Abstract
Background and purpose:
Erectile dysfunction is highly prevalent in diabetic patients and PDE V inhibitors are effective and safe for the treatment of erectile dysfunction in men with diabetes. Therefore, in this study we investigated whether a pharmacokinetic interaction occurs between DA-8159 and metformin, as both drugs are metabolized via hepatic CYP3A1/2 in rats.
Experimental approach:
DA-8159 (30 mg kg–1) and metformin (100 mg kg–1), both separately and together, were administered to rats either intravenously or orally. The V max, K m, CLint, apparent inhibition constants (K i), [I]/K i and concentrations of each drug in the liver and intestine were then measured.
Key results:
After i.v. administration of both drugs simultaneously, the AUC of DA-8159 and metformin was significantly greater (21.2 and 33.9% increase for DA-8159 and metformin, respectively) than that of each drug alone. After p.o. administration of the drugs, the AUC of metformin was also significantly greater (20.7% increase) in the presence of DA-8159 than in its absence. However, the AUC of DA-8159 was similar in the absence and presence of metformin.
Conclusions and implications:
The significantly greater AUC of metformin and DA-8159 after i.v. administration of both drugs and of metformin after p.o. administration of both drugs is probably due to competitive inhibition for the metabolism of these drugs via hepatic CYP3A1/2. However, the similar AUCs of DA-8159 in the absence and presence of metformin, after p.o. administration, indicates that the dose of metformin used was insufficient to inhibit the hepatic and intestinal metabolism of DA-8159.
Keywords: pharmacokinetic interaction, DA-8159, metformin, competitive inhibition, hepatic CYP3A1/2
Introduction
A new inhibitor of cyclic guanosine monophosphate (cGMP)-specific phophodiesterase type V, DA-8159 (udenafil; 5-(2-propyloxy-5-(1-methyl-2-pyrollidinylethylamidosul-phonyl)phenyl)-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo-(4,3-d)pyrimidine-7-one) has recently been marketed in Korea as Zydena for the treatment of male erectile dysfunction (Research Laboratory of Dong-A Pharmaceutical Company, Yongin, South Korea). Based on the in vitro data of DA-8159 obtained in microsomes containing Baculovirus-expressed rat hepatic microsomal cytochrome P450 (CYP) isozymes, the inhibitor was found to have three metabolites: DA-8164 (N-dealkylated DA-8159; 5-[2-propyloxy-5-(aminosulphonyl)phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo-(4,3-d)pyrimidine-7-one); M1 (hydroxyl DA-8159); M2 (N-demethyl DA-8159) (Choi et al., 2002). DA-8164 is the main metabolite of DA-8159 in humans and the pharmacological effect of DA-8164, in terms of its phophodiesterase type V inhibitory activity, is half that of DA-8159 (Kim et al., 2005b). Glucuronide- and sulphate-conjugations are not involved in the metabolism of DA-8159 in rats (Choi et al., 2002). Based on in vivo studies, DA-8159 is found to be metabolized to DA-8164 in mice, rats, rabbits, dogs and humans (Shim et al., 2005). In male Sprague–Dawley rats, Kim et al. (2005a) found that DA-8159 was primarily metabolized and DA-8164 was formed via hepatic CYP3A1/2, but not CYP1A1/2, 2B1/2, 2D1 and 2E1. Kim et al. (2005b) have recently reviewed the pharmacological actions, pharmacokinetics and metabolism, toxicity and clinical studies on DA-8159.
Metformin, a biguanide antihyperglycaemic agent, has been widely used in the management of type 2 diabetes; it lowers blood glucose without causing hypoglycaemia (Scheen, 1996). Scheen (1996) found that following intravenous (i.v.; at doses of 0.25–1.0 g) and oral (per os (p.o.); at doses of 0.5–1.5 g) administration of metformin to four healthy volunteers, the terminal half-lives (t1/2) were 1.52–4.50 h and the percentages of the doses excreted in the urine (via active renal tubular secretion) were 78.9–99.9%. Absorption was not complete (20–30% of the p.o. dose was recovered from the faeces), possibly because the absorption process becomes saturated, and the absolute oral bioavailability (F) values after oral administration were 33–55%. Choi and Lee (2006) recently demonstrated, in male Sprague–Dawley rats, that metformin is primarily metabolized via hepatic CYP2C11, 2D1 and 3A1/2, but not via CYP1A2, 2B1/2 and 2E1. Metformin has been found to be a better substrate for renal organic cation transporter 2 (OCT2) than for hepatic OCT1, and renal OCT2 has an important role in the pharmacokinetics of metformin (Kimura et al., 2005). However, no study has yet been published on whether DA-8159 is a substrate for renal OCT2 in rats.
