Abstract
The endocannabinoid lipid 2-arachidonoylglycerol (2-AG) is deactivated by intracellular hydrolysis catalyzed by monoacylglycerol lipase. 2-AG also serves as a substrate for oxidative metabolism catalyzed by cyclooxygenase 2 (COX-2). However, products of COX-2-mediated metabolism of endocannabinoids have not been identified in vivo. Hu and colleagues in this issue of the BJP demonstrate that COX-2 converts 2-AG into a biologically active, pro-nociceptive compound, prostaglandin E2 glycerol ester (PGE2-G). PGE2-G produces hyperalgesia in vivo and activates a rapidly acting transcription factor, nuclear factor kappa-B in vitro. These biological actions may be attributed to a unique receptor. This report of pro-nociceptive actions of an endogenous COX-2 metabolite of 2-AG that are largely opposite to known anti-nociceptive and anti-inflammatory actions of endocannabinoids has physiological relevance. These discoveries place renewed emphasis on the importance of understanding the highly interactive nature of lipid signalling pathways in the nervous system and the physiological roles of these lipid mediators in controlling homeostasis.
Keywords: endocannabinoid, 2-arachidonoylglycerol, anandamide, prostaglandin, cyclooxygenase, lipoxygenase, cytochrome P450, monoacylglycerol lipase, nociception, peripheral
Endocannabinoids, the brain's own cannabis-like compounds, are implicated in a vast array of physiological and pathological conditions, including the regulation of food intake, immunomodulation, analgesia and inhibition of cancer cell proliferation. Endocannabinoids are endogenous lipid-signalling molecules that produce physiological effects by activating cannabinoid receptors (see Piomelli, 2005). Anandamide and 2-arachidonoylglycerol (2-AG) are the best-studied endocannabinoids isolated so far. Anandamide is hydrolyzed by fatty-acid amide hydrolase (FAAH), whereas 2-AG is hydrolyzed predominantly by monoacylglycerol lipase (MGL) (Piomelli, 2005). However, the presence of an arachidonyl moiety in the structures of both 2-AG and anandamide suggests that these lipids also serve as substrates for metabolism by other enzymes, including a multitude of oxygenases (Kozak and Marnett, 2002).
The enzyme cyclooxygenase (COX) catalyzes the formation of a subclass of biological mediators known as prostanoids from arachidonic acid (see Kozak and Marnett, 2002). Prostanoids belong to the eicosanoid family of lipids that include the prostaglandins. Pharmacological inhibitors of COX, such as aspirin and ibuprofen, are widely used to suppress inflammation and pain. For almost three decades, COX enzymes were believed to act solely on free fatty acids. However, work by Walker's group now demonstrates that a COX enzyme also metabolizes an endocannabinoid into a biologically active endogenous compound that acts at a unique receptor (Hu et al., 2008).
Two isoforms of COX have been described (see Kozak and Marnett, 2002). COX-1 is a constitutive enzyme whose actions mediate a wide spectrum of cellular housekeeping functions whereas COX-2 is highly inducible in response to inflammatory stimuli within the CNS. Spinal COX-2 has been specifically implicated in the initiation of thermal hyperalgesia induced by carrageenan inflammation (Dirig et al., 1998). By contrast, non-spinal sources of prostanoids (derived from COX-2 and possibly COX-1-mediated metabolism) have been implicated in the maintenance of thermal hyperalgesia and inflammation (Dirig et al., 1998).
In vitro studies suggest that COX is capable of oxygenating both anandamide (Yu et al., 1997) and 2-AG (Kozak and Marnett, 2002) to generate prostaglandin ethanolamides and glyceryl prostaglandins, respectively. The COX-2 side pocket and the Arg 513 residue are critical determinants of the ability of this enzyme to generate prostaglandin ethanolamide efficiently (Kozak et al., 2003). By contrast, COX-1, which lacks this critical Arg residue, is less effective than COX-2 in metabolizing endocannabinoids (Kozak et al., 2003). COX-2 oxygenates both anandamide (Yu et al., 1997) and 2-AG (Kozak and Marnett, 2002) in vitro as effectively as it oxygenates arachidonic acid (AA). Thus, it is noteworthy that both anandamide- and 2-AG-derived prostanoids are metabolically more stable than free-acid prostaglandins. These observations suggest that COX-2 may catalyze the formation of a family of oxygenated lipids from various endocannabinoid precursors, and that these endogenous COX-2 metabolites may themselves act as biological mediators or pro-drugs. Prostaglandin ethanolamides and prostaglandin glycerol esters can be generated in vitro following incubation of high concentrations of synthetic endocannabinoids with the recombinant enzyme. However, whether or not COX-2-derived metabolites of endocannabinoids are produced in vivo and are biologically active has, until recently, remained unknown.
