Abstract
Background and purpose:
The bladder urothelium is now known to have active properties. Our aim was to investigate the contractile properties of the urinary mucosa in response to the tachykinin neurokinin A (NKA) and carbachol.
Experimental approach:
Discrete concentration–response curves for carbachol and NKA were obtained in matched strips of porcine detrusor, mucosa and intact bladder, suspended in organ baths. The effects of inhibitors and tachykinin receptor antagonists were studied on NKA-mediated contractions in mucosal strips. Intact sections of bladder and experimental strips were processed for histology and immunohistochemistry.
Key results:
All types of strips contracted to both carbachol and NKA. Mucosal responses to NKA (pD2 7.2) were higher than those in intact strips and were inhibited by the NK2 receptor antagonist SR48968 (pKB 9.85) but not the NK1 receptor antagonist SR140333, tetrodotoxin or indomethacin. Immunostaining for smooth muscle actin and vimentin occurred under the urothelium and on blood vessels. Desmin immunostaining and histological studies showed only sparse smooth muscle to be present in the mucosal strips. Removal of smooth muscle remnants from mucosal strips did not alter the responses to NKA.
Conclusions and implications:
This study has shown both functional and histological evidence for contractile properties of the mucosa, distinct from the detrusor. Mucosal contractions to NKA appear to be directly mediated via NK2 receptors. The main cell type mediating mucosal contractions is suggested to be suburothelial myofibroblasts. Mucosal contractions may be important in vivo for matching the luminal surface area to bladder volume.
Keywords: urinary bladder, neurokinin A, NK2 receptor, urothelium, mucosa, myofibroblasts, detrusor, smooth muscle actin, smooth muscle, SR48968
Introduction
Once regarded as a passive, impermeable barrier, the epithelial layer of the bladder (urothelium) is now known to have an active role in bladder function. Like neurons, urothelial cells express vanilloid (Birder et al., 2001) and muscarinic receptors (Wang et al., 1995), and can release mediators such as nitric oxide (Birder et al., 1998), ATP (Ferguson et al., 1997) and acetylcholine (Yoshida et al., 2006). Other cell types within the mucosa, such as myofibroblasts, have been proposed to act as stretch receptors involved in afferent sensation (Wiseman et al., 2003; Fry et al., 2004). Furthermore, it has been suggested that a urothelium-derived inhibitory factor (Hawthorn et al., 2000) is released, which modulates the contractile properties of the detrusor muscle. Isolated smooth muscle strip studies have shown that removal of the mucosa (urothelium plus lamina propria) allows the detrusor to produce significantly greater contractions in response to agonists (for example, carbachol) than when the mucosa is attached (Hawthorn et al., 2000). However, no studies have investigated whether the mucosa itself might have contractile properties.
Tachykinins are peptides that share a common C-terminal region and interact with tachykinin receptors with different potencies. The neuropeptides substance P (SP), neurokinin A (NKA) and neurokinin B have high affinity for NK1, NK2 and NK3 receptors, respectively, whereas the newly described haemokinin derived from inflammatory cells also has high affinity for NK1 receptors (Pennefather et al., 2004). NKA is coexpressed with SP and calcitonin gene-related peptide in the capsaicin-sensitive afferent fibres in the suburothelium and the detrusor of various species (Smet et al., 1997), and their receptors are located on smooth muscle and blood vessels in the human bladder (Burcher et al., 2000; Candenas et al., 2005). Tachykinin receptors are involved in the sensory mechanisms of micturition and are thought to relay information about bladder fullness, distension and visceral pain to the central nervous system (Lecci and Maggi, 2001). Although NKA is a potent contractile agent of the detrusor (Sjogren et al., 1982; Zeng et al., 1995; Candenas et al., 2005), almost nothing has been published about its role in the bladder mucosa.
It has been previously demonstrated that the pig is a suitable model for studies of human bladder function (Sibley, 1984; Crowe and Burnstock, 1989; Templeman et al., 2003; Kumar et al., 2004). The pig bladder is structurally similar to the human bladder, and the NK2 receptors in both species are analogous (Templeman et al., 2003). Moreover, it has been shown that the NK2 receptors are the predominant tachykinin receptor subtype mediating the contractile responses to NKA in the detrusor muscle of both pig and human (Templeman et al., 2003). In the present study, we have utilized the pig bladder as a model to investigate (1) the contractile properties of the detrusor muscle and mucosa in response to NKA, and (2) the mechanism(s) and structures involved in mucosal contraction.
Methods
Tissue preparation
Organs of the lower urinary tract were obtained from female pigs (6–9 months, ∼55 kg) killed at a local abattoir and transported on ice. Bladders were placed in cold Krebs–Henseleit solution upon arrival and dissected within 4 h of removal from the animal.
