Abstract
Despite progression in anticancer drug development and improvements in the clinical utilization of therapies, current treatment regimes are still dependent upon the use of systemic antiproliferative cytotoxic agents. Although these agents are unquestionably potent, their efficacy is limited by toxicity towards ‘normal' cells and a lack of tumour selective targeting, resulting in a therapeutic index which is modest at best. Consequently, the development of more tumour selective cancer treatments, with better discrimination between tumour and normal cells is unequivocally an important goal for cancer drug discovery. One such strategy is to exploit the tumour phenotype as a mechanism for tumour-selective delivery of potent therapeutics. An exciting approach in this area is to develop anticancer therapeutics as prodrugs, which are non-toxic until activated by enzymes localized specifically in the tumour. Enzymes suitable for tumour-activated prodrug development must have increased activity in the tumour relative to non-diseased tissue and an ability to activate the prodrug to its active form. One class of enzyme satisfying these criteria are the tumour endoproteases, particularly the serine- and metallo-proteases. These proteolytic enzymes are essential for tumour angiogenesis, invasion and metastasis, the major defining features of malignancy. This review describes the concept behind development of tumour-endoprotease activated prodrugs and discusses the various studies to date that have demonstrated the huge potential of this approach for improvement of cancer therapy.
Keywords: cancer therapy, endoprotease, prodrug, drug delivery, matrix metalloproteinase (MMP), prostate-specific antigen (PSA), cancer pharmacology
Introduction
Despite vast improvements in detection, diagnosis and treatment over the past decade, cancer still remains one of the most frequent causes of death worldwide (Varmus, 2006). Therefore, new more effective treatment strategies are required to impact upon this issue. To date, surgical resection remains the number one priority for treatment of solid tumours. Despite this, for the majority of solid tumours, cure by surgical resection is not feasible, either as a consequence of tumour accessibility, tumour pathology or the presence of tumour spread and metastasis. Therefore, chemotherapeutic interventions are required and essential both as a treatment in their own right and as an adjuvant to localized treatment such as surgery and radiotherapy. While many advances have been made in the area of anticancer drug development (reviewed in Newell, 2005; Collins and Workman, 2006), classical antiproliferative cytotoxic agents still form the basis of many current treatment regimens (Collins and Workman, 2006). Despite being potent with the ability to kill large number of tumour cells, the clinical efficacy of these classical agents is limited by their unavoidable toxicity to ‘normal' cells and their lack of tumour-selective targeting. With such compounds, the therapeutic index for tumour versus normal tissue is modest, toxic side effects are the norm and the development of resistance often occurs. As such, most currently utilized anticancer agents have a very small therapeutic index based on the high frequency of systemic toxicities and the need for large concentrations of active drug at the tumour site (Verweij and de Jonge, 2000). Consequently, a plateau of effectiveness has been reached with these agents.
An increased understanding of the molecular basis of cancer has led to many advancements in cancer chemotherapy, which has now entered an era of targeted molecular therapeutics—agents exploiting defined abnormalities responsible for the causation, maintenance, expansion or metastatic potential of cancer (reviewed in Newell (2005) and Collins and Workman (2006)). The development of new therapeutics with increased tumour selectivity, better discrimination between tumour and normal tissue, lower systemic toxicity and thus a larger therapeutic index, is possibly the most important aim of current cancer drug discovery. One such strategy is the development of agents to perturb the dysregulated molecular pathways involved in cancer development and progression, for example, Gleevec (imatinib) and Zolinza (vorinostat), resulting in therapies directed at the precise molecular pathology driving progression of specific tumour types (reviewed in Newell (2005) and Collins and Workman (2006)). Despite these successes, the identification of such critical targets for drug development is complicated due to both the characteristic genetic instability of cancer and the diverse nature of genetic changes involved in the tumorigenic process (Huang and Oliff, 2001; Collins and Workman, 2006).
An alternative strategy for more effective and targeted cancer therapeutics is the development of agents that take advantage of the tumour phenotype rather than intracellular signalling pathways, that is differential cell–matrix interactions, altered cell surface receptor expression and increased capacity for proteolytic degradation (Huang and Oliff, 2001). In this respect, epidermal growth factor receptor has been demonstrated as a clinically viable drug target as evidenced by the success of the small molecule drugs Iressa (gefitinib) and Tarceva (erlotinib) (Collins and Workman, 2006).
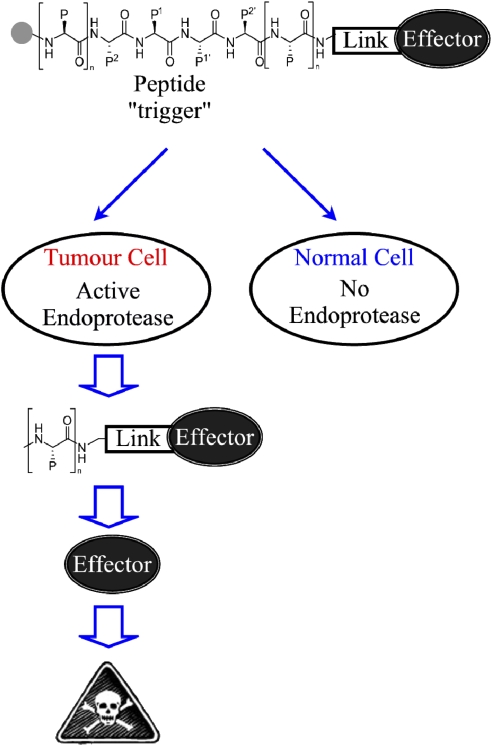
A further strategy to increase drug targeting and decrease systemic toxicities is to deliver potent chemotherapeutics selectively to their intended site, that is the tumour microenvironment. One such approach is the development of therapeutic agents as non-toxic prodrugs, termed tumour-activated prodrugs (TAPs), in a form whereby the potent therapeutic entity is masked until being activated by exploitation of unique phenotypic differences present in the tumour environment (depicted in Figure 1) (Denny, 2001).
Figure 1.
Schematic representation of tumour endoprotease-activated prodrugs (TAPs). The prodrug is composed of three domains: a potent therapeutic agent (effector), an endoprotease-cleavable peptide sequence (trigger) and a linker to join the trigger to the effector. This TAP remains inactive until activation of the trigger. The presence of the endoprotease in the tumour but not in the ‘normal' tissues results in activation of the TAP trigger selectively in the tumour, causing the release of the potent effector and a subsequent therapeutic effect.
