Abstract
Cotton rats (Sigmodon hispidus and S. fulviventer) are susceptible to many viruses that infect humans (e.g., poliovirus, respiratory syncytial virus, influenza virus, adenovirus, and parainfluenza virus) and have been influential in developing therapeutic clinical intervention strategies for many viral infections of man. This study set out to determine whether cotton rats are susceptible to infection with HIV type 1 (HIV-1). Results indicate that HIV-1 does infect the cotton rat and S. fulviventer is more susceptible than S. hispidus. The virus was passaged from animal to animal for a total of three serial passages; but HIV replicated poorly in vivo, was only detectable as proviral DNA, and never exceeded one provirus per 1.8 × 105 cotton rat peripheral blood mononuclear cells. Infection induced a distinct and characteristic anti-HIV antibody response that, in some animals, included neutralizing antibodies, recognized all of the major HIV-1 antigens and the antibodies lasted out to 52 wk post-infection. Neonate S. fulviventer were not more susceptible to infection than adults. In vitro culture studies produced indirect evidence of viral replication by detection of viral gag gene RNA in reverse transcriptase–PCR assays on viral culture supernatants. Collectively, these results indicate that HIV-1 can replicate in a nontransgenic rodent and that this system may have potential as an animal model for HIV-1 infection if viral replication rates can be improved in vivo.
Experimental lentiviral models have aided in research efforts to discern the biology and pathogenic process of infection leading to AIDS associated with HIV-1 and have provided a convenient platform with which to study the basic biological processes of lentiviral infection ranging from prophylactic vaccine design (1), to therapeutic intervention strategies (2). Unfortunately, HIV-1 does not infect monkeys and onset of disease is not induced in chimpanzees until greater than 10 yr post-infection (p.i.) (3). Although permissive for HIV infection, chimpanzees are enormously expensive, an endangered species, and difficult to maintain in research environments (4). Asian monkeys can be readily infected with simian immuno-deficiency virus (SIV), which can induce a disease similar to human AIDS. However, expense and the incomplete homology of SIV to HIV, especially in the envelope region, which is a crucial determinant for viral infectivity, somewhat limit the utility of this model (5).
Severe combined immunodeficient mice reconstituted with human peripheral blood mononuclear cells (PBMC) allow replication of HIV-1 in vivo (6). Although good HIV-1 replication occurs in the reconstituted human PBMC, the rest of the mouse’s innate immune system is intact with fully conserved natural killer cell activity (7) leading to problems of standardization and reproducibility thus limiting its usefulness for vaccine and antiviral screening (6, 8).
The rabbit is susceptible to HIV-1 infection (9) but requires large doses of virus to induce infection; pathogenic changes do not develop, and reproducibility is a problem (10). By transfecting rabbit cells with humanCD4 (hCD4) (11) and also producing transgenic rabbits expressing hCD4 (12) investigators improved the susceptibility of the rabbit to infection by including the principal receptor for HIV-1 attachment (13), but this present form of the model is not popular. With the recent discovery of the coreceptors that HIV-1 uses for entry into human cells (14, 15), however, workers have attempted to improve the mouse and rabbit systems further by developing transgenic animals and cell lines expressing hCD4 and CCR5 and/or CXCR4 (12, 16, 17, 18). However, post-cell entry restrictions severely limit HIV-1 replication in mice with such transgenes (16). Rabbit cells support low levels of viral replication, and when viral entry restrictions are removed by incorporating hCD4 and human CCR5 receptor transgenes into a rabbit cell line, HIV replication and functional virus production is facilitated (18). This result suggests that a transgenic small animal model for the study of HIV disease may be feasible.
We initiated an investigation to determine the susceptibility of the cotton rat (S. fulviventer and S. hispidus) to infection with HIV-1 with a view toward improving any observed susceptibility by inclusion of the human coreceptors for HIV. Historically this host has been indispensable in virological studies, being susceptible to poliovirus (19), several important human respiratory viruses including respiratory syncytial virus (20), influenza A and B viruses (21), parainfluenza virus types 1, 2, and 3, (22, 23) and several serotypes of adenovirus (24, 25) as well as typhus (26) and filariasis (27). Recently, HIV-1 was injected into cotton rats to determine their potential as a model for AIDS and the results of this study (28) are compared in this communication.
METHODS
Animals.
Cotton rats S. hispidus (inbred) and S. fulviventer (almost inbred) were obtained from Virion Systems (Rockville, MD). Animals were housed in the certified central facility at the National Institutes of Health and at Biocon (Rockville, MD). Blood was obtained from the retro-orbital sinus for plasma, PBMC, and viral isolations. In all cases, animals were bled and found to be negative for HIV-1 antibodies before inoculation. The Institutional Animal Care and Use Committee of the National Institutes of Health approved all animal protocols. Inhalation of methoxyflurane (Mallinkrodt) was used to sedate animals. Carbon dioxide inhalation was used for killing. Manipulations and procedures were conducted in biosafety level 2 facilities observing biosafety level 3 practices (29).
Experimental Design.
Four main experiments were conducted to determine the susceptibility of cotton rats to HIV-1 infection.
