Abstract
Background and purpose:
The finding that obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's stimulatory effect on food intake and gastric emptying has been questioned. The effect of obestatin has been mostly investigated in fasted rodents, a condition associated with high blood levels of ghrelin which may mask the effect of obestatin. We therefore investigated the effect of obestatin on food intake, gastric emptying and gastric contractility in ghrelin knockout mice.
Experimental approach:
The effect of obestatin on 6-h cumulative food intake was studied in fasted wildtype (ghrelin+/+) and ghrelin knockout (ghrelin−/−) mice. In both genotypes, the effect of obestatin and/or ghrelin was studied in vivo on gastric emptying measured with the 14C-octanoic acid breath test and in vitro on neural responses elicited by electrical field stimulation (EFS) of fundic smooth muscle strips.
Key results:
Administration of obestatin did not influence fasting-induced hyperphagia or gastric emptying in both genotypes. Injection of ghrelin accelerated gastric emptying in ghrelin+/+ and ghrelin−/− mice but the effect was not reversed by co-injection with obestatin. In fundic strips from ghrelin+/+ and ghrelin−/− mice, ghrelin increased EFS-induced contractions, but obestatin was without effect. However, co-administration with obestatin tended to reduce the excitatory effect of ghrelin in both genotypes.
Conclusions and implications:
In ghrelin−/− mice, obestatin failed to affect food intake and gastric motility. These results suggest that endogenous ghrelin does not mask the effect of obestatin and confirm that obestatin administered peripherally is not a major regulator of satiety signalling or gut motility.
Keywords: obestatin, food intake, gastric emptying, 14C octanoic breath test, in vitro contractility, ghrelin, ghrelin knockout
Introduction
In 1999, the endogenous ligand of the growth hormone secretagogue receptor was cloned from the stomach and named ghrelin (Kojima et al., 1999). This 28-amino-acid peptide characterized by an unique octanoyl group at Ser3 is not only a positive regulator of the somatotropic axis (Kojima et al., 1999; Wren et al., 2000), but is now considered as the first systemically active orexigenic hormone that induces weight gain by stimulating food intake (Nakazato et al., 2001; Druce et al., 2006) and promoting adipogenesis (Tschop et al., 2000). In addition, ghrelin has profound effects on gastric emptying, which may contribute to appetite signalling (Asakawa et al., 2001; Depoortere et al., 2005; Kitazawa et al., 2005; Tack et al., 2005).
Using a bioinformatic approach, it was recently demonstrated that preproghrelin also encodes another peptide secreted from the stomach, named obestatin (Zhang et al., 2005). This peptide was reported to have effects opposite to those of ghrelin, that is, it inhibits food intake, body weight gain, gastric emptying and jejunal contractility (Zhang et al., 2005). In the same paper, it was claimed that obestatin is the endogenous ligand of the GPR39 receptor, a receptor structurally related to the motilin–ghrelin–neurotensin receptor family of G-protein-coupled receptors. Even before the discovery of obestatin, Moechars et al. (2006) reported altered control of food intake and gastrointestinal motility in GPR39-knockout mice. A few months after its discovery, the biological effect of obestatin and its identity as the natural ligand for GPR39 had already become the subject of intense research and controversy. Indeed, several groups (Lauwers et al., 2006; Chartrel et al., 2007; Holst et al., 2007) reported that obestatin is not the native ligand of the GPR39 receptor, since in their experiments obestatin did not bind to GPR39-transfected cells and did not have any effect in various functional assays in this cell line. In a recent technical comment in Science, Zhang et al. (2007) admitted that their initial batch of obestatin contained impurities. With the purified obestatin they could not repeat obestatin binding and signalling in GPR39 receptor-transfected cells, but could repeat their original in vivo findings (decreased food intake, gastric emptying, body weight gain). However, the vast majority of subsequent studies, except four, could not reproduce the anorexigenic effects of obestatin either after intraperitoneal or after intracerebroventricular administration (Gourcerol and Taché, 2007; Gourcerol et al., 2007). Moreover, convergent evidence is also present for a lack of effect of obestatin on gastrointestinal motility, since none of the studies published so far could mimic the inhibitory effects seen with obestatin on gastric emptying in vivo and on the contractile activity of jejunal muscle strips in vitro (Gourcerol et al., 2006; Bassil et al., 2007; De Smet et al., 2007).