Erectile dysfunction is highly prevalent in diabetic patients (Musicki and Burnett, 2007), and phophodiesterase type V inhibitors are effective and safe drugs for the treatment of erectile dysfunction in men with diabetes (Buvat et al., 2006). Thus, in the present study, the pharmacokinetic interactions between DA-8159 and metformin were evaluated in rats, because both drugs are primarily metabolized via hepatic CYP3A1/2 in this species (Kim et al., 2005a; Choi and Lee, 2006). No studies on whether or not both drugs are inhibitors or inducers of CYP isozymes in rats have yet been published. Hence, the pharmacokinetic interaction between DA-8159 and metformin was determined after i.v. and p.o. administration of the two drugs, either simultaneously or alone, to male Sprague−Dawley rats.
Methods
Rats
The protocol for this animal study was approved by the Animal Care and Use Committee of the College of Pharmacy of Seoul National University, Seoul, South Korea. Male Sprague−Dawley rats, 6–9 weeks old and weighing 200–310 g, were purchased from the Taconic Farms Inc. (Samtako Bio Korea, O-San, South Korea). They were kept in a clean room (Animal Center for Pharmaceutical Research, College of Pharmacy, Seoul National University) at a temperature of 20–23 °C with 12-h light (0700–1900 hours) and dark (1900–0700 hours) cycles and a relative humidity of 50±5%. The rats were housed in metabolic cages (Tecniplast, Varese, Italy) under filtered pathogen-free air and with food (Samyang Company, Pyeongtaek, South Korea) and water available ad libitum.
Pretreatment of rats for i.v. and p.o. studies
In the early morning, each rat was lightly anaesthetized with ether before the jugular vein (for drug administration for i.v. study only) and the carotid artery (for blood sampling) were cannulated with a polyethylene tube (Clay Adams, Parsippany, NJ, USA) (Choi et al., 2006). Both cannulae were exteriorized to the dorsal side of the neck, where each cannula was terminated with a long silastic tube (Dow Corning, Midland, MI, USA). Both silastic tubes were inserted into a wire sheath to allow free movement of the rat. Each rat was housed individually in a rat metabolic cage (Daejong Scientific Company, Seoul, South Korea) and allowed to recover from the anaesthetic for 4–5 h before the experiments were started. Thus, the rats were not restrained in the present study.
Intravenous study
Either DA-8159 (30 mg kg–1, n=9; dissolved in a 0.05 M citric acid (pH 4) to produce a concentration of 15 mg ml–1), or metformin (100 mg kg–1, n=9; metformin hydrochloride was dissolved in a 0.05 M citric acid (pH 4) to produce a concentration of 50 mg ml–1 as metformin base) alone or both the drugs together (n=9) were infused (total infusion volume of 2 ml kg–1) for 1 min via the jugular vein. A blood sample (approximately 0.12 ml for DA-8159 and metformin alone, and 0.22 ml for both DA-8159 and metformin together) was collected via the carotid artery at 0 (control), 1 (at the end of the infusion), 5, 15, 30, 60, 120, 180, 240, 360, 480, 600 and 720 min after the start of the i.v. infusion of the drug(s). Blood samples were immediately centrifuged and a 50 μl plasma sample (two 50-μl samples for both drugs) was collected in a 1.5 ml polyethylene tube and stored at −70 °C (Revco ULT 1490 D-N-S; Western Mednics, Ashville, NC, USA) until used for the high-performance liquid chromatography (HPLC) analysis of DA-8159, DA-8164 and metformin. At the end of the experiment (24 h), each metabolic cage was rinsed with 10 ml of distilled water, and the rinsings were combined with the 24-h urine sample. After the exact volume of the combined urine sample had been measured, two 100-μl aliquots of the combined urine samples were stored at –70 °C until used for the HPLC analysis of DA-8159, DA-8164 and metformin. At the same time (24 h), each rat was exsanguinated and killed by cervical dislocation. Then, the abdomen was opened and the entire gastrointestinal tract (including its contents and faeces) of each rat was removed, transferred into a beaker containing 100 ml of methanol (to facilitate the extraction of DA-8159, DA-8164 and metformin), and cut into small pieces with scissors. After stirring with a glass rod for 1 min, two 100-μl aliquots of the supernatant were collected from each beaker and stored at −70 °C until used for the HPLC analysis of DA-8159, DA-8164 and metformin.