Comparison of S.
fulviventer and S. hispidus as hosts for HIV-1 infection. Seven S. fulviventer and five S. hispidus were each infected i.v. with 1 × 105.83 TCID50 (HIV-1LAI). Bleeds for control PBMC DNA isolation and plasma were obtained before infection. After viral inoculation, sample bleeding for detection of provirus in the PBMC DNA and antibodies in the plasma were taken weekly and at monthly intervals after 6 wk p.i. A repeat experiment by using the same conditions was conducted to confirm results. On this occasion, however, six S. fulviventer and six S. hispidus were used.
Serial passage of HIV-1 in cotton rats.
Whole blood (0.1 ml for all passages) was transferred via i.v. inoculation from an HIV-1 PCR and antibody positive S. fulviventer at 37 wk p.i. to two normal S. hispidus (A1, A2) and two S. fulviventer (A3, A4). At 37 wk p.i., blood was passaged i.v. from A3 (PCR-SB positive) to three S. fulviventer (A5, A6, A7). At 13 wk p.i., blood from A6 (PCR-SB positive) was inoculated into animals A8 and A9 (S. fulviventer). At 20 wk p.i., blood from A8 (PCR-SB positive) was inoculated into animals A10, A11, and A12. All animals were bled at weekly intervals, and plasma and PBMC were taken and examined for HIV-1 antibodies and provirus, respectively.
A further determination of susceptibility of S.
fulviventer to HIV-1 infection. To determine if a freshly isolated clinical strain (HIV-1#101) could produce a more virulent infection, mature (>10 wk) S. fulviventer were inoculated i.v. with TCID50 virus and assayed by initial weekly (6 wk) and then monthly bleeds for a total of 52 wk. To monitor the presence of provirus in the tissue of cotton rats, two animals were killed at each of two time points (20 and 38 wk p.i.), and the spleen, PBMC, kidney, bone marrow, and brain were taken for histological and PCR analysis.
Susceptibility of neonate S.
fulviventer to HIV-1 infection. Twenty-one 3-day-old S. fulviventer were inoculated i.v. with TCID50 HIV-1#101 in 0.1 ml of tissue culture fluid on two occasions 1 wk apart. Due to their small size, pre-bleeds on the newborn cotton rats were not possible. Samples were taken for PBMC DNA isolation and antibody determination from 2 wk p.i. at weekly intervals for 6 wk and then monthly for a period of 52 wk.
Virus and Cell Lines.
HIV-1(LAI) (HTLV-III/B10), was a gift from Thomas Kindt (Laboratory of Immunogenetics, NIH) and was passaged in vitro in H9 cells. A fresh clinical isolate, HIV-1(#101), was obtained from College of Physicians and Surgeons, Columbia University, NY (laboratory of H.S.G.) and propagated in human PBMC.
HIV-1 virus of both strains were grown in CEMX174 cells in vitro for the purpose of assay development (see below). Inoculations of virus into cotton rats used cell-free virus propagated to a high titer [as determined by p24 ELISA and reverse transcription (RT) activity] and suspended in cell culture medium.
Sample Collection.
Blood was obtained via the retro-orbital sinus. Following heparin addition, blood was centrifuged, plasma removed, complement inactivated, and plasma stored at −20°C for antibody analysis. The buffy coat was removed for separation of PBMC, centrifuged over lymphocyte separation medium, washed, and frozen at −20°C for DNA extraction.
Portions of tissue from spleen, liver, brain, and kidney were placed in ice-cold PBS and processed into a single cell suspension. After centrifugation, cells were washed and frozen at −20°C for DNA extraction. Aliquots of cells (1–5 × 106 per tube) were placed in fetal bovine serum/dimethyl sulfoxide and stored over liquid nitrogen. PBMC (1 × 104 to 2 × 105) and spleenocytes (1–5 × 106) were frozen over liquid nitrogen. The remaining organ portions were then subdivided. One portion was fixed in 10% formalin/saline for 24 h and processed for histological analysis. The remaining portions were subdivided equally and processed for DNA, RNA extractions and cocultured (1–5 × 105 cells) with human PBMC (cells) for viral isolation. For coculture, tissue cells were placed into RPMI complete medium and mixed with human PBMC, stimulated with human IL-2 and peanut haemaglutinin for 3 days, and cultured for 8 wk and then the entire contents of the well were examined by PCR-SB assay for HIV-1 DNA. Bone marrow samples were prepared by cutting free one femur, washing the bone marrow contents into a sterile container followed by washes and treated as described above.
Serology.