In most studies, the effect of obestatin on food intake and gastric emptying has been investigated in fasted rodents, a condition associated with the highest endogenous circulating levels of ghrelin (Tschop et al., 2000). We hypothesized that during these conditions obestatin's anorexigenic effects may be masked by the orexigenic effects of endogenous ghrelin and may vary according to the time of administration. We therefore tested the effect of obestatin on food intake, gastric emptying and in vitro gastric contractility in wild-type and ghrelin-knockout (ghrelin+/−) mice and investigated whether administration of obestatin could counteract the effects of exogenously applied ghrelin in these models.
Materials and methods
Animals
All animal procedures and experiments were approved by the Ethical Committee for animal experiments of the Catholic University of Leuven.
Male (25–30 weeks/30–35 g) wild-type (ghrelin+/+) and ghrelin−/− mice (C57Bl/6 genetic background) were developed by Lexicon Genetics Incorporated (The Woodlands, TX, USA). The generation of the ghrelin−/− mice was previously described in detail by De Smet et al. (2006). Mice were housed in a temperature-controlled environment (20–22 °C) under a 12 h:12 h light:dark cycle, with lights on at 7 hours and off at 19 hours. Standard commercial mouse chow (Ssniff, Soest, Germany; 12.8 MJ kg−1) and tap water were available ad libitum.
Experimental methods
Effect of obestatin on cumulative food intake
The effect of obestatin on food intake was investigated in ghrelin+/+ (n=7) and ghrelin−/− (n=7) mice in a cross-over design (4-day time interval). Fasted (18 h) mice were injected intraperitoneally (i.p.) with either vehicle (0.9% NaCl) or obestatin (60, 125, 250 or 500 nmol kg−1). The doses were selected on the basis of the doses shown to be effective in the study by Zhang et al. (2007). Mice were group-caged before start of the experiment, and were habituated twice weekly during 3 weeks before the start of the experiment, to the intraperitoneal injections and the single housing. Immediately after the injection, mice were given free access to a pre-weighed amount of food. Food intake was measured at 30 min, 1, 2, 3, 4, 5 and 6 h after the injection. Cumulative food intake was calculated and plotted as a function of time.
Effect of obestatin on gastric emptying
Gastric emptying was measured in ghrelin+/+ (n=12) and ghrelin−/− mice (n=12) by the 14CO2 octanoic acid breath test as described by Kitazawa et al. (2005). On the day of the experiment, fasted mice (overnight for 19 h with free access to water) were injected i.p. with 0.1 ml vehicle (0.9% NaCl), ghrelin (60 nmol kg−1), obestatin (60, 125 or 250 nmol kg−1) or the combination of ghrelin (60 nmol kg−1) and obestatin (125 nmol kg−1). After injection, the mice were put in an airtight plastic tube, which was adapted with an inlet valve and through which a continuous airflow (80% N2, 20% O2, 360 ml min−1) was maintained. The air outflow was bubbled through a vial containing a CO2 trapper. Fifteen minutes after injection, the mice received a test meal (0.2 g) of chow and baked egg yolk, doped with 0.01 μCi 14C octanoic acid. Sampling of the exhaled breath was performed every 5 min during the first 30 min and then every 15 min for the next 3.5 h. From the 14CO2 excretion curve, two parameters, gastric half excretion time (Thalf) (time at which 50% of the total amount of 14CO2 was excreted) and lag time (Tlag) (initial delay in gastric emptying due to the time required for the stomach to grind the meal into fine particles), were calculated as described earlier (Kitazawa et al., 2005). The time interval between two breath tests was set at 3–4 days. The mice were trained to adapt to the experimental conditions before the start of the experiments.
Contractility studies
Ghrelin+/+ (n=8) or ghrelin−/− (n=8) mice were killed by cervical dislocation. The stomach was removed and rinsed with ice-cold saline. Fundic muscle strips (10 × 2 mm) freed from mucosa were cut and suspended along their circular axis in an organ bath containing Krebs buffer (37 °C) (120.9 mM NaCl, 2 mM NaH2PO4, 15.5 mM NaHCO3, 5.9 mM KCl, 1.25 mM CaCl2, 1.2 mM MgCl2, 11.5 mM glucose) gassed with 95% O2 and 5% CO2. After equilibration at optimal stretch, electrical field stimulation (EFS) was applied via two parallel platinum rod electrodes with a Grass S88 stimulator (Grass, Quincy, MA, USA). Contractions were measured with an isometric force transducer/amplifier (Harvard Apparatus, South Natick, MA, USA), recorded on a multicorder and sampled for digital analysis with the Windaq data acquisition system and a DI-2000 PGH card (Dataq Instruments, Akron, OH, USA).