Classically, the term prodrug encompasses all compounds that are activated after administration either enzymatically, chemically or spontaneously to form a pharmacologically active species. In this sense, the concept of prodrugs within cancer chemotherapy is not new as demonstrated by cyclophosphamide, a clinically utilized prodrug of a nitrogen mustard alkylating agent activated in the liver by the cytochrome P450 enzyme system (Torkelson et al., 1974; Connors, 1986; Boddy and Yule, 2000). In the context of cyclophosphamide and the majority of cancer chemotherapeutics classified as prodrugs to date, these agents are not designed as TAPs, but rather as derivatives to modify drug uptake or pharmacokinetics. Unlike the TAP strategy, the use of prodrugs such as these is still limited by normal tissue toxicities due to their potential activation outside of the tumour environment (Rooseboom et al., 2004). As such, this family of prodrugs can be regarded as that in which the pharmacological properties of the active drug are modified to facilitate increased tumour delivery rather than those which are directed specifically at the tumour (Rooseboom et al., 2004; Bruno and Njar, 2007; Ganesh, 2007).
With the rapid expansion in our understanding of the molecular pathology of cancer and improved knowledge of classical and more recently targeted cancer therapies, we now have a robust framework by which to progress the development and assessment of therapeutics directed to the tumour for selective conversion to active therapeutic agents, specifically TAPs.
Tumour-activated prodrugs
As mentioned above, TAPs are systemic compounds that are activated selectively in tumour tissue by exploiting a unique physiological, metabolic or genetic difference between tumour and normal cells (Denny, 2001). TAPs must undergo selective cellular metabolism in tumours to generate their potent therapeutic agent. In general, TAPs are comprised of a minimum of three domains: trigger, linker and effector, where the trigger (controlling selectivity) is joined to the effector (responsible for therapeutic effect) by a linker, the prodrug remaining non-toxic until activation of the trigger (shown in Figure 1) (Denny, 2001).
The primary step in the development of TAPs is the identification of enzymes capable of metabolizing these agents selectively within tumour tissue. There is considerable evidence demonstrating differential expression of a panoply of enzymes between normal and tumour tissue, although consistent patterns of enzyme upregulation so far remain elusive (Denny, 2001). The ideal characteristics of tumour enzymes suitable for targeting by TAPs are as follows:
good characterization of the enzyme or family of enzymes possessing a known role in tumour development and/or progression;
demonstration of a high affinity of the enzyme for the prodrug;
significantly elevated expression in the disease state and activity in the tumour environment;
low or negative expression and lack of enzymatic activity in non-diseased tissue;
no presence of the enzyme in a prodrug-activating form in patient serum and
capability of the enzyme for selective and rapid activation of the prodrug.
In the context of TAPs, several phenotypic characteristics of the tumour have been suggested as selective prodrug targets involving a range of endogenous enzyme classes including oxidoreductases, transferases, hydrolases and lyases (reviewed in Rooseboom et al., 2004). An example of one area that has attracted considerable attention as a target for TAP therapeutics is tumour hypoxia, induced as a consequence of the poorly defined vascular network of many solid tumours. Bioreductively activated prodrugs for example, tirapazamine and banoxantrone (AQ4N) (Patterson and McKeown, 2000; McKeown et al., 2007), specifically target hypoxic cells by becoming selectively activated under low oxygen tension by enzymes including NQ01 and cytochrome P450 reductase (McKeown et al., 2007). This class of TAP is showing great promise in cancer clinical trials (McKeown et al., 2007). Targeting enzymes centrally involved in the main defining features of cancer is an attractive strategy for TAP development as these will provide optimal targets for TAPs by virtue of the phenotypic differences between normal and tumour cells. One class of enzyme that satisfies all criteria for TAP development and has been heavily implicated in tumour development and progression is the proteolytic endoproteases, the main focus of this review.
Central involvement of endoproteases in cancer development
The major defining features of malignant tumours are their ability to acquire an improved vasculature system, penetrate into surrounding normal tissues and disseminate to distant sites. Each one of these processes relies heavily upon the increased expression and activity of diverse extracellular endoproteases from multiple enzymatic classes, namely the metalloproteases and the serine, threonine, cysteine and aspartic proteases. These proteases constitute the cancer degradome—the repertoire of proteases that cells and tissues coordinately regulate to modulate their local environment (Lopez-Otin and Overall, 2002; Overall and Blobel, 2007). These endoproteases are frequently upregulated within tumour tissue where they promote the development and expansion of the tumour, formation of new blood vessels to support the burgeoning energy demands of the rapidly growing tumour and facilitate the metastasis of cancer cells to distant organs (Egeblad and Werb, 2002).
It has long been recognized that cellular invasion of basement membranes and connective tissue stroma and angiogenesis and metastasis involve the actions of diverse extracellular proteases, particularly the matrix metalloproteinase (MMP) and serine protease families. The initial belief that these proteases function solely to mediate tissue destruction and clear an invasive path allowing cell migration and thus tumour expansion is now known to be oversimplistic, with these enzymes having major roles in growth factor activation, cellular adhesion, cellular survival and immune surveillance, to name a few (McCawley and Matrisian, 2001; Egeblad and Werb, 2002; Hojilla et al., 2003). There is now a considerable body of literature demonstrating elevated expression of many of these endoproteases in primary tumours and metastases, with the majority demonstrating an association with tumour progression or validity as prognostic indicators of clinical outcome (Brinckerhoff et al., 2000; Kamat et al., 2006; Vizoso et al., 2007). Taken together their broad but essential role in tumorigenesis and their relationship to disease prognosis, these enzymes can be defined as a significant force in the phenotypic evolution of cancer.