Viral neutralization assay. Due to the small amount of blood available from individual cotton rats on serial bleedings, plasma rather than serum was used to conduct viral neutralization assays. This facilitated simultaneous isolation of PBMC from blood samples, thus minimizing animals’ distress. Heat inactivated plasma from cotton rats with a positive antibody response to gag antigens and to either gp41 or gp120 were selected for viral neutralizing antibody assays. A positive control serum (human) with a strong antibody response to all the HIV-1 antigens also was used. Plasma was diluted by using twofold serial dilutions (1/2 out to 1/256) in RPMI complete medium. Preinfection cotton rat plasma was used at a dilution of 1/4. HIV-1(#101) (final concentration of 100 infectious units) was then added to each dilution and incubated at 37°C for 30 min. Viral and plasma dilutions were then added to triplicate individual culture wells containing 5 × 105 CEMx174 cells in RPMI complete medium plus 10% human interleukin-2 (R & D Systems). Cultures were incubated for 2 days, then washed in Hanks’ balanced salt solution to remove free virus, and replated in fresh wells at the original concentration (media was changed every 3 days). RT activity was used to monitor viral growth at weekly intervals. The highest plasma dilution that prevented viral growth after culture for 8 wk was determined to be the neutralization titer.
Western blots.
HIV-1 antibodies were detected by using a Western blot kit (DuPont). Plasma samples from control or HIV-1-inoculated cotton rats were incubated according to manufacturers’ instructions with modification. After washes, all strips were exposed to cotton rat plasma and incubated for 1 h with a rabbit anti-cotton rat IgG antibody preparation (Virion Systems) at 1/1,000 dilution. After three washes, cotton rat plasma-exposed strips were incubated with anti-rabbit horse radish peroxidase-linked antibody (1/2,000 dilution; DAKO) for 1 h. Strips were then developed as normal. Control strips used strongly and weakly positive human sera and a serum negative for anti-HIV-1 antibodies.
DNA Isolation.
Total cellular DNA was isolated by using an ABI 341 nucleic acid purification system (Applied Biosystems). An average of 1.8 × 105 cotton rat PBMC were isolated from each bleeding and processed according to manufacturer’s instructions. After isolation, the DNA was suspended in 200 μl of dH2O and stored at −20°C until examined for provirus by PCR assay (see below). All of the sample (1.8 × 105 cells) was used to assay for proviral DNA.
Detection of HIV-1 in Genomic DNA.
The PCR assay for HIV-1 gag sequence used primers 5′-ATAATCCACCTATCCCAGTAGGAGAAAT-3′ (sense), 5′-TTGCCAAGTCCCTAGTTCTGCATT-3′ (anti-sense - outer) and 5′-GTTGTTGTCTCTACCCCAGAGC-3′ (anti-sense - inner) designed to amplify a 700-bp target from the gag gene of the provirus. Perkin–Elmer Cetus reagents and thermal cycler were used. DNA samples (0.5–1.0 μg) were amplified through 30 cycles at: 94°C for 60 sec; 55°C for 60 sec; and 72°C for 60 sec. Taq polymerase (0.5 unit) was used in a final volume of 50 μl. Ten identical samples were run from each animal. After initial amplification for 30 cycles, 10 μl was taken from the first reaction and placed into a second tube containing fresh reagents and the sense and nested anti-sense primers and amplified for a further 30 cycles. Ten microliters of these samples were electrophoresed on a 0.9% agarose gel. Standard Southern blotting was performed by using the previously cloned target region of the HIV-1 gag gene labeled with 32P dCTP (Amersham), hybridized to the blot under high stringency conditions, and processed for detection (30).
To determine the sensitivity of the PCR DNA assay, known numbers of HIV-1(LAI)-infected CMMT cells (an adherent monkey cell line) were added to suspensions of cotton rat PBMC titrated such that a mixture of 1.8 × 105 cotton rat PBMC (the average number of PBMC contained in a single sampling of cotton rat blood) contained 1,000, 100, 10, 1, and a theoretical 0.1 or 0.01 infected cells. After addition of virus, the CMMT cells were cultured for 2–3 days in duplicate, separate plates until almost confluent. One set of wells was fixed with ice-cold acetone/ethanol for 1 min, washed in saline, and blocked in 1% normal goat serum/saline for 30 min (all incubations at room temperature). Anti-HIV rabbit serum (against whole-inactivated virus) was diluted 1:200 in goat serum/saline (100 μl/well) and incubated for 30 min. After washes, anti-rabbit IgG was added [horse radish peroxidase linked (KPL), 100 μl/well diluted (1:1,000) in goat serum/saline] and incubated for 1 h. After final washes, wells were developed in nitroblue tetrazolium and viewed by microscopy. Positive cells stained dark brown against a light background. The entire contents of wells were counted to yield an estimate of the number of infected cells present. The replicates with the highest number of cells were selected, and the duplicate (still-living) cultures were washed and trypsinized. Cell suspensions were washed twice and titrated such that the required number of HIV-1-infected cells were mixed with 1.8 × 105 uninfected cotton rat PBMC. Suspensions were pelleted and the DNA extracted then stored at −20°C for PCR assay. The semi-nested PCR assay was sensitive, reproducible, and able to detect proviral DNA from an estimated one HIV-1-infected human cell in the extracted DNA from 1.8 × 105 uninfected cotton rat PBMC. PCR assays on experimental cotton rat DNA were run and signals compared with the above described control assays. Signals exposed on film were compared with those from the standards and scored 3+ if intensity was similar to 100 HIV-1 proviral copies; 2+ for 10 viral copies; and 1+ for 1 provirus copy in genomic DNA (data not shown). In practice, all cotton rat samples scored no greater than 1+ indicating low proviral load in genomic DNA.