The maximal contractility of each strip was investigated by applying pulse trains (pulse 1 ms, train 10 s, 6 V) at increasing frequencies of stimulation (0.5, 1, 2, 4, 8, 16 and 32 Hz). Each pulse train was followed by a 90-s interval. The effect of obestatin and ghrelin was tested during continuous stimulation. The frequency of EFS was set to obtain sub-maximal off-contractions (between 30 and 60% of the maximum as determined from the individual frequency spectra). When stable responses were obtained, obestatin (1 nM or 1 μM) and/or ghrelin (1 μM) were added to the tissue bath to investigate their effect on neuro-effector transmission.
For data analysis, following compound administration the averages of five consecutive off-responses (calculated as area under the curve) to EFS stimulation were measured over a 50-min period to obtain five separate data points. The change in response was then calculated by subtracting the baseline activity determined by averaging the mean of five consecutive off-responses before addition of the compounds. All responses were normalized for the cross-sectional area of the strips and expressed in g mm−2.
Statistical analysis
Data are expressed as mean±s.e.mean. The effect of exogenous obestatin or ghrelin on food intake, gastric emptying and contractility changes was analysed by repeated-measures analysis of variance analysis, with one (treatment) or two (time and treatment) repeated-measures factors. In case of significant factor effects, a Newman–Keuls post hoc test was performed. Data were analysed with Statistica 6.0 (StatSoft, Tulsa, OK, USA) and significance was accepted at the P<0.05 level.
Materials
Obestatin (H2N-FNAPFDVGIKLSGAQYQQHGRAL-CONH2; Eurogentec, Seraing, Belgium) and rat ghrelin (Tocris Bioscience, Bristol, UK) were dissolved in 0.9% NaCl and 0.1% bovine serum albumin, aliquoted and stored at −20 °C. Immediately before the start of the experiments, obestatin and ghrelin were diluted in 0.9% NaCl to reach the final concentration.
Results
Effect of obestatin on food intake in ghrelin+/+ and ghrelin−/− mice
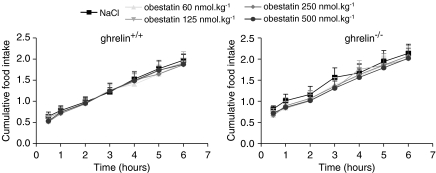
The effect of intraperitoneal injection of obestatin was tested in ghrelin+/+ and ghrelin−/− mice fasted for 18 h. In vehicle-treated mice, food intake did not differ (P=0.19) during the first 30 min after an overnight fast, and amounted to 0.64±0.10 and 0.82±0.07 g, respectively, in ghrelin+/+ and ghrelin−/− mice. Also the cumulative feeding response after 6 h did not differ between both genotypes (ghrelin+/+: 1.97±0.15 g, ghrelin−/−: 2.14±0.22 g). Injection of obestatin at doses ranging between 60 and 500 nmol kg−1 did not influence fasting-induced hyperphagia monitored between 30 min and 6 h post-injection in either the mutant or the wild-type mice (Figure 1).
Figure 1.
Effect of obestatin on cumulative food intake in fasted ghrelin+/+ and ghrelin−/− mice. Saline or obestatin (60, 125, 250, 500 nmol kg−1) was injected i.p. in overnight fasted ghrelin+/+ and ghrelin−/− mice and food intake was monitored during 6 h. Results are the mean±s.e.mean of seven mice from each genotype. I.p., intraperitoneally.
Effect of obestatin on gastric emptying in ghrelin+/+ and ghrelin−/− mice
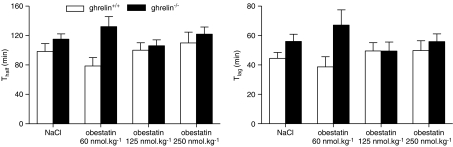
In vehicle-injected ghrelin+/+ and ghrelin−/− mice, the Thalf and Tlag did not differ significantly between both genotypes (Figure 2). Injection of obestatin at doses ranging between 60 and 250 nmol kg−1 did not affect the gastric emptying parameters either in the knockout mice (P=0.34) or in their wild-type littermates (P=0.26) (Figure 2).
Figure 2.
Effect of obestatin on gastric emptying in ghrelin+/+ and ghrelin−/− mice. Saline or obestatin (60, 125, 250 nmol kg−1) was injected i.p. in overnight fasted ghrelin+/+ and ghrelin−/− mice. The 14C octanoic acid-enriched solid meal was given 15 min after injection and gastric emptying was measured with the 14CO2 breath test during the next 4 h. Thalf (left) and Tlag (right) were calculated from the CO2 excretion curves. Results are the mean±s.e.mean of 12 mice from each genotype. I.p., intraperitoneally; Thalf, gastric half excretion time; Tlag, lag time.