From each dome, a segment (10 × 10 mm2) was removed for functional studies. One part was left intact and the adjacent part was dissected into mucosa and detrusor, following a natural plane of division. Three sets of strips (2 × 10 mm2; 5 mm between the ties) of intact bladder, detrusor only and mucosa only were cut longitudinally and mounted in 2 ml silanized organ baths, bathed in Krebs–Henseleit solution and bubbled with 95% O2 and 5% CO2, at 37 °C.
Functional studies
The preparations were equilibrated under a resting tension of 1.5 g, for 60 min. An initial test response of each strip was elicited by carbachol (1 μM) followed an hour later by a maximum response to carbachol (100 μM). Strips were allowed to equilibrate for a further 60 min with several washes throughout. Responses achieved on the day of collection to carbachol and NKA were significantly higher than those on the subsequent day; thus, further experiments were all performed on the day of collection.
Discrete concentration–response curves were obtained to carbachol (1 nM–100 μM) in all three types of strips simultaneously. Drug contact time was 2–4 min. At lower concentrations of carbachol (10–100 nM), 40–45 min was allowed to elapse between each contractile response, whereas at higher concentrations (1–100 μM), 60–90 min was allowed to elapse between responses. Similarly, discrete concentration–response curves were obtained to NKA (1 nM–10 μM), in intact, mucosal and detrusor strips. Drug contact time was 3–5 min with 60–120 min allowed to elapse between each contractile response. For the above experiments, a matched design was used whereby a duplicate set of intact, detrusor and mucosal strips was obtained from each animal. Throughout this study, only one concentration–response curve was obtained from each strip.
In preliminary experiments, the effect of the neutral endopeptidase inhibitor phosphoramidon (10 μM) was tested (Templeman et al., 2003). At the end of all experiments, a final maximum response to carbachol was elicited from each strip. The contractile portions of the strips were then weighed. The average weight was 115±12 (n=12), 95±13 (n=12), 24±3 mg (n=24) for intact, detrusor and mucosa, respectively. Strips were fixed in paraformaldehyde or Zamboni's fixative and later processed for histology and immunohistochemistry.
Mechanism of contractile action of NKA in mucosal strips
A further group of experiments was designed to investigate the mechanism of mucosal contraction to NKA. Concentration–response curves were obtained for the NK2-selective agonist [Lys5,MeLeu9,Nle10]NKA(4–10) (n=6), and to the NK1-selective agonist [Pro9]SP (n=5). Discrete concentration–response curves to NKA were obtained in the absence and presence of the NK1 receptor antagonist, SR140333 (100 nM) and the NK2 receptor antagonist, SR48968 (1–10 nM), both preincubated with the strips for 2 h before responses to NKA were elicited. The effects of tetrodotoxin (TTX, 1 μM) and indomethacin (1 μM) were also tested (incubation times 20 and 30 min, respectively; n=8).
Mucosal strips were also pretreated with trypsin (0.2 pg ml−1) or protamine sulphate (1 mg ml−1) for 5 min, to disrupt the epithelial layer and test for urothelial involvement in contraction. The strips were later processed histologically to ensure that the urothelial layer was indeed disrupted.
Studies with ‘dissected mucosal' strips
In a separate study, a mucosal square was pinned out under a dissecting microscope, luminal side down, and bands of smooth muscle and visible blood vessels were removed. The subsequent preparation was transparent and was then cut into several longitudinal strips of similar length (but not thickness) to the mucosal strips described above. These ‘dissected mucosa' strips weighed 12±1 mg and were mounted in organ baths as described above.
The preparations were equilibrated under a resting tension of 1.0 g for 60 min. An initial test response of each strip was elicited by carbachol (1 μM) followed an hour later by a maximum response to carbachol (100 μM). Strips were allowed to equilibrate for a further 60 min with several washes throughout. Concentration–response curves to NKA were then elicited as described above.
Data analysis
Contractile responses were measured initially as increase in tension in grams, using Grass FT isometric force transducers and recorded using Polygraph software (Mr E Crawford, University of NSW). Responses were expressed as increase in gram tension per strip weight and also as a percentage of the maximal response of the preparation to carbachol. The maximum responses to carbachol obtained at the beginning and the end of the day were not different when strips were used for carbachol, but in strips where concentration–response curves to NKA were elicited, the final response to carbachol was approximately half that of the first response, suggesting cross desensitization. For this reason, the final maximum response to carbachol was discarded when calculating responses to NKA in percentage terms. Responses obtained in duplicate strips from the same animal were then averaged. Data are expressed as mean±s.e.mean. The n value represents the number of animals rather than the number of strips.
Concentration–response data were analysed using PRISM version 5.0 software. Antagonist potency was estimated as pKB=log (concentration ratio–1)/log (molar concentration of antagonist) (Arunlakshana and Schild, 1959).