RT-PCR was performed on plasma from HIV-infected cotton rats, supernatants from primary cotton rat PBMC cultures, and cocultures of cotton rat and human PBMC. Samples were used fresh or were thawed from preservation at −70°C, centrifuged at 50,000 × g for 1 h at 4°C, the supernatant removed, and the pellet resuspended in 10 μl of dH2O then used immediately in RT-PCR. The Geneamp RNA PCR core kit (Perkin–Elmer) was used with the outer antisense primer for reverse strand synthesis, and the manufacturers’ protocol was followed thereafter by using the specific gag upstream primer.
RESULTS
Comparison of S. fulviventer and S. hispidus as Hosts for HIV-1 Infection.
Of 12 cotton rats inoculated, one died almost immediately probably from anesthesia effects. Nine of the remaining 11 animals became antibody positive (seven S. fulviventer), and six became PCR-SB positive (five S. fulviventer) (Table 1). Four cotton rats were killed for PCR-SB assay and histological examination at 24 wk p.i. The remainder of the cotton rats were apparently healthy throughout the course of the study. However, when DNA was extracted from samples from these animals at postmortem, viral DNA was detected from some (Table 1), indicating that PBMC from cotton rats could be infected with HIV-1.
Table 1.
Infection of the Cotton rat (S. fulviventer and S. hispidus) with HIV-1(LAI)
| Experiment | Animal no.* | Species | Sex† | Antibody positive | Tissues PCR positive | Time of death, D or sacrifice, S |
|---|---|---|---|---|---|---|
| 1 | S. hispidus | F | p17, p24 | − (negative) | S, wk 52 | |
| 2 | S. fulviventer | M | p17 | — | S, wk 24 | |
| 3 | S. hispidus | M | p24 | — | S, wk 24 | |
| 4 | S. hispidus | M | — | PBMC† | S, wk 24 | |
| 5 | S. fulviventer | M | p51, p55 | PBMC | S, wk 52 | |
| No. 1 | 6 | S. fulviventer | F | p17 | SPL, LN, BM | S, wk 24 |
| 7 | S. hispidus | M | — | — | S, wk 52 | |
| 8 | S. fulviventer | F | p24 | — | S, wk 52 | |
| 9 | S. fulviventer | F | p17, p24, p51, p55 | PBMC | S, wk 52 | |
| 10 | S. fulviventer | M | p51, p55 | PBMC, SPL | S, wk 52 | |
| 11 | S. fulviventer | F | p17, p51, p55 | PBMC, SPL, THY | S, wk 52 | |
| 12 | S. hispidus | F | — | — | D, day 0 | |
| 23 | S. hispidus | F | — | PBMC | D, wk 42 | |
| 24 | S. hispidus | F | — | PBMC | S, wk 60 | |
| 26 | S. hispidus | M | — | — | D, day 9 | |
| 27 | S. hispidus | M | — | — | D, day 0 | |
| 28 | S. hispidus | M | — | — | D, day 0 | |
| No. 2 | 29 | S. hispidus | M | — | — | D, day 0 |
| 30 | S. fulviventer | F | p17 | PBMC | S, wk 60 | |
| 31 | S. fulviventer | F | — | PBMC, BM, THY | S, wk 60 | |
| 32 | S. fulviventer | M | — | BM | S, wk 60 | |
| 33 | S. fulviventer | M | — | BM, SPL | S, wk 60 | |
| 34 | S. fulviventer | M | — | — | S, wk 60 | |
| 35 | S. fulviventer | F | — | — | D, day 0 |
Animal nos. 1–12 were from experiment no. 1 and animals nos. 23–24, 26–35 were from a confirmatory experiment (no. 2) as described in Materials and Methods. F, female, M, male.
In most cases at killing or death, the following tissues were taken for histology and PCR analysis: abbreviations; SPL, spleen; BM, bone marrow (femur); THY, thymus; LN, lymph node. Brain, lung, and kidney also were sampled but none were positive by PCR-SB and so are not included in the table.
A confirmatory experiment gave results similar to those of the first, and similarly deaths occurred early (animals numbers 23–24, 26–35): of the 12 animals inoculated, four died on day of inoculation, probably from anesthesia; one animal died on day 9 p.i. and one died at week 42 p.i. Of the six animals that died, four were S. hispidus. All tissues were taken and assayed (PCR). There was no evidence of large viral burdens or pathology associated with viral infection in animals that died. Four S. fulviventer and only two S. hispidus were PCR positive (Table 1). Only animal 30 (S. fulviventer) was positive for HIV-1 antibodies, a much reduced number compared with the initial experiment despite both groups receiving the same viral strain and infective dose (Table 1). Other than a possible variation in the lots of the immunoblot kits used, we can offer no obvious explanation as to why the immune responses of the two groups of animals in this experiment varied so much. A reduction in the number of lymphocytes surrounding the spleen blood vessels (data not shown), was a minor pathological change that was not evident in samples from uninfected animals.