Tumour endoproteases as targets for tumour-selective anticancer drug delivery
The involvement of endoproteases in tumour development, expansion and metastasis is unequivocal, as outlined above and reviewed extensively elsewhere (Egeblad and Werb, 2002; Borgono et al., 2004; Lee et al., 2004; Mohamed and Sloane, 2006; Overall and Kleifeld 2006; Overall and Blobel, 2007). In terms of drug development, proteases provide a significant opportunity as they account for approximately 2% of mammalian genes and represent 5% of all drug targets (Lee et al., 2004; Overall and Dean, 2006; Overall and Blobel, 2007). Within the protease family, the serine and metalloproteases constitute the largest group (accounting for 65% of all proteases) with central roles in neoplastic and malignant processes (Lopez-Otin and Overall, 2002; Borgono et al., 2004; Deryugina and Quigley, 2006; Mohamed and Sloane, 2006; Overall and Blobel, 2007). The main driving principles behind utilization of an enzyme for TAP development include increased activity in the tumour environment relative to non-diseased tissue and an ability to selectively cleave the prodrug to its active form (Denny, 2001). In this respect, the serine and metalloproteases are ideal candidates for TAP development due to both their elevated activity in the extracellular tumour environment and their ability to selectively and specifically cleave short peptide sequences. Consequently, the increased endoprotease activity within tumours relative to non-diseased tissue can be harnessed to activate peptide-conjugated prodrugs of potent anticancer therapeutics, resulting in high levels of the active agent at the tumour and low or negative drug levels in ‘normal' tissues. The concentration of active therapeutic selectively released and deposited in the tumour is highly dependent upon both the catalytic efficiency of the targeted endoprotease and the level to which the enzyme is expressed within the tumour. As such, when developing such strategies, careful consideration must be given to target efficiency and suitability for TAP activation, as well as the potency and mechanism of action of the active therapeutic agent. In recent years, several endoprotease-targeted TAPs have been reported in the literature, initially against the serine protease prostate-specific antigen (PSA), and latterly the MMPs family, as discussed below.
Serine proteases: prostate-specific antigen as a target for prodrugs
The serine protease PSA, is a secreted member of the kallikrein gene family, which as the name implies is expressed selectively in prostatic tissue (Borgono et al., 2004). In cancers of the prostate, PSA levels are significantly elevated relative to non-diseased tissue (Denmeade et al., 2001; Wong et al., 2001). In addition, PSA is a clinically utilized serological diagnostic marker of prostate cancer, with higher levels being indicative of a larger tumour burden or metastatic disease (Rao et al., 2007; Reynolds et al., 2007). In terms of PSA as a target for TAP development via the criteria outlined above, PSA is elevated and proteolytically active at high levels in prostatic tumours while all normal tissues lack detectable PSA activity, and it is capable of selective and rapid activation of peptide-conjugated prodrugs (Denmeade et al., 1998; DeFeo-Jones et al., 2000; Wong et al., 2001; Reynolds et al., 2007). Furthermore, PSA present in patient serum is proteolytically inactive and incapable of prodrug activation due to formation of a complex with the plasma protease inhibitors α1-antichymotrypisn and α2-microglobulin, thereby fulfilling another major criteria for TAP development (Otto et al., 1998; Reynolds et al., 2007). Taken together, these characteristics of PSA strongly supported its potential for TAP development, as discussed below.
Matrix metalloproteinases as targets for tumour-activated prodrugs
Matrix metalloproteinases, a family of at least 24 zinc-dependent endoproteases, possess the ability to degrade most, if not all components of the extracellular matrix and basement membrane, contributing to the formation of a microenvironment permissive of tumour growth, angiogenesis and metastasis (Egeblad and Werb, 2002; Deryugina et al., 2006). A number of MMPs have been associated with tumour cell invasion evidenced by a significant correlation between the extent of local tissue penetration and MMP levels (McCawley and Matrisian, 2000, 2001; Deryugina et al., 2006).
In addition to the classical role of MMPs in the degradation of the extracellular matrix, MMPs are also able to cleave a wide range of non-matrix substrates including those involved in apoptosis, cell dissociation, cell–cell communication and cell division, thereby negating the initial view that MMPs functioned purely to bulldoze the extracellular matrix and facilitate cell invasion (McCawley et al., 2001; Egeblad and Werb, 2002; Hojilla et al., 2003; Deryugina et al., 2006). Extensive studies have demonstrated frequent overexpression of several MMPs in many forms of human tumour (Brinckerhoff et al., 2000; Hoekstra et al., 2001; Kamat et al., 2006; Vizoso et al., 2007; Atkinson et al., 2007b) with a clear relationship between increased MMP expression and poor clinical outcome in a number of cancers including breast (MMP-11), colon (MMP-1), gastric (MMP-2 and MMP-9), non-small cell lung cancer (MMP-13), oesophageal (MMP-7), small-cell lung cancer (MMP-3, MMP-11 and MMP-14) (Hoekstra et al., 2001; Vizoso et al., 2007). Furthermore, the expression of specific MMPs has been shown in many independent studies to serve as both prognostic indicators of clinical outcome and markers of tumour progression in a diverse range of tumour types (Brinckerhoff et al., 2000; Vihinen and Kahari, 2002; Zucker and Vacirca, 2004; Kamat et al., 2006; Vizoso et al., 2007).
Matrix metalloproteinases may be divided into eight distinct groups; five are secreted and three are membrane-bound (membrane-type matrix metalloproteinases, MT-MMPs) (Egeblad and Werb, 2002). The majority of MMPs, with the exception of the MT-MMPs and MMP-11, -21, -23 and -28, are secreted by the cell as inactive zymogens and require activation extracellularly by cleavage of the N-terminal pro-domain resulting in a fully functional enzyme (Brinckerhoff and Matrisian, 2002). In contrast, the MT-MMP family is not secreted from the cell but is activated intracellularly and presented on the cell surface in a proteolytically active state (Brinckerhoff and Matrisian, 2002; Osenkowski et al., 2004). Therefore, all MMPs possess proteolytic activity in the extracellular microenvironment within the tumour, distinct from the lack of activity present in ‘normal' tissues.
Many eloquent experiments have been performed in recent years that undeniably demonstrate a crucial role for MMPs in tumorigenesis through a number of potential mechanisms, detailed extensively in the literature (McCawley et al., 2000, 2001; Egeblad and Werb, 2002; Hojilla et al., 2003; Deryugina et al., 2006). For example, overexpression of MMP-14 (MT1-MMP) in cell lines null for MMP-14 or non-malignant epithelial cells afforded the cells the capability to invade (Soulie et al., 2005). Conversely, in many models in which MMP-14 is knocked down, a reduction in cellular invasiveness and tumour progression is observed (Ueda et al., 2003; Sabeh et al., 2004; Rutkauskaite et al., 2005; Deryugina et al., 2006; Zhang et al., 2006). Therefore, MMPs are a family of extracellular proteases that demonstrate the potential to become efficient drug targets for TAP development, facilitating release of therapeutic agents selectively in the tumour microenvironment.