Oral study
DA-8159 (the same solution used in the i.v. study) at a dose of 30 mg kg–1 (n=6), metformin (the same solution used in the i.v. study) at a dose of 100 mg kg–1 (n=7) or both (n=8) were orally administered (total p.o. volume of 6 ml kg–1) using a feeding tube to rats. A blood sample (approximately 0.12 ml for DA-8159 alone and metformin alone, and 0.22 ml for both DA-8159 and metformin) was collected via the carotid artery at 0, 15, 30, 60, 90, 120, 180, 240, 360, 480, 600, 720, 960, 1200 and 1440 min after oral administration of the drug(s). Other procedures were similar to those for the i.v. study.
Competitive inhibition for the metabolism of DA-8159 and metformin by each other in rat hepatic microsomal fractions
The procedures used for the preparation of hepatic microsomal fractions were similar to those used previously (Kim et al., 2006). Protein content in the hepatic microsomes was measured by the method used by Bradford (1976).
To determine whether DA-8159 inhibits metabolism of metformin, the above hepatic microsomal fractions (equivalent to 0.5 mg protein), a 5-μl aliquot of 0.9% NaCl-injectable solution containing 1, 2.5, 5, 7.5, 10, 20, 50, 100 or 200 μM as metformin base (as a substrate), a 5-μl aliquot of a 0.05 M citrate buffer (pH 4) with or without 2, 10, 20, 100 or 200 μM DA-8159 (as an inhibitor) and 50 μl of 0.1 M phosphate buffer (pH 7.4) containing 1 mM NADPH, in a final volume of 0.5 ml, were incubated in a water-bath shaker (kept at 37 °C, 500 oscillations per min). The reaction was terminated by addition of 1 ml of acetonitrile after 15-min incubation.
The procedures used to determine whether metformin inhibits metabolism of DA-8159 were similar to those used to investigate DA-8159 inhibition of metformin; a 5-μl aliquot of 0.05 M citrate buffer (pH 4) containing 1, 2.5, 5, 10, 20, 50, 100 or 200 μM DA-8159 (as a substrate) and a 5-μl aliquot of 0.9% NaCl solution with or without 2, 10, 20, 100 or 200 μM metformin base (as an inhibitor) were used. The reaction was terminated by addition of 1 ml of ether after 5-min incubation.
Measurement of Vmax, Km, CLint and Ki for the disappearance of DA-8159 and for the formation of DA-8164 with and without metformin, and for the disappearance of metformin with or without DA-8159 in rat hepatic and intestinal microsomal fractions
The procedures used for the preparation of hepatic and intestinal microsomal fractions were as described previously (Peng et al., 2004; Kim et al., 2006, respectively). Protein content in the hepatic and intestinal microsomes was also measured using the method by Bradford (1976).
The Vmax (the maximum velocity) and Km (the Michaelis–Menten constant—the concentration at which the rate is one-half of Vmax) for the disappearance of DA-8159 and for the formation of DA-8164 with or without metformin were determined after incubating the above microsomal fractions (equivalent to 0.5 mg protein for both hepatic and intestinal microsomes), a 5-μl aliquot of 0.05 M citrate buffer (pH 4) containing 0.5, 2, 5, 10, 20, 50, 100 or 200 μM DA-8159 (hepatic microsomes) or 5, 10, 20, 50 or 200 μM DA-8159 (intestinal microsomes), and a 5-μl aliquot of 0.9% NaCl solution in the absence or presence of 10 μg (hepatic microsomes) or 1 μg (intestinal microsomes) metformin base. The reaction was terminated by addition of 1 ml of ether after 5 and 45 min incubation for hepatic and intestinal microsomes, respectively.