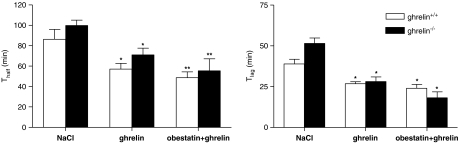
Administration of ghrelin at 60 nmol kg−1 accelerated gastric emptying and decreased Thalf in ghrelin+/+ (P<0.05) and ghrelin−/− mice (P<0.01) (Figure 3). To investigate whether obestatin could antagonize ghrelin's effect on gastric emptying, obestatin (125 nmol kg−1) was co-injected with ghrelin (60 nmol kg−1). Co-injection with obestatin could not reverse the effect of ghrelin on gastric emptying. Emptying was still accelerated in ghrelin+/+ (P=0.52 vs ghrelin alone) and ghrelin−/− mice (P=0.15 vs ghrelin alone) (Figure 3). Similar results were observed for Tlag (Figure 3).
Figure 3.
Effect of obestatin on the pro-kinetic effects of ghrelin in ghrelin+/+ and ghrelin−/− mice. Saline, ghrelin (60 nmol kg−1) or a combination of ghrelin (60 nmol kg−1) and obestatin (125 nmol kg−1) was injected i.p. in overnight fasted ghrelin+/+ and ghrelin−/− mice. The 14C octanoic acid-enriched solid meal was given 15 min after injection and gastric emptying was measured with the 14CO2 breath test during the next 4 h. Thalf (left) and Tlag (right) were calculated from the CO2 excretion curves. Results are the mean±s.e.mean of 12 mice from each genotype. *P<0.05; **P<0.01 vs NaCl-injected mice. I.p., intraperitoneally; Thalf, gastric half excretion time; Tlag, lag time.
Effect of obestatin on neural smooth-muscle responses in fundic strips from ghrelin+/+ and ghrelin−/− mice
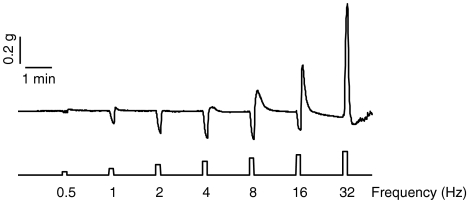
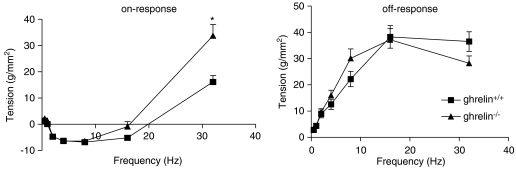
Electrical field stimulation of fundic strips from wild-type mice resulted in on-relaxations, on-contractions and off-contractions (Figure 4). The magnitude of the on-relaxations, which appeared between 2 and 16 Hz, was similar in both genotypes. On-contractions were only apparent at 32 Hz and were higher in ghrelin−/− than in ghrelin+/+ mice (Figure 5). No significant differences were observed between both genotypes regarding the off-contractions, which appeared after cessation of stimulation over the entire frequency spectrum (Figure 5).
Figure 4.
Representative tracing of responses elicited by EFS of fundic smooth-muscle strips from ghrelin+/+ mice at increasing frequencies of stimulation. EFS, electrical field stimulation.
Figure 5.
Comparison of the responses elicited by EFS of fundic smooth-muscle strips from ghrelin+/+ and ghrelin−/− mice. Data shown are the tension generated by the strips during (on-response) or after (off-response) increasing frequencies of stimulation. Results are the mean±s.e.mean of eight mice from each genotype. *P<0.01 vs the response in ghrelin+/+ mice. EFS, electrical field stimulation.