An interesting point when considering the value of MMPs in TAP development is the actual subtumoral expression of the specific protease. In many cases, MMPs within the tumour are not specifically produced by the tumour cells themselves, but rather by the host stromal cells within or adjacent to the invading tumour (Egeblad and Werb, 2002; Jodele et al., 2006). Although initially thought to be problematic for TAP development, in reality this may actually prove to be beneficial for this strategy for two reasons. First, the important issue for TAP success is the elevation of active MMP selectively within the tumour microenvironment, leading to local release of the active therapeutic and toxicity towards tumour cells. As MMP activity is known to be restricted to the tumour mass, the expression of MMPs by stromal cells increases only the level of target for tumour-selective TAP activation. Second, stromal cells producing MMPs are known to facilitate malignant behaviour (Egeblad and Werb, 2002; Jodele et al., 2006); therefore, toxicity against these cells may, in itself, prove beneficial. As such, the expression of MMPs by many cell types within the tumour microenvironment is perceived as a positive factor for development and success of tumour-selective MMP-activated TAPs.
Tumour-activated prodrugs directed to the serine protease, prostate-specific antigen
There are now numerous examples in the literature regarding the targeting of PSA with a variety of TAPs. Drug conjugates including doxorubicin (Denmeade et al., 1998; Wong et al., 2001; DeFeo-Jones et al., 2002; DiPaola et al., 2002), vinblastine (DeFeo-Jones et al., 2002), 5-fluorodeoxyuridine (Mhaka et al., 2002), thapsigargin (Denmeade et al., 2003) and paclitaxel (Kumar et al., 2007) have been investigated and resulted in promising preclinical data. The creation of a proteolytic cleavage map of PSA against its physiological substrate, semenogelin I, led to the identification of a heptapeptide sequence rapidly hydrolysed by PSA (DeFeo-Jones et al., 2000). Covalent linkage of this peptide sequence to the aminoglycoside portion of doxorubicin (a topoisomerase II poison) led to the creation of L-377202, a TAP hydrolysed by PSA resulting in the release of leucine–doxorubicin and subsequently doxorubicin (DeFeo-Jones et al., 2000). When evaluated in preclinical studies, a substantial therapeutic index was demonstrated for L-377202 and molar equivalent doses of the prodrug eight- to ninefold higher than doxorubicin alone could be administered in vivo without additional toxicity (DeFeo-Jones et al., 2000; DiPaola et al., 2002). In clinical trials, only a 1.3-fold molar increase in prodrug relative to doxorubicin alone was administered due to dose-limiting neutropaenia, although at this dose a substantial reduction was noted in mean PSA percentage, an indicator of tumour volume (DiPaola et al., 2002). Further clinical trials are yet to be reported for this agent.
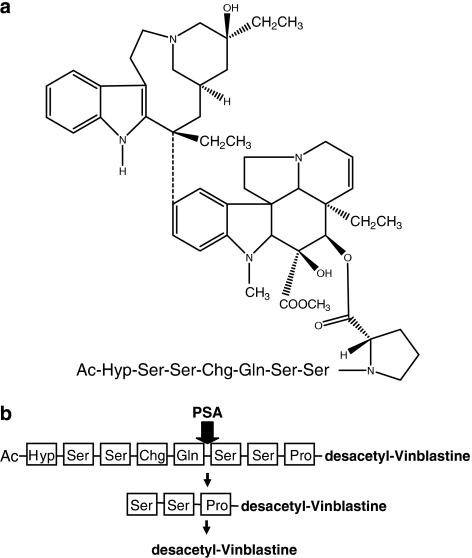
Following demonstration of proof of concept with L-377202, several other PSA-directed chemotherapeutic TAPs have been now reported with relative success (DeFeo-Jones et al., 2002; Mhaka et al., 2002; Denmeade et al., 2003; Kumar et al., 2007). Using the chemical strategy outlined for L-377202, DeFeo-Jones et al. (2002) subsequently developed a PSA-directed TAP incorporating the microtubule-targeted agent vinblastine (depicted in Figure 2), with the aim of increasing efficacy over L-377202 against advanced prostate cancer. In preclinical studies, this TAP demonstrated PSA-dependent activation, significantly lower toxicity in both mice and dogs compared with desacetyl–vinblastine (effector) alone and a significant inhibition in the growth of PSA-producing tumours in vivo (DeFeo-Jones et al., 2002). In addition, the vinblastine conjugate was shown to have a superior therapeutic index compared with the doxorubicin conjugate, L-377202 (DeFeo-Jones et al., 2002). Clinical evaluation of this agent is yet to be reported. In another study, PSA-targeted TAPs have been developed incorporating the potent SERCA pump inhibitor thapsigargin, the cytotoxicity of which is regardless of proliferative state and thus effective against characteristically slow-growing prostate tumours (Denmeade et al., 2003). In agreement with previous approaches, thapsigargin TAPs were selectively effective against PSA-producing prostate cells both in vitro and in vivo (Denmeade et al., 2003). Continuous administration of this TAP in vivo resulted in complete growth inhibition of PSA-producing prostate cancer xenografts but not PSA-negative renal carcinoma xenografts (Denmeade et al., 2003). This thapsigargin TAP is reportedly being evaluated in clinical trials for metastatic prostate cancer (Denmeade et al., 2003). The development of PSA-activated prodrugs has also been described for both paclitaxel and 5-fluorodeoxyuridine, although only in vitro results have been reported to date (Mhaka et al., 2002; Kumar et al., 2007).
Figure 2.
(a) Structure of tumour-activated prodrug incorporating the microtubule-targeted agent vinblastine targeted at the serine protease, prostate-specific antigen (PSA) (DeFeo-Jones et al., 2002). Vinblastine is attached at position 4 to an octapeptide incorporating a PSA-selective cleavage site. The prodrug is endcapped by acetylation to prevent nonspecific exopeptidase activation. (b) Schematic representation of prodrug activation by PSA. The cleavage site for PSA within the peptide is indicated by the arrow. Following the initial cleavage by PSA, the remaining amino-acid residues are removed rapidly by exopeptidases.
The success of PSA-targeted chemotherapeutics has recently resulted in the expansion of the TAP concept into the biological arena, incorporating a bacterial cytolytic protein (aerolysin) rather than a chemotherapeutic effector (Williams et al., 2007). This PSA-activated protoxin (PRX302) when administered intratumorally in vivo caused minimal effect against PSA-null tumours, but complete remission of PSA-secreting tumour models. In addition, no toxicity was observed in organs adjacent to the prostate following intratumoral injection, supporting the specificity of this agent towards PSA. Clinical trials are currently underway to assess PRX302 as an intraprostatic treatment for recurrent prostate cancer (Williams et al., 2007).