The procedures used to measure the Vmax and Km for the disappearance of metformin with or without DA-8159 were similar to those mentioned above for DA-8159. A 5-μl aliquot of 0.9% NaCl solution containing 1, 2.5, 5, 7.5, 10, 20, 50, 100 or 200 μM metformin base (hepatic microsomes) or 1, 2.5, 5, 7.5, 10, 20, 50, 100 or 150 μM (intestinal microsomes) metformin base, and a 5-μl aliquot of 0.05 M citrate buffer (pH 4) with or without 10 μg (hepatic microsomes) or 2 μg (intestinal microsomes) of DA-8159 were used. The reaction was terminated by addition of 1 ml of acetonitrile after 15 and 60 min incubation for hepatic and intestinal microsomes, respectively.
All of the above microsomal incubation conditions were linear. The kinetic constants (Km and Vmax) for the disappearance of DA-8159 and for the formation of DA-8164 with or without metformin, and for the disappearance of metformin with or without DA-8159 were calculated using a nonlinear regression method (Duggleby, 1995). The intrinsic clearance (CLint) was calculated by dividing the Vmax by the respective Km.
The apparent inhibition constant (Ki) of DA-8159 for the disappearance of metformin and of metformin for the disappearance of DA-8159 and for the formation of DA-8164 in hepatic and intestinal microsomal fractions were also measured. The Ki of an inhibitor (metformin or DA-8159) was calculated using the following equation for competitive inhibition (Segel, 1975);
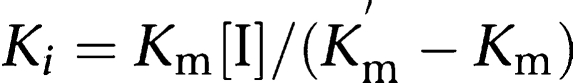 |
where [I] is the concentration of an inhibitor, and Km and Km′ are the Michaelis–Menten constants of the substrate without and with an inhibitor, respectively. In a competitive manner, the Ki value is equivalent to the concentration of an inhibitor that doubles the slope of the inverse of velocity for the disappearance versus the inverse of substrate concentration plot.
Bechmann and Lewis (2005) suggest that if, for a putative inhibitory drug–drug interactions, the [I]/Ki ratio is <0.01, the mechanism is not attributable to CYP inhibition, and the [I]/Ki ratio is >2, the drug–drug interaction is due to CYP inhibition.
Measurement of liver and intestinal concentrations of DA-8159, DA-8164 and metformin after iv. or p.o. administration of DA-8159 and metformin
The procedures used were similar to those described previously (Lee et al., 2005). The same doses of DA-8159 and metformin were administered intravenously and orally. At 1, 15, 30, 60, 120, 180 and 240 min (for i.v. administration) or 5, 15, 30, 60, 120, 180 and 240 min (for p.o. administration), as much blood as possible was collected via the carotid artery, and each rat was killed by cervical dislocation (n=3 or 4 at each time point for each route of administration). Approximately 1 g of the liver (i.v. and p.o. studies) and intestine (p.o. study) were excised and blotted with paper tissue. Then, each liver and intestine sample was homogenized with 4 volumes of a 0.9% NaCl solution and centrifuged (9000 g for 10 min). Two 100-μl aliquots of the supernatant were collected and stored at −70 °C until used for the HPLC analysis of DA-8159, DA-8164 and metformin.
HPLC analysis of DA-8159, DA-8164 and metformin
Concentrations of DA-8159 and DA-8164 in the above samples were determined by a slight modification of the HPLC method used by Shim et al. (2002); the biological sample was in a 50-μl aliquot instead of a 100-μl aliquot. A 0.1 ml aliquot of 0.1 N Na2CO3 containing 3 μg ml–1 of sildenafil (an internal standard) and 1 ml of ether were added to the 50-μl aliquot of biological sample. After vortex-centrifugation (16 000 g for 8 min), the ether layer was collected and dried (Dry thermobath, Eyela, Tokyo, Japan) under a gentle stream of nitrogen gas at 50 °C. A 100-μl aliquot of the mobile phase was added to reconstitute the residue and a 50-μl aliquot was directly injected onto a reversed-phase (C18) HPLC column. The mobile phase, 20 mM KH2PO4:acetonitrile (72:28 (v/v)), was run at a flow rate of 1.5 ml min–1 and the column eluent was monitored using an ultraviolet detector at 292 nm at room temperature. Retention times for DA-8159, DA-8164 and sildenafil (an internal standard) were approximately 9.7, 17.1 and 6.9 min, respectively. Shim et al. (2002) found that the limits of quantification (LOQs) of DA-8159 and DA-8164 in rat plasma and urine samples were all 0.05 μg ml–1. The inter- and intraday coefficients of variation (CVs) of both DA-8159 and DA-8164 are below 10.1% for rat plasma and 9.91% for rat urine samples.
