMed] [Google Scholar]
- Mudd J. B., Leavitt R., Ongun A., McManus T. T. Reaction of ozone with amino acids and proteins. Atmos Environ. 1969 Nov;3(6):669–682. doi: 10.1016/0004-6981(69)90024-9. [DOI] [PubMed] [Google Scholar]
- Vrijsen R., Boeyé A. Gel electrophoresis of protein-dodecyl sulfate complexes in a pH gradient and improved resolution of poliovirus polypeptides. Anal Biochem. 1978 Apr;85(2):355–366. doi: 10.1016/0003-2697(78)90231-2. [DOI] [PubMed] [Google Scholar]