Statistical analysis
The repeated measures ANOVA and Bonferroni post test was used where three or more paired groups of data were compared. Student's t-tests were used to compare two groups of data.
Histological and immunohistochemical studies
Sections of fresh intact bladder dome as well as strips taken from organ bath studies were fixed in paraformaldehyde fixative overnight, then transferred into 70% ethanol until paraffin-embedded blocks were created. Some strips were fixed in Zamboni's solution and frozen sections were later processed for immunohistochemistry. Slide-mounted sections were stained histologically using Masson's trichrome or haematoxylin and eosin. Other sections were processed for immunohistochemistry using antibodies against α-smooth muscle actin (SMA), desmin, vimentin and c-kit. Nonspecific binding was quenched using 0.3% hydrogen peroxide applied for 10 min, then sections were incubated with primary antibody for 30 min, washed with Tris buffer, followed by incubation with Dako DualEnvisionLink, washed again with buffer and then DAB applied for 10 min. Sections were counterstained with haematoxylin.
Materials
Neurokinin A, [Lys5,MeLeu9,Nle10]NKA(4–10) and [Pro9]SP were purchased from Auspep (Melbourne, Vic, Australia). Carbachol, indomethacin, TTX, trypsin and protamine sulphate were purchased from Sigma Chemical Company (Sydney, NSW, Australia). SR48968 and SR140333 were gifts from Dr X Emonds-Alt, Sanofi-Synthélabo, Montpellier, France. The antibodies against desmin (catalogue M0760, 1:100), SMA (catalogue M0851, 1:200), vimentin (M7020, 1:150) and DualEnvisionLink were from Dako (Sydney, NSW, Australia). The c-kit antibody (SC-13508, 1:400) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other reagents were of high analytical grade.
Results
Responses to carbachol
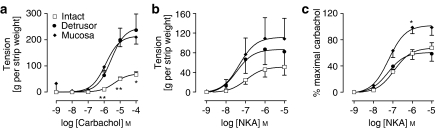
Intact, detrusor and mucosal strips all contracted in response to carbachol. The magnitude of responses in mucosal strips was significantly greater than that in intact strips and was not significantly different from responses in detrusor (Figure 1a). There was a greater contractile response obtained from detrusor strips compared with intact strips (P<0.05) at the maximum concentration of carbachol.
Figure 1.
Increases in tension in response to carbachol (a) and neurokinin A (NKA; b, c) were elicited in matched sets of intact, detrusor and mucosal strips from the pig bladder (n=6). In this and subsequent figures, responses represent the mean±s.e.mean from duplicate strips, and n=number of animals. (a) Responses to carbachol in detrusor and mucosal strips were higher than those in intact strips. (b) NKA data are expressed as increase in tension per strip weight. (c) NKA data are expressed as a percentage of the maximum carbachol response in the same strip. For (a) and (c), responses in mucosal strips were significantly higher than those in intact strips (*P<0.05, **P<0.01, repeated measures ANOVA followed by Bonferroni test).
Responses to NKA
Intact, detrusor and mucosal strips also contracted in response to NKA (Figures 1b and c), with experimental traces shown in Figure 2. When expressed as increased tension per strip weight (Figure 1b), the responses to NKA in mucosal strips were similar to those in the detrusor strips and were significantly greater than intact strips (P<0.05, n=6). When expressed as a percentage of the maximal carbachol response, mucosal strips showed significantly greater responses than intact strips (P<0.05) (Figure 1c, Table 1). Phosphoramidon (10 μM) had no significant effect on NKA-induced contractile responses of the mucosa or detrusor (data not shown). Thus, subsequent experiments were conducted without the use of phosphoramidon.
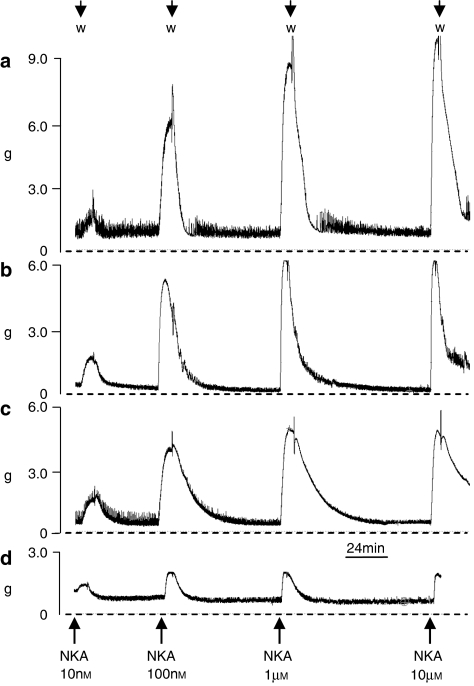
Figure 2.