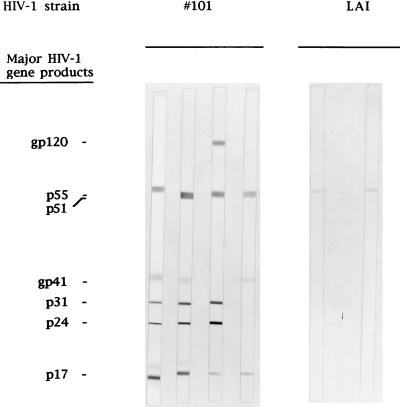
Thirteen S. fulviventer animals were infected in these two experiments of which nine were PCR positive. Of the 11 S. hispidus inoculated, three were positive (PCR) from PBMC samples and fewer organs were PCR positive on postmortem analysis for this species (Table 1). These data suggested that S. fulviventer is more susceptible to HIV-1 infection than S. hispidus. Tissue culture of HIV-1 inoculated cotton rat PBMC gave no indication of viral replication by p24 ELISA or RT activity. However, a weak antibody response was demonstrated by serological reactions to gag proteins in a small number of animals. The weakness of this response may be correlated with the laboratory strain of virus inoculated, since in later experiments using a fresh clinical isolate of HIV-1, the response in cotton rats was stronger (Fig. 1).
Figure 1.
Specificity of the Cotton rat (S. fulviventer) serological response to HIV-1(#101) and HIV-1(LAI) by Western blot.
Serial Passage of HIV-1 in Cotton Rats.
The sequence of blood donors were A3, A6, and A8 for the first, second, and third passage, respectively. The original blood inoculum donor was an antibody and PCR-positive S. fulviventer. Inoculation of blood from this donor resulted in all four recipients becoming infected (PCR assay). In this first passage of HIV-1(LAI), the two S. hispidus (A1 and A2) were PCR positive at 2 wk p.i. Thereafter, neither S. hispidus had any detectable provirus in PBMC DNA until A1 became PCR positive at 32 wk p.i. and A2 at 53 wk p.i. At killing, A1 was positive for HIV-1 DNA in bone marrow, spleen, and PBMC. S. fulviventer animals (A3 and A4), although not as rapidly positive for provirus, first tested positive at 6 wk p.i; at 28 wk, and at 32 wk p.i., both animals were PCR-positive. Blood (0.1 ml) from animal A3 (most consistently positive) was passaged to three S. fulviventer (A5, A6, and A7). A6 was positive (PCR) at 4 wk p.i. At 6 wk p.i., all three were positive. At 13 wk p.i., blood from animal A6, which was consistently positive for three consecutive samples, was used to infect two more S. fulviventer (A8 and A9). At 3 wk p.i., both were positive by PCR. Blood from A8, PCR positive at 20 wk p.i., was used to passage the virus for a fourth time, but provirus was undetectable in recipients. No animal had a detectable antibody response throughout the course of this experiment. At various times throughout the course of this experiment, viral isolation was attempted but was not successful.
A Further Determination of Susceptibility of S. fulviventer to HIV-1 Infection.
The primary isolate HIV-1(#101) was used to infect cotton rats on this occasion, and all 19 animals were negative for antibodies and provirus before inoculation. Sixteen of nineteen animals had an antibody response by 4 wk p.i., and all had an antibody response to at least two gag proteins (Table 2). These responses remained constant until near the end point of the study at 52 wk p.i., at which time only 86% of the animals had an antibody response, down from 100% at wk 44 p.i. (Table 2). Analysis of individual Western blots, however, indicated a diminution of the levels of circulating antibodies from some animals in terms of the number of antigens detected (Table 2), wherease other antibody responses were steady out to 52 wk p.i. Unlike infection with HIV-1(LAI), however, infection with HIV-1(#101) resulted in a much more pronounced antibody response with 9/19 cotton rats responding to either gp120 or gp41 envelope glycoproteins (Table 2, Fig. 1). Antibody responses to envelope glycoproteins were not evident in plasma from animals infected with HIV-1(LAI) (Fig. 1). Antibody responses to certain antigens decreased with time in 13 of 19 animals (Table 2). Viral neutralizing antibody was present in the plasma from three animals with responses to HIV-1 envelope antigens with titers of 1/40, 1/20, and 1/20 for animals 5B, 15B, and 17B, respectively, at 12 wk p.i. The human positive control plasma gave a consistent titer of >1/160. No viral neutralizing antibody activity was evident in plasma from other animals.
Table 2.