Tumour-activated prodrugs directed to the matrix metalloproteinases
To date, a small number of MMP-targeted prodrugs have been developed, most commonly towards the secreted family members MMP-2 and MMP-9 (Timar et al., 1998; Kratz et al., 2001; Mincher et al., 2002; Mansour et al., 2003; Young et al., 2003; Kline et al., 2004; Albright et al., 2005; Van Valckenborgh et al., 2005; Atkinson et al., 2007a). One of the first studies to assess MMP-2-mediated tumour-selective prodrug activation involved the incorporation of the alkylating agent melphalan into an MMP-2-cleaveable hexapeptide (termed MHP), a derivation of the TAP conjugate depicted in Figure 1 (Timar et al., 1998). Although in vitro cytotoxicity of MHP proved disappointing, the melphalan effector was successfully liberated in the presence of MMP-2/-9 and conditioned media from MMP-positive tumour cells, thereby supporting the potential of MMP-targeted TAPs (Timar et al., 1998). Subsequent strategies to develop MMP-targeted TAPs were based on the structure outlined in Figure 1, in which the effector therapeutic was conjugated to the terminus of the peptide sequence rather than being incorporated within it (as for MHP). One such TAP was a water-soluble maleimide derivative of doxorubicin, incorporating a peptide sequence suggested to be selectively cleaved by MMP-2 (Kratz et al., 2001; Mansour et al., 2003). In addition to having doxorubicin attached at one end of the conjugate, this TAP was also developed to bind to serum albumin, with the aim of using albumin as a macromolecular carrier to increase tumour accumulation of the TAP (Kratz et al., 2001; Mansour et al., 2003). Efficient activation of this TAP to release doxorubicin was demonstrated using both purified MMP-2 and MMP-2-positive tissue homogenates. In vivo, the maximum-tolerated dose for albumin-bound doxorubicin was substantially higher than that for doxorubicin alone and subsequent studies showed superior activity against A375 melanoma at equitoxic doses (Mansour et al., 2003). The concept of macromolecular delivery of MMP-activated TAPs has also been investigated by several other groups with differing success (Chau et al., 2004; Tauro and Gemeinhart, 2005; Chau et al., 2006a, 2006b).
In contrast to the use of macromolecules as delivery vehicles, MMP-activated TAPs conforming to the simple ‘trigger–linker–effector' structure, shown in Figure 1, have shown significant potential as anticancer therapeutics. In one approach, an anthraquinone topoisomerase inhibitor (NU:UB31) was linked to the C terminus of an MMP-9-cleavable heptapeptide, and the N terminus of the peptide was ‘capped' with fluorescein isothiocyanate (FITC), to produce a TAP, EV1–FITC (Mincher et al., 2002; Young et al., 2003; Van Valckenborgh et al., 2005). FITC in this prodrug allows for prodrug cleavage to be observed fluorescently, as the FITC is quenched by anthraquinone in the intact TAP, a factor that is not present following TAP activation. Using a murine myeloma model, TAP metabolism in tissues ex vivo was higher in MMP-9-expressing tumour-bearing organs (bone marrow, spleen) relative to other tissues (heart, lung, kidney), as determined by fluorescence from FITC release (Van Valckenborgh et al., 2005). There was however a significant level of prodrug activation in non-diseased tissue homogenates, suggesting a lack of TAP specificity towards MMP-9 (Van Valckenborgh et al., 2005). An attempt to further increase MMP selectivity was addressed using peptidomimetic analogues of TAPs, incorporating doxorubicin, auristatins and the duocarmycins (Kline et al., 2004). However, these peptidomimetic TAPs were either not activated by MMPs or demonstrated no selective activity against MMP-positive versus MMP-negative cells. These studies suggest that careful consideration of the effector molecule and the peptide sequence is paramount for the success of these TAPs.
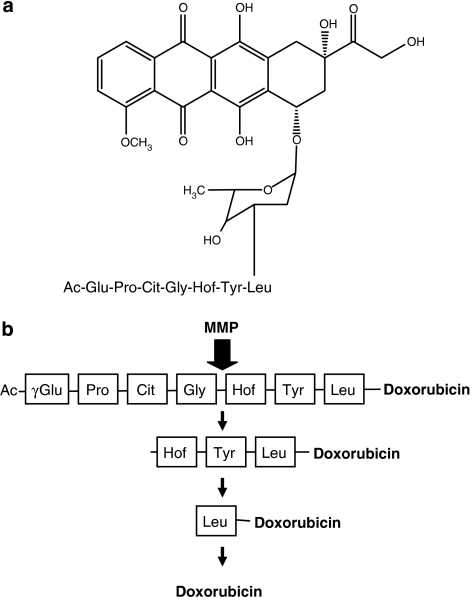
In the most detailed study reported to date, MMP-targeted TAPs of doxorubicin were demonstrated to show a much higher therapeutic index than doxorubicin alone, using the MMP-expressing HT1080 preclinical model (Albright et al., 2005). In this strategy, the peptide length was shown to be central to both compound stability and MMP-cleavage efficiency, with a heptapeptide containing three or four amino acids to the carboxy side of the scissile bond being optimal (depicted in Figure 3) (Albright et al., 2005). Similar to EV1–FITC, these prodrugs were capped on the free peptide end to prevent unrequired exopeptidase degradation (Figure 3). The optimized TAPs were activated by the secreted MMPs, MMP-2 and MMP-9, and the membrane-type MMP, MMP-14 (MT1-MMP), but not the endoprotease neprilysin, reinforcing their selectivity towards the MMP family. In an advancement of previous approaches, these TAPs were shown to be pharmacologically stable in vivo and preferentially metabolized in MMP-expressing tumours relative to heart and plasma, resulting in a 10-fold increase in the tumour/heart ratio relative to administration of doxorubicin alone (Albright et al., 2005). In addition, the optimized TAP resulted in an 80% cure rate against the HT1080 xenograft model, compared with only 10% with doxorubicin alone. One potential downside of this approach was that a significant fraction of leucine–doxorubicin formed in the tumour, as a consequence of TAP activation, was not rapidly metabolized to release doxorubicin (Figure 3), allowing for diffusion of leucine–doxorubicin away from the tumour to other tissues before conversion to doxorubicin, with potential consequences for induction of normal tissue toxicity (Albright et al., 2005). Further evaluation of these TAPs and their progression towards clinical trials are yet to be reported.