Walker's group (Hu et al., 2008) is the first to show that COX-2 converts the endocannabinoid 2-AG into prostaglandin E2 glycerol ester (PGE2-G) in vivo. This study suggests a physiological role for PGE2-G as an endogenous pro-nociceptive compound. Intraplantar administration of PGE2-G enhanced behavioural sensitivity to thermal and mechanical stimulation in a manner likely to be independent of cannabinoid and prostanoid receptors. Notably, these effects were only partially reversed by co-administration of PGE2-G with a cocktail of antagonists for prostanoid receptors. Thus, the physiological effects of PGE2-G are likely to be mediated by a unique and previously uncharacterized receptor. The discovery of novel endogenous ligands and previously unidentified receptor systems should be expected, given that numerous ‘orphan' receptors have been identified that have no known endogenous ligands. Recent advances in analytic chemistry and mass spectrometry have fostered the development of lipidomics, a field aimed at identifying the vast array of lipids formed in biological tissues. Thus, lipidomics-based approaches hold considerable promise for identifying endogenous ligands for orphan receptors and exploring their functional roles in the nervous system.
PGE2-G also modulated activity of the transcription factor nuclear factor kappa-B in a macrophage cell line in a concentration-dependent manner; high doses of PGE2-G increased and low doses of PGE2-G decreased activation of nuclear factor kappa-B (Hu et al., 2008). These findings suggest a physiological role for endogenous COX-2-mediated metabolites of 2-AG in enhancing nociception through a mechanism that may involve nuclear factor kappa-B-mediated signal transduction. However, more work is necessary to better understand the functional significance of prostaglandin ethanolamides/glycerol esters in other biological systems. Most notably, the consequences of interactions between classic eicosanoid and endocannabinoid pathways under physiological and pathological conditions require further investigation.
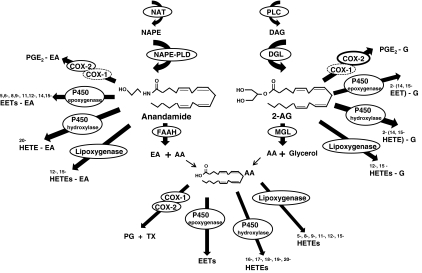
It is critical to recognize that COX enzymes are not the only oxygenases capable of metabolizing endocannabinoids (Figure 1). Therefore, the ramifications of this work extend beyond the findings reported by Hu et al. (2008). In vitro studies demonstrate that endocannabinoid lipids such as 2-AG (Kozak et al., 2002) and anandamide (Ueda et al., 1995) also undergo lipoxidative oxygenation by lipoxygenase enzymes. Moreover, cytochrome P450 enzymes have also been implicated in metabolizing anandamide and 2-AG, further adding to the complexity of endocannabinoid metabolism (Snider et al., 2007). Oxidative metabolism of both of these endocannabinoids by microsomal P450 enzymes produces a diverse array of oxygenated products (Figure 1), including epoxides (epoxyeicosatetraenoic acids) and hydroxy-metabolites (hydroxyeicosatetraenoic acids) (Snider et al., 2007). More work is necessary to demonstrate that these endocannabinoid-derived metabolites occur in vivo and are biologically active.
Figure 1.
The endocannabinoids 2-AG and anandamide serve as substrates for metabolism by a complex array of enzymes. MGL catalyzes the hydrolysis of 2-AG into arachidonic acid (AA) and glycerol, whereas FAAH catalyzes hydrolysis of anandamide into arachidonic acid and ethanolamine. COX-2 and other enzymes catalyze metabolism of these same endocannabinoid lipids into various oxygenated compounds. COX-1, cyclooxygenase-1; DAG, diacylglycerol; DGL, diacylglycerol lipase; EA, ethanolamide; EETs, epoxyeicosatetraenoic acids; FAAH, fatty-acid amide hydrolase; G, glycerol ester; HETEs, hydroxyeicosatetraenoic acids; NAPE, N-arachidonoyl-phosphatidylethanolamine; NAT, N-acyl-transferase; P450, cytochrome P450; PG, prostaglandins; PGE2, prostaglandin E2; PLC, phospholipase C; PLD, phospholipase D; TX, thromboxanes.