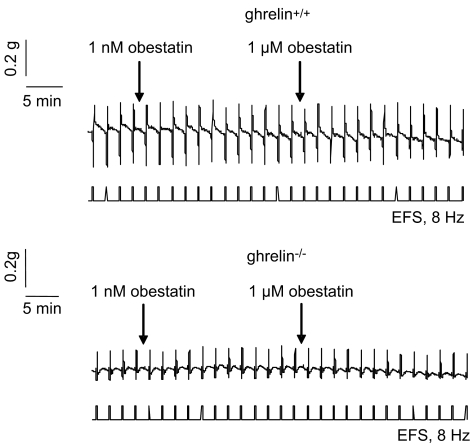
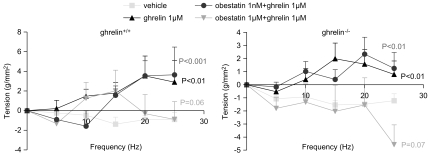
The effect of obestatin was investigated during continuous stimulation at 8 Hz in strips from ghrelin+/+ and ghrelin−/− mice. A representative tracing is shown in Figure 6. Obestatin at 1 nM or 1 μM did not affect EFS-induced neural off-responses in strips from both genotypes (Figure 6). The effect of obestatin on the excitatory effects induced by ghrelin was also tested. Ghrelin (1 μM) increased EFS-induced off-responses (P<0.01 vs vehicle) in both genotypes (Figure 7). Co-administration of 1 μM ghrelin with 1 nM of obestatin still enhanced EFS-induced off-contractions in both ghrelin+/+ (P<0.001 vs vehicle) and ghrelin−/− mice (P<0.01 vs vehicle) (Figure 7). In contrast, in the presence of 1 μM obestatin, the excitatory effect of 1 μM ghrelin tended to be blocked in ghrelin+/+ (P=0.06 vs vehicle) and ghrelin−/− (P=0.07 vs vehicle) mice, but this did not reach statistical significance.
Figure 6.
Representative tracing showing the effect of obestatin on neural responses in fundic smooth-muscle strips from ghrelin+/+ and ghrelin−/− mice. Strips from mouse fundus were continuously stimulated by EFS at 8 Hz. When stable responses were obtained, obestatin (1 nM) was added and 12 stimulations later obestatin (1 μM). EFS, electrical field stimulation.
Figure 7.
Interactions between obestatin and ghrelin on EFS-induced neural responses in fundic smooth-muscle strips from ghrelin+/+ and ghrelin−/− mice. Data shown are the tension (g mm−2) of the off-responses generated after continuous stimulation at 8 Hz of mouse fundic strips after administration of saline, ghrelin (1 μM) or co-administration of ghrelin (1 μM) with obestatin (1 nM or 1 μM). Results are the mean±s.e.mean of eight mice from each genotype. P-values are calculated vs the vehicle response in each genotype. EFS, electrical field stimulation.
Discussion
In both wild-type and ghrelin−/− mice, peripheral administration of obestatin failed to inhibit food intake and gastric motility in vivo and in vitro. It is therefore unlikely that endogenous ghrelin levels counteract and mask the effect of exogenously applied obestatin. Although in vitro obestatin tended to reduce the excitatory neural responses evoked by ghrelin in fundic strips, in vivo obestatin (intraperitoneal) failed to block the pro-kinetic effects of ghrelin on gastric emptying. Our results therefore suggest that obestatin administered peripherally is not an important physiological opponent of ghrelin and is not a major regulator of satiety signalling and gut motility.
A positive control showing that we were able to detect changes in food intake was not included in the current experiment. Nevertheless, in a previous study (De Smet et al., 2006), using the same strain of ghrelin+/+ and ghrelin−/− mice, we have shown that ghrelin is able to stimulate both food intake and gastric emptying in these mice in a dose-dependent manner. Hence, these results emphasize that we are able to detect changes in food intake in these mice, and that in addition ghrelin−/− mice display an intact orexigenic and gastric motor-stimulating pathway. It is therefore highly unlikely that the lack of effect observed with obestatin is related to the fact that the mice have become insensitive to anorexigenic peptides. We would rather conclude that our negative findings are related to an absence of a physiological role of obestatin. Although we have selected several doses of obestatin, it cannot be excluded that a shift in sensitivity to anorexigenic peptides may have occurred. However, it should be stressed that also in ghrelin+/+ mice, obestatin at doses effective in the study of Zhang et al. (2005) was without effect.
Our observations in wild-type mice are in line with the vast majority of previous studies, which failed to reproduce the anorexigenic properties of obestatin as initially reported by Zhang et al. (2005). Indeed, De Smet et al. (2007) showed that obestatin, injected i.p. at a dose that was efficient in Zhang's report, did not affect food intake in fasted mice. Similar findings were reported by Gourcerol et al. (2006) in rats and mice. In addition these authors showed that obestatin did not modify the inhibition of food intake elicited by the satiety signal, cholecystokinin. Other studies failed to reproduce the inhibitory effects on food intake after intracerebroventricular injection of obestatin during either acute or chronic treatment, a condition which was not tested in the present study (Nogueiras et al., 2007; Samson et al., 2007; Yamamoto et al., 2007).