Experimental traces from (a) intact, (b) detrusor, (c) mucosal and (d) dissected mucosal strips of porcine bladder. Strips weighed 100, 60, 11 and 8 mg, respectively. Discrete responses, measured in grams tension (g), were obtained to increasing concentrations of neurokinin A (NKA), indicated by arrows. W, indicates washing of preparation.
Table 1.
Summary data of functional responses to NKA in porcine mucosal strips
| Agonist | Inhibitor (concentration) | pD2 | Emax at 10 μM (% maximal carbachol) | n | |
|---|---|---|---|---|---|
| NKA | — | — | 7.2±0.2 | 98.8±12.0 | 12 |
| [Lys5,MeLeu9,Nle10]NKA(4–10) | — | — | 7.9±0.1 | 105.2±5.0 | 6 |
| NKA | TTX | 1 μM | 7.8±0.1 | 95.1±4.5 | 8 |
| NKA | Indomethacin | 1 μM | 7.8±0.1 | 108.0±4.7 | 8 |
| NKA | SR140333 | 1 μM | 7.5±0.2 | 97.6±5.2 | 5 |
| 1 nM | 6.6±0.3 | 94.2±7.7 | 5 | ||
| NKA | SR48968 | 3 nM | 5.9±0.2** | 72.8±11.7 | 6 |
| 10 nM | 5.5±0.4** | 36.8±11.0** | 4 | ||
Abbreviations: NKA, neurokinin A; TTX, tetrodotoxin.
**P<0.01 (ANOVA followed by Dunnett's test).
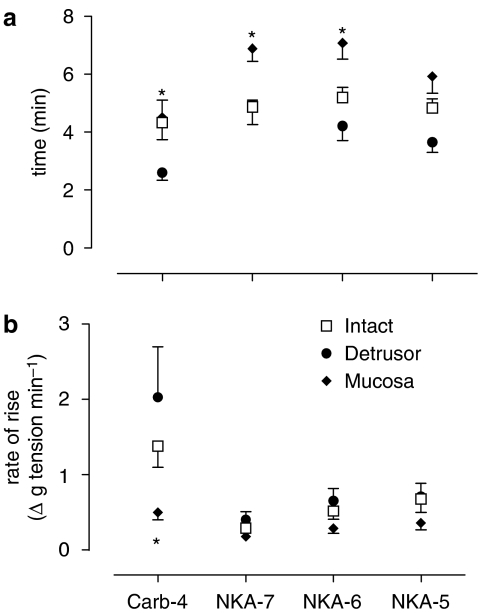
The time course of these responses was examined (Figure 3). In general, responses to carbachol achieved their peak in a shorter time than responses to NKA (Figure 3a). For NKA, responses in mucosal strips were significantly slower than those in intact, and particularly in detrusor strips (Figure 3a). For carbachol, the rate of increase in tension was three- to fourfold less in mucosal strips than in corresponding intact and detrusor strips (Figure 3b). For NKA, there was much less variation between different types of strips for this parameter (Figure 3b). When comparing responses to carbachol (10−4 M) with those to the highest concentration of NKA (10−5 M), the rate of increase in tension of detrusor strips to NKA was 34.2% of that to carbachol. All mucosal strips showed similar values.
Figure 3.
Time course of contractile responses to neurokinin A (NKA; 10−7, 10−6 and 10−5 M) and carbachol (10−4 M) in intact, detrusor and mucosal strips (n=6). (a) Time taken to reach peak response at each concentration. For both NKA and carbachol, responses in mucosal strips were significantly slower than those in detrusor or intact strips (one-way ANOVA, P<0.0001). (b) Rate of rise to peak contraction. The mean carbachol response in mucosal strips was significantly less than in detrusor strips (one-way ANOVA, P<0.05). For NKA, there was no difference between intact, detrusor and mucosal strips. The mucosal responses were not significantly different from each other.
Mechanism of contractile effect of NKA in mucosal strips
Responses of mucosal strips to the NK2-selective agonist [Lys5,MeLeu9,Nle10]NKA(4–10) were similar to those of NKA (Figure 4a; Table 1), whereas those to the NK1-selective agonist, [Pro9]SP were extremely weak (response at 1 μM was 13.4±7.8% (n=5) of carbachol maximum).
Figure 4.
Experiments to characterize the neurokinin A (NKA)-induced response of mucosal strips. (a) The concentration–response curve to the NKA analogue [Lys5,MeLeu9,Nle10]NKA(4–10) was very similar to that of NKA (both n=6). (b) Preincubation with NK1 receptor antagonist, SR140333 (100 nM), had no effect on the NKA-mediated mucosal response (n=5). (c) Tetrodotoxin (TTX) and (d) indomethacin preincubation (both 1 μM, n=8) resulted in no significant change in responses to NKA in mucosal strips (Student's paired t-test). (e) Responses to NKA in mucosal strips, measured as percentage of maximal carbachol response, were reduced in the presence of SR48968 at 1, 3 and 10 nM, compared with the control (n=4–6). (f) Schild plot of the data in (e). Slope 1.06±0.46; pKB 9.85.