Figure 3.
(a) Structure of matrix metalloproteinase (MMP)-targeted tumour-activated prodrug incorporating the cytotoxic agent doxorubicin (Albright et al., 2005). Doxorubicin is attached to a heptapeptide incorporating an MMP-selective cleavage site. The prodrug is endcapped by acetylation to prevent nonspecific exopeptidase activation and promote in vivo drug stability. (b) Schematic representation of prodrug activation by MMP with expected cleavage products. The cleavage site for MMP within the peptide is indicated by the arrow. Following the initial cleavage by MMP, the remaining amino-acid residues are removed rapidly by exopeptidases.
In addition to exploiting MMPs to activate cytotoxic agents linked to peptides, preliminary work has been undertaken to develop MMP-activated biotoxins, incorporating anthrax toxin, measles toxin, cytolysin, CD95-L and tumour-necrosis factor alpha (Liu et al., 2000; Potrich et al., 2005; Gerspach et al., 2006; Springfeld et al., 2006; Watermann et al., 2007). The strategy for these agents is similar to that for MMP-activated cytotoxins in that the therapeutic moiety is bound to a MMP-cleavable sequence that acts to inactivate the agent until released selectively in the tumour. Although the potential for all these strategies was successfully demonstrated in vitro, antitumour data are only reported for the measles virus biotoxin, which demonstrated potent MMP-dependent activity (Springfeld et al., 2006).
While a number of studies, outlined above, have endeavoured to harness the proteolytic power of MMPs to selectively metabolize and thus activate TAPs, these attempts have, in many cases, not proved as fruitful as anticipated. The most likely explanation for this is the primary focus of strategies towards peptide conjugation of the ‘effector' and the subsequent inactivation of this agent as a TAP rather than upon the design of the ‘trigger' or peptide sequence. Determination of the optimal peptide sequence to facilitate both pharmacological stability in vivo and selective cleavage by MMPs (or even specific MMP family members) is now known to be the most crucial and difficult step in the design of TAPs against endoproteases, specifically the MMPs.
Tumour endoprotease-activated agents: the future?
As discussed herein, development of agents targeted towards the tumour by exploiting their increased endoprotease activity is an area of drug development showing great potential and promise. Several strategies and approaches have been evaluated with mixed results, all of which have led to a significant increase in our knowledge and understanding of this area. It is important to remember that in addition to these prodrugs improving tumour-selective delivery of cancer therapeutics, they function in parallel to reduce the unrequired activity of these agents against normal tissues, including liver, heart and bone marrow. In this sense, these TAPs could be developed to deliver agents to the tumour, which in practice could not be delivered due to extensive normal tissue toxicities and a consequent narrow therapeutic index.
The determination of new endoprotease targets, protease substrates and overall substrate sequence requirements for individual proteases will be essential in the future development of TAPs. It is only once these enzyme requirements are fully understood that truly proficient and selective TAPs may be designed. As proteases account for 2% of the human genome and regulate virtually every biological process, determining protease substrate specificities will be essential if TAPs are to be designed to be selectively activated by only one enzyme family, or indeed, one enzyme. Overlapping substrate specificities between protease families must be understood and addressed in the future design of these agents. In addition, improvements in our knowledge and understanding of other endoprotease systems overactive in cancer will further improve and increase the potential of this approach. In support of this, urokinase plasminogen activator, a serine endoprotease, was suggested as a valid target for TAP development (Romer et al., 2004), against which tumour-selective protoxins have been developed recently (Rono et al., 2006).
In summary, studies to date have demonstrated the worth of endoprotease-targeted TAPs. On the basis of these encouraging studies, drug development strategies need to be accelerated to realize the clinical potential of these prodrugs.
Acknowledgments
We thank Paul Loadman and Robert Falconer for their help and encouragement with this research. We would also like to thank Jon Laye for critical review of this manuscript. This work was supported by Cancer Research UK (programme Grant A5953).
Abbreviations
- FITC
fluorescein isothiocyanate
- MMP
matrix metalloproteinase
- MT-MMP
membrane-type matrix metalloproteinase
- PSA
prostate-specific antigen
- TAP
tumour-activated prodrug
Conflict of Interest
The authors state no conflict of interest.
References
- Albright CF, Graciani N, Han W, Yue E, Stein R, Lai Z, et al. Matrix metalloproteinase-activated doxorubicin prodrugs inhibit HT1080 xenograft growth better than doxorubicin with less toxicity. Mol Cancer Ther. 2005;4:751–760. doi: 10.1158/1535-7163.MCT-05-0006. [DOI] [PubMed] [Google Scholar]
- Atkinson JM, Falconer RA, Pennington CJ, Martin SW, Edwards DR, Patterson LH, et al. Membrane type 1-matrix metalloproteinase (MT1-MMP) targeted antitumour agents American Association for Cancer Research Annual Proceedings 2007a. Abstract: 2453
- Atkinson JM, Pennington CJ, Martin SW, Anikin VA, Mearns AJ, Loadman PM, et al. Membrane type matrix metalloproteinases (MMPs) show differential expression in non-small cell lung cancer (NSCLC) compared to normal lung: Correlation of MMP-14 mRNA expression and proteolytic activity. Eur J Cancer. 2007b;43:1764–1771. doi: 10.1016/j.ejca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Boddy AV, Yule SM. Metabolism and pharmacokinetics of oxazaphosphorines. Clin Pharmacokinet. 2000;38:291–304. doi: 10.2165/00003088-200038040-00001. [DOI] [PubMed] [Google Scholar]