The diversity of potential, functionally active, products formed by oxygenation of anandamide and 2-AG is noteworthy (Figure 1). Moreover, the fact that the biological activities of these metabolites may differ so dramatically from that of the parent compound merits special attention. The fates of endocannabinoids are likely to differ based upon the level of activation or expression of enzymes involved in hydrolysis (MGL or FAAH) and/or oxidative metabolism (COX-2, lipoxygenase, cytochrome P450) within a given cell type. Thus, the enzymatic phenotype of the cell, in addition to the more classically recognized neurochemical phenotype of a cell, can be expected to influence the spectrum of biological effects produced downstream of endocannabinoid mobilization. These alternative pathways for oxidative metabolism of endocannabinoids may take on the predominant physiological role under conditions in which the primary enzymes catalyzing endocannabinoid hydrolysis (MGL or FAAH) are inhibited or absent (Fowler, 2007). PGE2-G could be formed preferentially at the spinal, as opposed to peripheral, level under conditions in which COX-2 expression is specifically up-regulated at this site by inflammation. Endocannabinoid-derived products of oxidative metabolism could act on different types of receptors, such as the peroxisome proliferator-activated receptor alpha or the transient receptor potential vanilloid 1 receptor and others that remain to be identified and produce compensatory changes in the activity of other enzymes (MGL or FAAH) that contribute to analgesic or pro-nociceptive effects under different physiological or pathological conditions.
Walker's group (Hu et al., 2008) is the first to demonstrate the in vivo production of an endogenous pro-nociceptive compound by COX-2-mediated metabolism of 2-AG. This discovery may have clinical relevance, given that pharmacological inhibitors of endocannabinoid hydrolysis (for example, the FAAH inhibitor URB597) are currently being evaluated for therapeutic potential in animal models of inflammation and pain. Further elucidation of the complexities of endocannabinoid metabolism, along with simultaneous measurements of endocannabinoids, their precursors and metabolites, should improve our knowledge of the physiological roles of these lipid mediators in the nervous system. Such discoveries may, in turn, benefit patients and clinicians alike by improving pharmacotherapies for inflammation and chronic pain. A better understanding of pathways controlling in vivo metabolism of endocannabinoids may be used to optimize the therapeutic potential of endocannabinoid-based pharmacotherapies, whereas limiting the profile of potentially adverse side effects.
Acknowledgments
JG is supported by a Fonds de la recherche en santé du Québec (FRSQ) postdoctoral fellowship. AGH is supported by DA021644, DA022478 and DA022702.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- CNS
central nervous system
- COX-1
cyclooxygenase 1
- COX-2
cyclooxygenase-2
- FAAH
fatty-acid amide hydrolase
- MGL
monoacylglycerol lipase
- PGE2-G
prostaglandin E2 glycerol ester
Conflict of interest
The authors state no conflict of interest.
References
- Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp Ther. 1998;285:1031–1038. [PubMed] [Google Scholar]
- Fowler CJ. The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol. 2007;152:594–601. doi: 10.1038/sj.bjp.0707379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SSJ, Bradshaw HB, Chen JSC, Tan B, Walker JM.Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol that produces hyperalgesia and modulates NFκB activity Br J Pharmacol 20081531538–1549.this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, et al. 15-Lipoxygenase metabolism of 2-arachidonylglycerol: generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66:211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JJ, Rowlinson SW, Prudhomme DR, Marnett LJ. Amino acid determinants in cyclooxygenase-2 oxygenation of the endocannabinoid anandamide. Biochemistry. 2003;42:9041–9049. doi: 10.1021/bi034471k. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- Snider NT, Kornilov AM, Kent UM, Hollenberg PF. Anandamide metabolism by human liver and kidney microsomal cytochrome P450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J Pharmacol Exp Ther. 2007;321:590–597. doi: 10.1124/jpet.107.119321. [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, et al. Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochim Biophys Acta. 1995;1254:127–134. doi: 10.1016/0005-2760(94)00170-4. [DOI] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]