HIV-1 antigens* recognized by Cotton rat (S. fulviventer) plasma antibodies after infection with HIV-1(#101)
| Animal No. | Weeks p.i.
|
||||
|---|---|---|---|---|---|
| 4 | 12 | 24 | 36 | 52 | |
| 3B | negative | negative | p17, p31 | ND | S† (38 wk) |
| 5B | negative | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | S (38 wk) |
| gp41, p51, p55 | gp41, p51, p55 | gp41, p51, p55 | |||
| 6B | p51, p55 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | p24, p31 |
| p55 | p51, p55 | ||||
| 7B | p17, p51, p55 | p17, p24, p31 | p24, p31 | p17, p24, p31 | p24, p31 |
| p51, p55, gp120 | p51, p55, gp120 | p51, p55, gp120 | p51, p55, gp120 | ||
| 9B | p51, p55 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 |
| p51, p55 | p51, p55 | p51, p55 | p51, p55 | ||
| 11B | p51 | p17, p24, p31 | p17, p51, p55 | p17, p51, p55 | p17, p51, p55 |
| 14B | p51 | p17, p24, p31 | p51, p55 | ND | p51, p55 |
| 15B | p24, p51, p55 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 |
| p51, p55, gp120 | p51, p55 | p51, gp41 | p51, p55 | ||
| 16B | p51, p55 | p17, p51, p55 | p51, p55 | p17, p24, p31 | p51, p55 |
| p51, p55 | |||||
| 17B | p51, p55 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 |
| p51, p55, gp120 | p51, p55 | p51, p55 | p51, p55 | ||
| 19B | p55 | p51, p55 | p51, p55 | p51, p55 | negative |
| 22B | p51, p55 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 |
| p51, p55 | p51, p55, gp41 | p51, p55, gp41 | gp41 | ||
| 23B | p51, p55 | ND | p17, p51, p55 | p17, p51, p55 | p17, p51, p55 |
| 24B | negative | p55 | p51, p55 | p17, p55 | negative |
| 25B | p51, p55 | p17, p55, gp41 | p17, p55, gp41 | p17, p55 | negative |
| 28B | p51, p55 | p17, p24, p31 | S (20 wk) | ||
| p51, p55 | |||||
| 29B | p51, p55 | p17, p51, p55 | S (20 wk) | ||
| 30B | p51, p55 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | p24, p31 |
| p51, p55 | p51, p55 | p51, p55 | p51, p55 | ||
| 31B | p55 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 | p17, p24, p31 |
| p51, p55 | p51, p55 | p51, p55 | p51, p55 | ||
| 32B‡ | negative | negative | negative | negative | negative |
| 33B‡ | negative | negative | negative | negative | negative |
Western blots were conducted according to the protocol described in Materials and Methods.
S denotes that an animal was killed for histological analysis and tissue viral isolation/detection.
Uninoculated negative control animals.
ND, not determined.
Susceptibility of Neonate S. fulviventer to HIV-1 Infection.
Antibody responses in neonate versus mature animals were much reduced in terms of numbers reacting (only 8 of 19 had a response) and in the number of antigens recognized (p31, p51, p55). All 19 animals showed evidence of infection by 8 wk p.i. (PCR). The detection rate declined as infection progressed to 52 wk p.i., indicating that neonates are not more susceptible to infection than mature animals. Attempts to passage the virus in vitro from infected neonate cotton rat PBMC to susceptible human PBMC were unsuccessful.
In Vitro Culture Studies.
Viral infection of cultures could not be established with combinations of (i) PBMC from infected animals, (ii) normal cotton rat PBMC cocultured with PBMC from infected animals, and (iii) PBMC from an infected cotton rat cocultured with human cells (activated PBMC and CEMss cell line). However, on one occasion viral gag gene RNA was amplified by RT-PCR from a PBMC culture supernatant derived from an infected, PCR positive cotton rat, suggesting the presence of minute amounts of virus in the culture supernatant (the culture supernatant was filtered for cell removal and ultracentrifuged, and the pellet was assayed by PCR).
DISCUSSION
This study demonstrates that cotton rats, particularly S. fulviventer, are permissive for low-level HIV-1 infection. Provirus was detected consistently in PBMC and other tissues from infected animals. Virus, particularly a fresh human isolate, stimulated a strong, specific and long-lasting immune response and was maintained out to 1 yr p.i. with viral neutralizing antibodies present in some animals. Although not demonstrable by direct culture of PBMC and tissues from infected animals, clearly infectious virus replicated at a low level in PBMC of these animals because it was present for three serial passages of blood. The presence of viral RNA activity in the supernatant of one culture and from plasma from an infected animal also is suggestive of infrequent but significant viral replication and correlates with the level of viral DNA detection by PCR-SB observed from cotton rat genomic DNA samples. Viral p24 antigen was never detected by enzyme immunoassay in culture supernatants or from plasma of infected animals. This indicates that viral presence originated from the PBMC of the animal and not from an in vitro infection of cultured cells (12). Additionally, p24 ELISA is not sufficiently sensitive to detect HIV-1 antigen from rabbits in vitro compared with hCD4 transgenic rabbit PBMC despite the occurrence of viral DNA in the infected, nontransgenic rabbit PBMC (12).
Numerous small animal species have been inoculated with blood from seropositive humans but none were replication permissive for HIV-1 (31). Most attention has focused on the mouse as a potential model, but several significant blocks to viral replication may exist in mice (32). S. hispidus supports low-level HIV-1 infection and was associated with host death in a recent study (28), a finding that is not confirmed by our more exhaustive study.