Responses to NKA were completely unaffected by the NK1-selective antagonist, SR140333 (100 nM; Figure 4b; Table 1). Incubation with TTX (n=8) or indomethacin (n=8) had no effect on the response to NKA (Figures 4c and d).
Responses to NKA were markedly inhibited in the presence of 1–10 nM SR48968 (Figures 4e and f; Table 1). Rightward shifts of the concentration–response curves occurred at 1, 3 and 10 nM SR48968. At 3 and 10 nM SR48968, the maximal concentration of NKA (10 μM) failed to elicit the maximum NKA response. The pKB (affinity) value for SR48968 was estimated at 9.85±0.1 (n=4–6).
Experiments were designed to determine the role of the bladder epithelium (urothelium) in contractile responses of the mucosa. Histological analysis showed that treatment with protamine sulphate and trypsin resulted in loss of the urothelium, but did not affect the contractile response of mucosal strips to carbachol or NKA (data not shown). In another study, responses to a submaximal concentration of NKA (100 nM) were repeated five times at hourly intervals, with responses 44.9±4.1, 56.1±3.9, 58.2±4.4, 55.7±4.8 and 52.2±4.1% (n=6) of the carbachol maximum response. Similarly, submaximal responses to carbachol (1 μM) were 13.3±2.4, 23.0±4.8, 24.3±6.5, 28.1±6.51 and 28.7±7.1% (n=4). Thus, contractile responses to agonists were not diminished on repetition, although histological analysis of the strips showed that the urothelium had largely disappeared by the end of the experiment (Figures 5a and c).
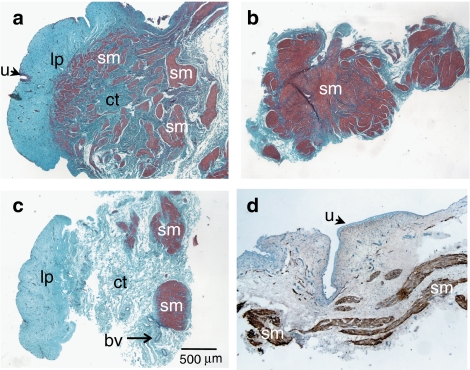
Figure 5.
Low-power photomicrographs showing histological and immunostaining of strips after an organ bath procedure. (a–c) Masson's trichrome blue staining on paraffin-embedded (a) intact, (b) detrusor and (c) mucosal strips. Muscle was stained purple/red and connective tissue was stained blue. Note the almost total absence of urothelium (arrowed) in (a and c). (d) Frozen section of a mucosal strip immunostained with desmin and counterstained with haematoxylin. Discrete desmin immunoreactivity was associated with muscle bundles. bv, blood vessels; ct, connective tissue; sm, smooth muscle; lp, lamina propria; u, urothelium.
Histological and immunohistochemical studies
In strips previously used in the organ bath, Masson's trichrome stain clearly delineated smooth muscle from connective tissue (Figures 5a–c). In mucosal strips (Figure 5c), only minimal amounts of muscle were present. Frozen sections of mucosal strips immunostained with SMA resulted in moderate to dense immunolabelling throughout the tissue except for the urothelium (not shown), whereas adjacent sections with desmin immunostaining (Figure 5d) showed narrow bands of muscle fibres running within the lower lamina propria (close to where the detrusor had been removed).
In paraffin sections of whole bladder photographed at higher magnification (Figure 6), desmin immunostaining corresponded to the ‘red' staining of the muscle bundles seen with Masson's trichrome (Figures 6a and b). SMA immunostaining revealed dense immunolabelling of a discrete layer under the urothelium, within small muscle bundles in the mucosa and larger muscle bundles in the detrusor, as well as staining of blood vessels within the mucosa (Figures 6c and d). In fact, there was light SMA immunostaining over the entire section, except for the urothelium itself. Vimentin staining was widespread, particularly in the suburothelial layer (Figures 6e and f), where it appeared to correspond to SMA-like immunoreactivity (Figure 6d), although smooth muscle did not stain. Staining with the c-kit antibody was weak and nonspecific (not shown).
Figure 6.
Sections from paraffin-embedded intact pig bladder dome. (a) Section stained with α-smooth muscle actin (SMA) shows dense immunolabelling of small muscle bundles in the mucosa and larger muscle bundles in the detrusor, and also on blood vessels and underneath the urothelium. The urothelium itself (stained blue) is not SMA-immunoreactive. (b) High-power photomicrograph of part of (a), showing dense labelling of a discrete layer (indicated by arrowheads) directly beneath the urothelium. SMA-immunoreactive blood vessels are present in the lamina propria. (c) Adjacent section stained with vimentin shows extensive immunolabelling throughout the section, particularly on the suburothelial layer and blood vessels. (d) High-power photomicrograph of part of (c), shows dense labelling of the same discrete suburothelial layer (indicated by arrowheads) as depicted in (b). M, mucosal layer; D, detrusor layer; bv, blood vessels; lp, lamina propria; sm, smooth muscle; u, urothelium.