- Borgono CA, Michael IP, Diamandis EP. Human tissue kallikreins: physiologic roles and applications in cancer. Mol Cancer Res. 2004;2:257–280. [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–4830. [PubMed] [Google Scholar]
- Bruno RD, Njar VC. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg Med Chem. 2007;15:5047–5060. doi: 10.1016/j.bmc.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau Y, Dang NM, Tan FE, Langer R. Investigation of targeting mechanism of new dextran–peptide–methotrexate conjugates using biodistribution study in matrix-metalloproteinase-overexpressing tumor xenograft model. J Pharm Sci. 2006a;95:542–551. doi: 10.1002/jps.20548. [DOI] [PubMed] [Google Scholar]
- Chau Y, Padera RF, Dang NM, Langer R. Antitumor efficacy of a novel polymer–peptide–drug conjugate in human tumor xenograft models. Int J Cancer. 2006b;118:1519–1526. doi: 10.1002/ijc.21495. [DOI] [PubMed] [Google Scholar]
- Chau Y, Tan FE, Langer R. Synthesis and characterization of dextran–peptide–methotrexate conjugates for tumor targeting via mediation by matrix metalloproteinase II and matrix metalloproteinase IX. Bioconjug Chem. 2004;15:931–941. doi: 10.1021/bc0499174. [DOI] [PubMed] [Google Scholar]
- Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Biol. 2006;2:689–700. doi: 10.1038/nchembio840. [DOI] [PubMed] [Google Scholar]
- Connors TA. Prodrugs in cancer chemotherapy. Xenobiotica. 1986;16:975–988. doi: 10.3109/00498258609038977. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D, Brady SF, Feng DM, Wong BK, Bolyar T, Haskell K, et al. A prostate-specific antigen (PSA)-activated vinblastine prodrug selectively kills PSA-secreting cells in vivo. Mol Cancer Ther. 2002;1:451–459. [PubMed] [Google Scholar]
- DeFeo-Jones D, Garsky VM, Wong BK, Feng DM, Bolyar T, Haskell K, et al. A peptide-doxorubicin ‘prodrug' activated by prostate-specific antigen selectively kills prostate tumor cells positive for prostate-specific antigen in vivo. Nat Med. 2000;6:1248–1252. doi: 10.1038/81351. [DOI] [PubMed] [Google Scholar]
- Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, et al. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- Denmeade SR, Nagy A, Gao J, Lilja H, Schally AV, Isaacs JT. Enzymatic activation of a doxorubicin–peptide prodrug by prostate-specific antigen. Cancer Res. 1998;58:2537–2540. [PubMed] [Google Scholar]
- Denmeade SR, Sokoll LJ, Chan DW, Khan SR, Isaacs JT. Concentration of enzymatically active prostate-specific antigen (PSA) in the extracellular fluid of primary human prostate cancers and human prostate cancer xenograft models. Prostate. 2001;48:1–6. doi: 10.1002/pros.1075. [DOI] [PubMed] [Google Scholar]
- Denny WA. Prodrug strategies in cancer therapy. Eur J Med Chem. 2001;36:577–595. doi: 10.1016/s0223-5234(01)01253-3. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- DiPaola RS, Rinehart J, Nemunaitis J, Ebbinghaus S, Rubin E, Capanna T, et al. Characterization of a novel prostate-specific antigen-activated peptide–doxorubicin conjugate in patients with prostate cancer. J Clin Oncol. 2002;20:1874–1879. doi: 10.1200/JCO.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Ganesh T. Improved biochemical strategies for targeted delivery of taxoids. Bioorg Med Chem. 2007;15:3597–3623. doi: 10.1016/j.bmc.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerspach J, Muller D, Munkel S, Selchow O, Nemeth J, Noack M, et al. Restoration of membrane TNF-like activity by cell surface targeting and matrix metalloproteinase-mediated processing of a TNF prodrug. Cell Death Differ. 2006;13:273–284. doi: 10.1038/sj.cdd.4401735. [DOI] [PubMed] [Google Scholar]
- Hoekstra R, Eskens FA, Verweij J. Matrix metalloproteinase inhibitors: current developments and future perspectives. Oncologist. 2001;6:415–427. doi: 10.1634/theoncologist.6-5-415. [DOI] [PubMed] [Google Scholar]
- Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer. 2003;89:1817–1821. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PS, Oliff A. Drug-targeting strategies in cancer therapy. Curr Opin Genet Dev. 2001;11:104–110. doi: 10.1016/s0959-437x(00)00164-7. [DOI] [PubMed] [Google Scholar]
- Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- Kamat AA, Fletcher M, Gruman LM, Mueller P, Lopez A, Landen CN, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12:1707–1714. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline T, Torgov MY, Mendelsohn BA, Cerveny CG, Senter PD. Novel antitumor prodrugs designed for activation by matrix metalloproteinases-2 and -9. Mol Pharm. 2004;1:9–22. doi: 10.1021/mp0340183. [DOI] [PubMed] [Google Scholar]
- Kratz F, Drevs J, Bing G, Stockmar C, Scheuermann K, Lazar P, et al. Development and in vitro efficacy of novel MMP2 and MMP9 specific doxorubicin albumin conjugates. Bioorg Med Chem Lett. 2001;11:2001–2006. doi: 10.1016/s0960-894x(01)00354-7. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Williams SA, Isaacs JT, Denmeade SR, Khan SR. Modulating paclitaxel bioavailability for targeting prostate cancer. Bioorg Med Chem. 2007;15:4973–4984. doi: 10.1016/j.bmc.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Lee M, Fridman R, Mobashery S. Extracellular proteases as targets for treatment of cancer metastases. Chem Soc Rev. 2004;33:401–409. doi: 10.1039/b209224g. [DOI] [PubMed] [Google Scholar]
- Liu S, Netzel-Arnett S, Birkedal-Hansen H, Leppla SH. Tumor cell-selective cytotoxicity of matrix metalloproteinase-activated anthrax toxin. Cancer Res. 2000;60:6061–6067. [PubMed] [Google Scholar]
- Lopez-Otin C, Overall CM. Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol. 2002;3:509–519. doi: 10.1038/nrm858. [DOI] [PubMed] [Google Scholar]
- Mansour AM, Drevs J, Esser N, Hamada FM, Badary OA, Unger C, et al. A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res. 2003;63:4062–4066. [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6:149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs: from concept to clinic. Clin Oncol (R Coll Radiol) 2007;19:427–442. doi: 10.1016/j.clon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Mhaka A, Denmeade SR, Yao W, Isaacs JT, Khan SR. A 5-fluorodeoxyuridine prodrug as targeted therapy for prostate cancer. Bioorg Med Chem Lett. 2002;12:2459–2461. doi: 10.1016/s0960-894x(02)00433-x. [DOI] [PubMed] [Google Scholar]