Good evidence for significant chronic viral replication in vivo is the detection of antibodies to HIV, which qualitatively rose 4–6 wk p.i. and persisted throughout the course of the study. The cotton rat can react against the complete spectrum of HIV-1 antigens including gp120 and gp41 and all of the major gag proteins. In some cases, the strength of the reaction to a number of antigens declined quantitatively as the infections progressed, particularly in the mature S. fulviventer infected with HIV-1(LAI). The general antibody response probably was a bona fide reaction to a constant low-level viral antigen production because the intensity of it increased with time in a number of animals. This finding, taken together with the results of the passage experiment, in which proviral DNA was detectable in the genome of cotton rats that received serially passaged blood from infected animals further indicates that low quantities of viral replication occurred and accounted for these phenomena.
Cellular markers specific for cotton rat CD4 and CD8 do not exist yet and so precluded accurate estimation of changes in the ratio of cell subsets as infection progressed. However, no changes in the ratio of the relative proportion of lymphocytes to polymorphonuclear cells was evident, perhaps indicating low replication of HIV-1 in the cotton rat. Transgenic rabbits expressing hCD4, showed minor changes in the CD4 and CD8 cell ratio, which was probably due to low viral load (12). In our study, the sensitivity of the PCR assay was optimal (1 copy of proviral DNA could be detected) and gave an estimate of <1 infected PBMC per 1.8 × 105 cells. This viral load estimate is much less than humans (33, 34, 35) and SIV-infected macaques (36). The HIV-1 viral load in chimpanzees can be as low as 1 copy per 1 × 106 PBMC (37). Certainly the load observed in infected cotton rats is comparable with that reported for normal or hCD4 transgenic rabbits (12).
The ability to passage the virus in vivo is convincing. This report presents such evidence in nontransgenic rodents. Passaged infections were consistently low-level, no overt disease was produced, and no strong antibody response was observed [probably due to the reduced immunogenicity of the LAI strain in the cotton rat (see Fig. 1)]. Viral persistence, free or cell-associated, from the original inoculum might be considered to have caused these observations. This is extremely unlikely, however, as the dilution factor involved is large (inocula were 0.1 ml into an adult cotton rat (up to 15.0 ml of total blood volume) with three positive passages, the final dilution equals 3.3 × 10−6) and would make such a possibility of persistence of the original inoculum occurring remote, if not impossible. Also, individual mammalian lymphocytes typically have a life span of 100–200 days (38) and the blood passage experiment spanned 812 days (2.2 yr) and thus precluding the possibility that any of the original inoculum remained.
We, and another group (28) have presented strong evidence that HIV-1 can infect the cotton rat. Although this infection is at an extremely low-level, it is nonetheless detectable, real, and stimulates an immune response qualitatively similar to that seen in humans. The current cotton rat model bears similarities to the nontransgenic rabbit/HIV model (9, 10), i.e., low viral replication rates are supported and the main barrier to infection may lie at the level of viral entry to the cell. Rabbits and rabbit cells expressing hCD4 show improved HIV-1 production, and rabbit cells can be rendered highly permissive for HIV replication and produce infectious particles by incorporation of the HIV coreceptors hCD4 and human CCR5 (18). Transgenic mice expressing human CD4, CXCR4, and CCR5 are made more permissible for HIV-1 entry and genomic incorporation of the provirus (16, 17). Similar approaches in expressing the necessary coreceptors on the surface of cotton rat cells could lead to an improvement of the model and provide a platform with which to establish a reliable, reproducible, inexpensive, and accessible inbred rodent laboratory host to study aspects of HIV infection conveniently in vivo. This exciting approach is now in progress. If successful, transgenic cotton rats will be produced. These will be of tremendous value for development of vaccines and successful anti-HIV-1 therapy.
Acknowledgments
This work was supported by the National Institutes of Health (National Institute of Allergy and Infectious Diseases Grant Z01 AI00730). We wish to thank Robert Goeken, Diane Adger-Johnson, George Dapolito, Anna Hahn, Miriam Darnell, and Lorraine Ward for technical assistance. Dr. Robert Chanock encouraged this study and provided facilities. Dr. Vanessa Hirsch and Dr. Simoy Goldstein provided helpful discussion.
ABBREVIATIONS
- p.i.