Functional and immunological studies in ‘dissected mucosal' strips
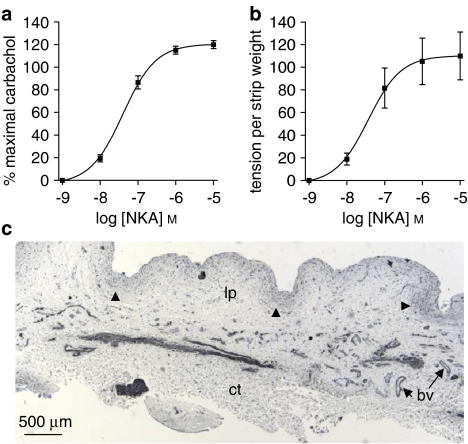
The ‘dissected mucosal' strips also contracted in response to NKA (Figure 2d) and to carbachol. Responses to the highest concentration of NKA were similar to those to the maximal carbachol response (Figure 7a). On a grams tension per strip weight basis (Figure 7b), the response to the highest concentration of NKA was equivalent to previous mucosal responses at the same concentration (Figure 2b). The pD2 value in both cases was 7.40. These strips also contracted to carbachol, which was significantly less efficacious than NKA.
Figure 7.
Responses to neurokinin A (NKA; n=7) were elicited in ‘dissected mucosal' strips, expressed as (a) percentage of maximal carbachol response and (b) as grams tension per strip weight. In both cases, NKA responses were of similar magnitude to those achieved in mucosal strips (Figures 1b and c). The maximum response to NKA (10 μM) was significantly greater (a, P<0.001; b, P<0.01; unpaired t-test) than that to carbachol (100 μM). (c) Photomicrograph of section of dissected mucosal strip after an organ bath procedure, immunostained with α-smooth muscle actin (SMA). Dense immunolabelling can be seen on blood vessels (bv), as well as moderately weak immunolabelling of the suburothelial layer (arrowheads). No smooth muscle bundles are present. Note the absence of the urothelium. ct, connective tissue; lp, lamina propria; u, urothelium.
Immunological studies on these strips showed dense SMA labelling of blood vessels in the lamina propria as well as weaker labelling of the myofibroblasts/suburothelial layer (Figure 7c). The ‘dissected mucosal' strips showed no histological evidence for the presence of smooth muscle staining by SMA (Figure 7c) or by desmin (not shown).
Discussion
While there is keen interest in the various functions of the bladder mucosa, this is the first study to investigate a novel and unexpected property of this tissue: its ability to contract in response to neurochemicals. Our most striking finding was that both carbachol and the tachykinin NKA were potent contractile agents of the pig bladder mucosa. Mucosal contractions to NKA were almost twofold greater than those of comparable intact strips, although potencies were similar. It was noteworthy that contractions to NKA and carbachol were independent of the presence of the transitional epithelium, as responses were unchanged after its passive loss in the organ bath with time, as well as by its active removal by protamine sulphate (Birder et al., 2001) and trypsin.
The mechanism(s) of action of NKA appear to be via tachykinin NK2 receptors in the mucosa, as verified using NK1 and NK2 receptor-selective agonists and antagonists. The pKB value for SR48968 was estimated at 9.85, somewhat higher than values (8.0–8.3) obtained previously in the pig neck and detrusor, human detrusor (8.9) (Templeman et al., 2003) and dog bladder (8.9) (Mussap et al., 1996), where noncompetitive antagonism was observed. Responses were unaffected by TTX, showing a lack of involvement of neuronal mechanisms. Indomethacin was also without effect on responses to NKA in this study, similar to findings in strips of human detrusor and intact bladder (Warner et al., 2002). Although the involvement of neural mechanisms and prostanoids was ruled out here, NKA may cause the release of other factors that, in turn, cause contraction of the mucosa. Responses to NKA in the mucosa (and the detrusor) were also, perhaps surprisingly, unaffected by phosphoramidon 10 μM, again similar to previous findings in human bladder (Warner et al., 2002). Phosphoramidon is a potent inhibitor of EC24.11 (neutral endopeptidase) (Ki 2 nM) but also inhibits EC3.4.24.71 at micromolar concentrations; both enzymes are found in the urothelium (see Warner et al., 2002).