Positive effects were observed with obestatin on food intake only in five (Zhang et al., 2005; Bresciani et al., 2006; Sibilia et al., 2006; Carlini et al., 2007; Lagaud et al., 2007) out of 14 studies (Gourcerol et al., 2006, 2007; Seoane et al., 2006; De Smet et al., 2007; Holst et al., 2007; Nogueiras et al., 2007; Samson et al., 2007; Tremblay et al., 2007; Yamamoto et al., 2007; Zizzari et al., 2007) and in some studies even with restrictions. Indeed, Sibilia et al. (2006) showed that intracerebroventricular infusion of obestatin significantly decreased food consumption only in fed rats during the first day of treatment, and that long-term administration of obestatin did not affect body weight.
From the above findings it appears that differences in species (rat/mouse), route of administration or feeding condition cannot explain the negative findings. Also with other anorexigenic peptides, such as PYY3−36, the majority of studies failed to show robust, reproducible effects in rodents in certain laboratories (Tschop et al., 2004), whereas the effect could be observed in other laboratories and in humans (Batterham et al., 2002; Halatchev et al., 2004; Degen et al., 2005). Apparently the same story holds true for obestatin.
The effects of obestatin on gastrointestinal motility are less well studied but are all negative. Gourcerol et al. (2006) showed that in rats, obestatin (i.p.) did not influence gastric emptying of a viscous non-caloric meal, nor did it delay the gastric emptying induced by intraperitoneal injection of cholecystokinin. Neither did obestatin (intravenous) affect the phasic nor the tonic motility of the stomach. To prevent rapid degradation of obestatin, Bassil et al. (2007) studied the effects of obestatin on gastric emptying of a liquid meal and on small bowel motility during continuous infusion but without result. Also in vitro, obestatin failed to affect the contractility of mouse and rat smooth muscle strip preparations from stomach or small intestine under different experimental conditions (De Smet et al., 2007). In the present study we showed that also in ghrelin−/− mice obestatin does not affect gastric contractility in vitro.
Zhang et al. (2007) claimed that the timing of the administration of obestatin relative to the presentation of food is important and should be exactly 15 min. In addition, Lagaud et al. (2007) showed that obestatin has an unusual U-shaped dose–response curve in vivo and is only effective over a narrow range of doses. In our food intake experiments, food was made available immediately after injection of obestatin, but not in our gastric emptying studies, where mice received food exactly 15 min after injection of obestatin. In addition, our doses were chosen within the active range.
Zhang et al. (2005) showed that obestatin behaves as a physiological antagonist and counteracts the effect of ghrelin. In the present study, we reported that obestatin was not able to counteract the pro-kinetic effect of ghrelin in vivo on gastric emptying either in the wild type or in the ghrelin−/− mice. However, the inability of obestatin to reduce the pro-kinetic activity of ghrelin in vivo contrasts with the in vitro findings. In both ghrelin+/+ and ghrelin−/− mice, obestatin at 1 μM, but not 1 nM, tended to reduce the excitatory response of ghrelin on neural responses in smooth muscle strip preparations. Similar findings have been reported in rats (Bassil et al., 2007). The reason for this discrepancy between in vivo and in vitro experiments is unclear. It is possible that the ability of obestatin to antagonize ghrelin's effect depends upon the relative concentrations of other orexigenic/anorexigenic factors at the time of injection. In vivo this may be more relevant than in vitro.
In the present study, the possibility that obestatin could counteract the orexigenic effect of ghrelin was not evaluated as we tested the effect of obestatin during the fasted state, a condition during which ghrelin does not stimulate food intake. Although Zizzari et al. (2007) showed that exogenous obestatin per se did not modify food intake in fasted and fed mice, these authors reported that obestatin can inhibit ghrelin's effects on food intake, but only in fed mice.
Furthermore, our data show that ablation of the ghrelin gene did neither affect food intake nor gastric contractility in vivo and in vitro. This suggests that endogenous ghrelin rather plays a redundant role in the regulation of food intake and gastric emptying and that its loss may be compensated by other redundant inputs. Nevertheless, in a previous study, we have shown that some subtle, age-dependent phenotypic changes occur in ghrelin−/− mice (De Smet et al., 2006). Especially in young animals, endogenous ghrelin seems to be involved in the selection of energy stores and in the partitioning of metabolizable energy between storage and dissipation as heat.