post-infection
- PBMC
peripheral blood mononuclear cells
- RT
reverse transcription
- SIV
simian immunodeficiency virus
References
- 1.Desrosiers R C. AIDS Res Hum Retroviruses. 1992;8:411–421. doi: 10.1089/aid.1992.8.411. [DOI] [PubMed] [Google Scholar]
- 2.Wyand M S. AIDS Res Hum Retroviruses. 1992;8:349–356. doi: 10.1089/aid.1992.8.349. [DOI] [PubMed] [Google Scholar]
- 3.Novembre F, Saucier M, Anderson D C, Klumpp S A, O’Neill S P, Brown C R, Hart C E, Guenther P C, Swenson R B, Fultz N P, et al. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moor-Janowski J, Mahoney C J. J Med Primatol. 1989;18:1–26. doi: 10.1002/ajp.1350180102. [DOI] [PubMed] [Google Scholar]
- 5.Kirchoff F, Mori K, Desrosiers R C. J Virol. 1994;68:3682–3692. doi: 10.1128/jvi.68.6.3682-3692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosier D E, Gulizia R J, Baird S M, Wilson D B, Spector D H, Spector S A. Science. 1991;251:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 7.Dorschkind K, Pollack S B, Bosma M J, Phillips R A. J Immunol. 1985;134:3798–3801. [PubMed] [Google Scholar]
- 8.Mosier D E, Gulizia R J, MacIsaac P D, Corey L, Greenberg P D. Proc Natl Acad Sci USA. 1993;90:2443–2447. doi: 10.1073/pnas.90.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filice G, Cereda R M, Varnier O E. Nature (London) 1988;335:366–369. doi: 10.1038/335366a0. [DOI] [PubMed] [Google Scholar]
- 10.Tseng C K, Hughes M A, Hsu P-L, Mohoney S, Dubric M, Sell S. Am J Pathol. 1991;138:1149–1164. [PMC free article] [PubMed] [Google Scholar]
- 11.Hague B F, Sawasdikosol S, Brown T J, Lee K, Recker D P, Kindt T J. Proc Natl Acad Sci USA. 1992;89:7963–7967. doi: 10.1073/pnas.89.17.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn C S, Mehtali M, Houdebine L M, Gut J-P, Kirn A, Aubertin A-M. J Gen Virol. 1995;76:1327–1336. doi: 10.1099/0022-1317-76-6-1327. [DOI] [PubMed] [Google Scholar]
- 13.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. Nature (London) 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 15.Doranz B J, Rucker J, Yanijie Y, Smyth R J, Samson M, Pieper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 16.Browning J, Horner J W, Pettoello-Mantovani M, Rakker C, Yurasov S, DePinho R A, Goldstein H. Proc Natl Acad Sci USA. 1997;94:14637–14641. doi: 10.1073/pnas.94.26.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tachibana K, Nakajima T, Sato A, Igarashi K, Shida H, Iizasa H, Yoshida N, Yoshie O, Kishimoto T, Nagasawa T. J Exp Med. 1997;185:1865–1870. doi: 10.1084/jem.185.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speck R F, Penn M L, Wimmer J, Esser U, Hague B F, Kindt T J, Atchison R E, Goldsmith M A. J Virol. 1998;72:5728–5734. doi: 10.1128/jvi.72.7.5728-5734.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong C. Public Health Rep. 1939;54:1719–1721. [Google Scholar]
- 20.Prince G A, Jenson A B, Horswood R L, Camargo E, Chanock R M. Am J Pathol. 1978;93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 21.Sadowski W, Wilczynski J, Semkow R, Tulimowska M, Krus S, Kantoch M. Med Dosw Mikrobiol. 1987;39:43–55. [PubMed] [Google Scholar]
- 22.Murphy T F, Dubovi E J, Clyde W A., Jr Exp Lung Res. 1981;2:97–109. doi: 10.3109/01902148109052306. [DOI] [PubMed] [Google Scholar]
- 23.Sadowski W, Semkow R, Wilczynski J, Krus S, Kantoch M. Med Dosw Mikrobiol. 1987;39:33–42. [PubMed] [Google Scholar]
- 24.Pacini D L, Dubovi E J, Clyde W A. J Infect Dis. 1984;150:92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg H S, Lundholm-Beauchamp U, Prince G A. Molecular Basis of Virus Disease. Cambridge, U.K.: Cambridge Univ. Press; 1987. pp. 245–258. [Google Scholar]
- 26.Frolova O M, Balaeva N M, Genig V A, Ignatovich V F. Zh Mikrobiol Epidemiol Immunobiol. 1987;2:12–15. [PubMed] [Google Scholar]
- 27.Beg M A, Ingram G A, Storey D M. J Nutr Immunol. 1993;2:23–36. [Google Scholar]
- 28.Rytik P G, Kucherov I I, Mueller V, Podol’skaya I A, Kruzo M, Duboiskaya G P, Poleshchuk N N. Immunologiia. 1997;1:19–21. [Google Scholar]
- 29.Centers for Disease Control. Morbid Mortal Wkly Rep. 1988;37:1. [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 31.Morrow W J W, Wharton M, Lau D, Levy J A. J Gen Virol. 1987;68:2253–2257. doi: 10.1099/0022-1317-68-8-2253. [DOI] [PubMed] [Google Scholar]
- 32.Mizrachi Y, Sternas L, Volsky D J. Virology. 1992;186:167–174. doi: 10.1016/0042-6822(92)90071-v. [DOI] [PubMed] [Google Scholar]
- 33.Hsia K, Spector S A. J Infect Dis. 1991;164:470–475. doi: 10.1093/infdis/164.3.470. [DOI] [PubMed] [Google Scholar]
- 34.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Nature (London) 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. Nature (London) 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Elkins W R, Jr, Alvord W G, Montefiori D C, et al. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novembre F J, Saucier M, Anderson D C, Klumpp S A, O’Neill S P, Brown C R, II, Hart C E, Guenther P A, Swenson R B, McClure H M. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reece W O. Physiology of Domestic Animals. Baltimore: Williams & Wilkins; 1997. p. 128. [Google Scholar]