Although the mucosa contains the urothelium, connective tissue, blood vessels, nerves and myofibroblasts, it is not considered to contain smooth muscle. In fact, only a few narrow smooth muscle bands (as defined by histological staining with Masson's and immunostaining with desmin) were seen in our mucosal strips, which had previously produced very marked contractions to NKA. Therefore, to eliminate the possibility that it was these smooth muscle remnants, which were responsible for mucosal contractions, we refined our dissections to produce ‘dissected mucosal' strips, which lacked smooth muscle (Figure 7). These strips produced contractions to NKA identical to if not larger than those of the original mucosal strips. The slower time course of the responses in mucosal and ‘dissected mucosal' strips is in line with contraction by nonsmooth muscle elements in the mucosa.
Apart from smooth muscle, SMA immunostaining was clearly absent from urothelial cells, but present in all other areas, such as blood vessels and suburothelial myofibroblasts, and even (more weakly) in connective tissue, suggesting that the mucosa has several distinct contractile elements. It was notable that the network of blood vessels in the lamina propria showed dense SMA and vimentin immunostaining, raising the possibility that these might contribute to contractile responses to NKA and carbachol. Vimentin is suggested to be a myofibroblast marker, but in the pig bladder, staining was widespread (although smooth muscle was unstained). Thus, vimentin was less helpful than SMA in delineating contractile structures, although the suburothelial layer was labelled. We hoped to identify interstitial cells of Cajal-like cells by use of c-kit staining, but this was unsuccessful, probably due to the lack of specificity of the antibody in the pig.
Myofibroblasts have contractile properties (Hinz, 2006) and the structures that mediate mucosal contraction are likely to include the network of SMA-immunoreactive cells and fibres underneath the urothelium (Figure 6c). In the identical region of the guinea-pig bladder, a band of similar ‘suburothelial interstitial cells' has been identified (Gillespie et al., 2006) by immunostaining with vimentin. SMA is commonly regarded as the most important marker for myofibroblasts (Drake et al., 2006); thus, it is probable that the suburothelial SMA immunoreactivity represents myofibroblasts or suburothelial interstitial cells whose specific roles in the bladder are still controversial (Wiseman et al., 2003; Fry et al., 2004; Gillespie et al., 2006). However, conclusive identification would require electron microscopic studies (Drake et al., 2006). We also observed that after removal of the mucosa from the underlying detrusor, which involves unfolding and stretching, the mucosa regains its original folds within a short time. This suggests passive elastic properties and/or active contractile properties allowing for self-organization. This is probably important as the mucosa folds and unfolds to accommodate urine.
The presence of tachykinin NK2 receptors in the bladder mucosa is a novel finding. Immunoreactivity for muscarinic receptors (M2 and M3) has previously been described, on human urothelium, suburothelial myofibroblasts, nerve fibres and detrusor (Mukerji et al., 2006). The sources of the endogenous agonists are not clear from this study. Tachykinin-like immunoreactivity is present in a subset of suburothelial nerves (Smet et al., 1997); acetylcholine might originate from neuronal sources, as well as from the urothelium (Yoshida et al., 2006). In bladder, the role of NK2 receptors has traditionally been in the mediation of smooth muscle contraction, for example of the detrusor, as reported previously in human (Zeng et al., 1995), pig (Templeman et al., 2003) as well as other species (reviewed in Candenas et al., 2005). The abundance of NK2 receptors in peripheral smooth muscle has made them a key therapeutic target in a number of human disorders (Lecci and Maggi, 2003). Furthermore, pathological conditions such as Crohn's disease and ulcerative colitis show marked changes of NK1 and NK2 receptors, suggesting their important role in inflammatory bowel diseases (Mantyh et al., 1988; Renzi et al., 2000; Menzies et al., 2001). This study has discovered a new (so far, unintended) target for drugs in the treatment of diseases involving the NK2 receptor.
Conclusions
This study has shown both functional and histological evidence for contractile properties of the pig bladder mucosa, distinct from the detrusor. Mucosal contractions to NKA appear to be directly mediated via NK2 receptors. The contractile elements are likely to include the SMA-immunoreactive layer underneath the urothelium, provisionally identified as myofibroblasts, and perhaps the extensive network of blood vessels. We require further understanding of the molecular and cellular characteristics of this layer, and of the location and density of the NK2 and other receptors present within the mucosa. Although this study focused on the urinary bladder mucosa, it is probable that mucosal layers in other organ systems also have contractile properties. This may be important in vivo for matching the mucosal surface area to volume.
Acknowledgments
The acquisition of pig bladders was made possible by the Biological Resource Centre, UNSW. We thank Mr Lawrence Young (Dako, Australia) for the gift of desmin antibody and for valuable assistance in immunohistochemical studies, Dr Fei Shang for assistance in functional studies and in photomicroscopy and Dr Lu Liu for advice on data analysis.