- Mincher DJ, Loadman PM, Lyle J, Di Salvo A, Turnbull A, Bibby MC, et al. Design of tumour-activated oligopeptide prodrugs that exploit the proteolytic activity of matrix metalloproteinases. Eur J Cancer. 2002;38:S121. [Google Scholar]
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- Newell DR. How to develop a successful cancer drug—molecules to medicines or targets to treatments. Eur J Cancer. 2005;41:676–682. doi: 10.1016/j.ejca.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- Otto A, Bar J, Birkenmeier G. Prostate-specific antigen forms complexes with human alpha 2-macroglobulin and binds to the alpha 2-macroglobulin receptor/LDL receptor-related protein. J Urol. 1998;159:297–303. doi: 10.1016/s0022-5347(01)64085-0. [DOI] [PubMed] [Google Scholar]
- Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- Overall CM, Dean RA. Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006;25:69–75. doi: 10.1007/s10555-006-7890-0. [DOI] [PubMed] [Google Scholar]
- Overall CM, Kleifeld O. Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- Patterson LH, McKeown SR. AQ4N: a new approach to hypoxia-activated cancer chemotherapy. Br J Cancer. 2000;83:1589–1593. doi: 10.1054/bjoc.2000.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrich C, Tomazzolli R, Dalla Serra M, Anderluh G, Malovrh P, Macek P, et al. Cytotoxic activity of a tumor protease-activated pore-forming toxin. Bioconjug Chem. 2005;16:369–376. doi: 10.1021/bc049873z. [DOI] [PubMed] [Google Scholar]
- Rao AR, Motiwala HG, Karim OM.The discovery of prostate-specific antigen BJU Int 2007. e-pub ahead of print, 30 August [DOI] [PubMed]
- Reynolds MA, Kastury K, Groskopf J, Schalken JA, Rittenhouse H. Molecular markers for prostate cancer. Cancer Lett. 2007;249:5–13. doi: 10.1016/j.canlet.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Romer J, Nielsen BS, Ploug M. The urokinase receptor as a potential target in cancer therapy. Curr Pharm Des. 2004;10:2359–2376. doi: 10.2174/1381612043383962. [DOI] [PubMed] [Google Scholar]
- Rono B, Romer J, Liu S, Bugge TH, Leppla SH, Kristjansen PE. Antitumor efficacy of a urokinase activation-dependent anthrax toxin. Mol Cancer Ther. 2006;5:89–96. doi: 10.1158/1535-7163.MCT-05-0163. [DOI] [PubMed] [Google Scholar]
- Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev. 2004;56:53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- Rutkauskaite E, Volkmer D, Shigeyama Y, Schedel J, Pap G, Muller-Ladner U, et al. Retroviral gene transfer of an antisense construct against membrane type 1 matrix metalloproteinase reduces the invasiveness of rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2005;52:2010–2014. doi: 10.1002/art.21156. [DOI] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulie P, Carrozzino F, Pepper MS, Strongin AY, Poupon MF, Montesano R. Membrane-type-1 matrix metalloproteinase confers tumorigenicity on nonmalignant epithelial cells. Oncogene. 2005;24:1689–1697. doi: 10.1038/sj.onc.1208360. [DOI] [PubMed] [Google Scholar]
- Springfeld C, von Messling V, Frenzke M, Ungerechts G, Buchholz CJ, Cattaneo R. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006;66:7694–7700. doi: 10.1158/0008-5472.CAN-06-0538. [DOI] [PubMed] [Google Scholar]
- Tauro JR, Gemeinhart RA. Matrix metalloprotease triggered delivery of cancer chemotherapeutics from hydrogel matrixes. Bioconjug Chem. 2005;16:1133–1139. doi: 10.1021/bc0501303. [DOI] [PubMed] [Google Scholar]
- Timar F, Botyanszki J, Suli-Vargha H, Babo I, Olah J, Pogany G, et al. The antiproliferative action of a melphalan hexapeptide with collagenase-cleavable site. Cancer Chemother Pharmacol. 1998;41:292–298. doi: 10.1007/s002800050742. [DOI] [PubMed] [Google Scholar]
- Torkelson AR, LaBudde JA, Weikel JH., Jr The metabolic fate of cyclophosphamide. Drug Metab Rev. 1974;3:131–165. doi: 10.3109/03602537408993740. [DOI] [PubMed] [Google Scholar]
- Ueda J, Kajita M, Suenaga N, Fujii K, Seiki M. Sequence-specific silencing of MT1-MMP expression suppresses tumor cell migration and invasion: importance of MT1-MMP as a therapeutic target for invasive tumors. Oncogene. 2003;22:8716–8722. doi: 10.1038/sj.onc.1206962. [DOI] [PubMed] [Google Scholar]
- Van Valckenborgh E, Mincher D, Di Salvo A, Van Riet I, Young L, Van Camp B, et al. Targeting an MMP-9-activated prodrug to multiple myeloma-diseased bone marrow: a proof of principle in the 5T33MM mouse model. Leukemia. 2005;19:1628–1633. doi: 10.1038/sj.leu.2403866. [DOI] [PubMed] [Google Scholar]
- Varmus H. The new era in cancer research. Science. 2006;312:1162–1165. doi: 10.1126/science.1126758. [DOI] [PubMed] [Google Scholar]
- Verweij J, de Jonge MJ. Achievements and future of chemotherapy. Eur J Cancer. 2000;36:1479–1487. doi: 10.1016/s0959-8049(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- Vizoso FJ, Gonzalez LO, Corte MD, Rodriguez JC, Vazquez J, Lamelas ML, et al. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer. 2007;96:903–911. doi: 10.1038/sj.bjc.6603666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermann I, Gerspach J, Lehne M, Seufert J, Schneider B, Pfizenmaier K, et al. Activation of CD95L fusion protein prodrugs by tumor-associated proteases. Cell Death Differ. 2007;14:765–774. doi: 10.1038/sj.cdd.4402051. [DOI] [PubMed] [Google Scholar]
- Williams SA, Merchant RF, Garrett-Mayer E, Isaacs JT, Buckley JT, Denmeade SR. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J Natl Cancer Inst. 2007;99:376–385. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BK, DeFeo-Jones D, Jones RE, Garsky VM, Feng DM, Oliff A, et al. PSA-specific and non-PSA-specific conversion of a PSA-targeted peptide conjugate of doxorubicin to its active metabolites. Drug Metab Dispos. 2001;29:313–318. [PubMed] [Google Scholar]
- Young L, Di Salvo A, Turnbull A, Lyle J, Bibby MC, Double JA, et al. Design of tumour-activated prodrugs that harness the ‘dark side' of MMP-9. Br J Cancer. 2003;88:S27. [Google Scholar]
- Zhang W, Matrisian LM, Holmbeck K, Vick CC, Rosenthal EL. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]