The effect of obestatin has not been tested yet in humans. A recent study showed that fasted obestatin levels were lower in obese patients, in patients with type 2 diabetes and in patients with impaired glucose regulation, than in controls, suggesting a role for obestatin in appetite regulation in these patients (Guo et al., 2007; Huda et al., 2007; Qi et al., 2007). However, contradictory findings were reported on the effect of food intake on obestatin levels (Guo et al., 2007; Huda et al., 2007). For a hormone postulated to affect food intake acutely, clear changes in circulating concentrations postprandially are essential. Recently, the existence of obestatin in mammalians was questioned again (Bang et al., 2007). These authors found no evidence for obestatin peptides, circulating as distinct entities in the human and rat circulation, or as a secretable hormone in rat tissues.
If obestatin does not play an important role in the regulation of food intake and gastrointestinal motility, it may have other physiological effects. Obestatin has been shown to suppress drinking responses (Samson et al., 2007), improve memory (Carlini et al., 2007), decrease growth hormone secretion in vivo (Zizzari et al., 2007), regulate sleep (Szentirmai and Krueger, 2006), activate cortical neurons (Dun et al., 2006) and stimulate proliferation of retinal pigment epithelial cells (Camina et al., 2007). In most cases these findings represent single observations and further studies are warranted to explore these effects, at least if the existence of obestatin as a unique endogenous peptide can be confirmed.
Acknowledgments
We thank L Nys for skilful technical assistance. This work was supported by grants from the Flemish Foundation for Scientific Research (contracts FWO G.0144.04 and 1.5.125.05) and the Belgian Ministry of Science (contracts GOA 03/11 and IUAP P5/20).
Abbreviations
- EFS
electrical field stimulation
- ghrelin−/−
ghrelin knockout
- ghrelin+/+
ghrelin wild type
- Thalf
gastric half excretion time
- Tlag
lag time
Conflict of interest
I Depoortere, T Thijs, B De Smet, TL Peeters have no conflict of interest. D Moechars and L Verdonck are employees of Johnson & Johnson Research and Development.
References
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Bang AS, Soule SG, Yandle TG, Richards AM, Pemberton CJ. Characterisation of proghrelin peptides in mammalian tissue and plasma. J Endocrinol. 2007;192:313–323. doi: 10.1677/JOE-06-0021. [DOI] [PubMed] [Google Scholar]
- Bassil AK, Haglund Y, Brown J, Rudholm T, Hellstrom PM, Naslund E, et al. Little or no ability of obestatin to interact with ghrelin or modify motility in the rat gastrointestinal tract. Br J Pharmacol. 2007;150:58–64. doi: 10.1038/sj.bjp.0706969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Bresciani E, Rapetti D, Dona F, Bulgarelli I, Tamiazzo L, Locatelli V, et al. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest. 2006;29:RC16–RC18. doi: 10.1007/BF03344175. [DOI] [PubMed] [Google Scholar]
- Camina JP, Campos JF, Caminos JE, Dieguez C, Casanueva FF. Obestatin-mediated proliferation of human retinal pigment epithelial cells: regulatory mechanisms. J Cell Physiol. 2007;211:1–9. doi: 10.1002/jcp.20925. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Schioth HB, Debarioglio SR. Obestatin improves memory performance and causes anxiolytic effects in rats. Biochem Biophys Res Commun. 2007;352:907–912. doi: 10.1016/j.bbrc.2006.11.112. [DOI] [PubMed] [Google Scholar]
- Chartrel N, Alvear-Perez R, Leprince J, Iturrioz X, Reaux-Le Goazigo A, Audinot V, et al. Comment on ‘Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake' Science 2007315766author reply 766 [DOI] [PubMed] [Google Scholar]
- De Smet B, Depoortere I, Moechars D, Swennen Q, Moreaux B, Cryns K, et al. Energy homeostasis and gastric emptying in ghrelin knockout mice. J Pharmacol Exp Ther. 2006;316:431–439. doi: 10.1124/jpet.105.091504. [DOI] [PubMed] [Google Scholar]
- De Smet B, Thijs T, Peeters TL, Depoortere I. Effect of peripheral obestatin on gastric emptying and intestinal contractility in rodents. Neurogastroenterol Motil. 2007;19:211–217. doi: 10.1111/j.1365-2982.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, et al. Effect of peptide YY3–36 on food intake in humans. Gastroenterology. 2005;129:1430–1436. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005;515:160–168. doi: 10.1016/j.ejphar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Druce MR, Neary NM, Small CJ, Milton J, Monteiro M, Patterson M, et al. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int J Obes (London) 2006;30:293–296. doi: 10.1038/sj.ijo.0803158. [DOI] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Brailoiu E, Yang J, Chang JK, Dun NJ. Distribution and biological activity of obestatin in the rat. J Endocrinol. 2006;191:481–489. doi: 10.1677/joe.1.06944. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, et al. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides. 2006;27:2811–2819. doi: 10.1016/j.peptides.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, Taché Y. Obestatin—a ghrelin-associated peptide that does not hold its promise to suppress food intake and motility. Neurogastroenterol Motil. 2007;19:161–165. doi: 10.1111/j.1365-2982.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Gourcerol G, St-Pierre DH, Taché Y. Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP) Regul Pept. 2007;141:1–7. doi: 10.1016/j.regpep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Guo ZF, Zheng X, Qin YW, Hu JQ, Chen SP, Zhang Z. Circulating preprandial ghrelin to obestatin ratio is increased in human obesity. J Clin Endocrinol Metab. 2007;92:1875–1880. doi: 10.1210/jc.2006-2306. [DOI] [PubMed] [Google Scholar]
- Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3–36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145:2585–2590. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148:13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- Huda MS, Durham BH, Wong SP, Deepak D, Kerrigan D, McCulloch P, et al. Plasma obestatin levels are lower in obese and post-gastrectomy subjects, but do not change in response to a meal Int J Obes (London) 2007. Jul 31; e-pub ahead of print [DOI] [PubMed]
- Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL. Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut. 2005;54:1078–1084. doi: 10.1136/gut.2005.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Lagaud GJ, Young A, Acena A, Morton MF, Barrett TD, Shankley NP. Obestatin reduces food intake and suppresses body weight gain in rodents. Biochem Biophys Res Commun. 2007;357:264–269. doi: 10.1016/j.bbrc.2007.03.138. [DOI] [PubMed] [Google Scholar]
- Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun. 2006;351:21–25. doi: 10.1016/j.bbrc.2006.09.141. [DOI] [PubMed] [Google Scholar]
- Moechars D, Depoortere I, Moreaux B, de Smet B, Goris I, Hoskens L, et al. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology. 2006;131:1131–1141. doi: 10.1053/j.gastro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, et al. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology. 2007;148:21–26. doi: 10.1210/en.2006-0915. [DOI] [PubMed] [Google Scholar]
- Qi X, Li L, Yang G, Liu J, Li K, Tang Y, et al. Circulating obestatin levels in normal subjects and in patients with impaired glucose regulation and type 2 diabetes mellitus. Clin Endocrinol (Oxford) 2007;66:593–597. doi: 10.1111/j.1365-2265.2007.02776.x. [DOI] [PubMed] [Google Scholar]
- Samson WK, White MM, Price C, Ferguson AV. Obestatin acts in brain to inhibit thirst. Am J Physiol Regul Integr Comp Physiol. 2007;292:R637–R643. doi: 10.1152/ajpregu.00395.2006. [DOI] [PubMed] [Google Scholar]
- Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest. 2006;29:RC13–RC15. doi: 10.1007/BF03344174. [DOI] [PubMed] [Google Scholar]
- Sibilia V, Bresciani E, Lattuada N, Rapetti D, Locatelli V, De Luca V, et al. Intracerebroventricular acute and chronic administration of obestatin minimally affect food intake but not weight gain in the rat. J Endocrinol Invest. 2006;29:RC31–RC34. doi: 10.1007/BF03349204. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Krueger JM. Obestatin alters sleep in rats. Neurosci Lett. 2006;404:222–226. doi: 10.1016/j.neulet.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology. 2007;148:501–506. doi: 10.1210/en.2006-1275. [DOI] [PubMed] [Google Scholar]
- Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, et al. Physiology: does gut hormone PYY3–36 decrease food intake in rodents Nature 20044301following 165; discussion 2 p following 165 [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Ikeshita N, Daito R, Herningtyas EH, Toda K, Takahashi K, et al. Neither intravenous nor intracerebroventricular administration of obestatin affects the secretion of GH, PRL, TSH and ACTH in rats. Regul Pept. 2007;138:141–144. doi: 10.1016/j.regpep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang JV, Klein C, Pei-Gen R, Kass S, Ver Donck L, Moechars D, et al. Response to comment on ‘Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake'. Science. 2007;315:766. doi: 10.1126/science.1135047. [DOI] [PubMed] [Google Scholar]
- Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology. 2007;148:1648–1653. doi: 10.1210/en.2006-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]