Abbreviations
- NKA
neurokinin A
- SMA
α-smooth muscle actin
- SP
substance P
- TTX
tetrodotoxin
Conflict of interest
The authors state no conflict of interest.
References
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcher E, Zeng XP, Strigas J, Shang F, Millard RJ, Moore KH. Autoradiographic localization of tachykinin and calcitonin gene-related peptide receptors in adult urinary bladder. J Urol. 2000;163:331–337. [PubMed] [Google Scholar]
- Candenas L, Lecci A, Pinto FM, Patak E, Maggi CA, Pennefather JN. Tachykinins and tachykinin receptors: effects in the genitourinary tract. Life Sci. 2005;76:835–862. doi: 10.1016/j.lfs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Crowe R, Burnstock G. A histochemical and immunohistochemical study of the autonomic innervation of the lower urinary tract of the female pig. Is the pig a good model for the human bladder and urethra. J Urol. 1989;141:414–422. doi: 10.1016/s0022-5347(17)40785-3. [DOI] [PubMed] [Google Scholar]
- Drake MJ, Fry CH, Eyden B. Structural characterization of myofibroblasts in the bladder. BJU Int. 2006;97:29–32. doi: 10.1111/j.1464-410X.2006.05818.x. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism. J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP.Control of bladder function by peripheral nerves: avenues for novel drug targets Urology 200463Suppl 124–31.review [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Markerink-van Ittersum M, De Vente J. Endogenous nitric oxide/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res. 2006;325:325–332. doi: 10.1007/s00441-005-0146-4. [DOI] [PubMed] [Google Scholar]
- Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol. 2006;85:175–181. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kumar V, Chapple CC, Chess-Williams R. Characteristics of adenosine triphosphate release from porcine and human normal bladder. J Urol. 2004;172:744–747. doi: 10.1097/01.ju.0000131244.67160.f4abstract. [DOI] [PubMed] [Google Scholar]
- Lecci A, Maggi CA.Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system Regul Pept 20011011–18.Review [DOI] [PubMed] [Google Scholar]
- Lecci A, Maggi CA.Peripheral tachykinin receptors as potential therapeutic targets in visceral diseases Expert Opin Ther Targets 20037343–362.Review [DOI] [PubMed] [Google Scholar]
- Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP, Jr, Vigna SR, et al. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci USA. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies JR, McKee R, Corbett AD. Differential alterations in tachykinin NK2 receptors in isolated colonic circular smooth muscle in inflammatory bowel disease and idiopathic chronic constipation. Regul Pept. 2001;99:151–156. doi: 10.1016/s0167-0115(01)00244-0. [DOI] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Grogono J, Underwood J, Agarwal SK, Khullar V, et al. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol. 2006;176:367–373. doi: 10.1016/S0022-5347(06)00563-5. [DOI] [PubMed] [Google Scholar]
- Mussap CJ, Stamatakos C, Burcher E. Radioligand binding, autoradiographic and functional studies demonstrate tachykinin NK-2 receptors in dog urinary bladder. J Pharmacol Exp Ther. 1996;279:423–434. doi: 10.1163/2211730x96x00225. [DOI] [PubMed] [Google Scholar]
- Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA.Tachykinins and tachykinin receptors: a growing family Life Sci 2004741445–1463.Review [DOI] [PubMed] [Google Scholar]
- Renzi D, Pellegrini B, Tonelli F, Surrenti C, Calabro A. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–1522. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley GNA. A comparison of spontaneous and nerve mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren C, Andersson KE, Husted S. Contractile effects of some polypeptides on the isolated urinary bladder of guinea-pig, rabbit, and rat. Acta Pharmacol Toxicol. 1982;50:175–184. doi: 10.1111/j.1600-0773.1982.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Smet PJ, Moore KH, Jonavicius J. Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest. 1997;77:37–49. [PubMed] [Google Scholar]
- Templeman L, Sellers DJ, Chapple CR, Rosario DJ, Hay DP, Chess-Williams R. Investigation of neurokinin-2 and -3 receptors in the human and pig bladder. BJU Int. 2003;92:787–792. doi: 10.1046/j.1464-410x.2003.04458.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- Warner FW, Shang F, Millard RJ, Burcher E. Enhancement of neurokinin A-induced smooth muscle contraction in human urinary bladder by mucosal removal and phosphoramidon: relationship to peptidase inhibition. Eur J Pharmacol. 2002;438:171–177. doi: 10.1016/s0014-2999(02)01316-x. [DOI] [PubMed] [Google Scholar]
- Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, et al. Non-neuronal cholinergic system in human bladder urothelium. Urology. 2006;67:425–430. doi: 10.1016/j.urology.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zeng X-P, Moore KH, Burcher E. Characterization of tachykinin NK2 receptors in human urinary bladder. J Urol. 1995;153:1688–1692. [PubMed] [Google Scholar]