Concentrations of metformin in the above samples were determined by a slight modification in the HPLC method described by Hale et al. (2002); ipriflavone instead of hydrocodeine was used as an internal standard. A 50-μl aliquot of biological sample was deproteinized with 100 μl of acetonitrile, and 50 μl of methanol containing 10 μg ml–1 of ipriflavone (an internal standard) was added. After the sample had been vortex-mixed and centrifuged (16 000 g for 10 min), a 50-μl aliquot was injected directly onto a reversed-phase (C18) HPLC column. The mobile phase (pH=6), 10 mM KH2PO4:acetonitrile (47.8:52.2 (v/v)) was run at a flow-rate of 1.5 ml min–1, and the column eluent was monitored using an ultraviolet detector at 235 nm at room temperature. The retention times of metformin and ipriflavone (an internal standard) were approximately 4 and 6.5 min, respectively. The LOQs of metformin in the rat plasma and urine samples were 0.05 and 1 μg ml–1, respectively. The inter- and intraday CVs of metformin were below 9.91 and 7.52% for rat plasma and urine samples, respectively, in the concentration ranges of 0.05–5000 and 1–1000 μg ml–1 for the plasma and urine samples, respectively.
Pharmacokinetic analysis
Standard methods (Gibaldi and Perrier, 1982) were used to calculate the following pharmacokinetic parameters using a noncompartmental analysis (WinNonlin; Pharsight Corporation, Mountain View, CA, USA): the total area under the plasma concentration–time curve from time zero to time infinity (AUC), the time-averaged total body (CL), renal (CLR) and nonrenal (CLNR) clearances, the terminal t1/2, the first moment of AUC (AUMC), the mean residence time, the apparent volume of distribution at a steady state (Vss) and F. The peak plasma concentration (Cmax) and time to reach Cmax (Tmax) were obtained directly from the experimental data.
Statistical analysis
A P-value <0.05 was deemed to be statistically significant, determined by using Student's t-test for unpaired data. All results are expressed as mean±s.d. except median (ranges) for Tmax.
Chemicals
Metformin hydrochloride was donated by Daelim Pharmaceutical Company (Seoul, South Korea). DA-8159, DA-8164 and sildenafil (an internal standard for the high-performance liquid chromatographic (HPLC) analysis of DA-8159 and DA-8164), ipriflavone (an internal standard for the HPLC analysis of metformin) were supplied from Research Laboratory of Dong-A Pharmaceutical Company. The reduced form of β-NADPH (as a tetrasodium salt), tris(hydroxymethyl)aminomethane (Tris)-buffer, and ethylenediamine tetraacetic acid (EDTA) were purchased from Sigma–Aldrich Corporation (St Louis, MO, USA). Other chemicals were of reagent grade or HPLC grade.
Results
Pharmacokinetics of DA-8159 and DA-8164 after i.v. administration of DA-8159 with or without metformin to rats
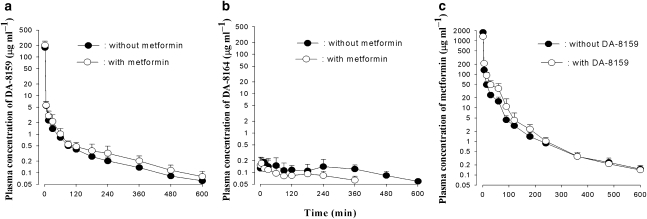
For the i.v. administration of DA-8159 with or without metformin, the mean arterial plasma concentration–time profiles of DA-8159 and DA-8164 are shown in Figures 1a and b, respectively, and relevant pharmacokinetic parameters are listed in Table 1. After simultaneous i.v. administration of both drugs, changes in pharmacokinetic parameters of DA-8159 compared with those without metformin (DA-8159 alone) are as follows: the AUC was significantly greater (21.2% increase); CL, CLR, and CLNR were significantly slower (18.1, 54.9, and 15.7% decrease, respectively); percentage of the i.v. dose of DA-8159 excreted in the 24-h urine sample as unchanged drug (Ae0–24 h) was significantly smaller (47.4% decrease) compared with that of DA-8159 alone. The Ae0–24 h of DA-8159 and contribution of the CLR to CL of DA-8159 (6.54 and 3.60% for without and with metformin, respectively) were very small. Thus, these changes are unlikely to have a marked effect on other pharmacokinetic interactions of DA-8159 with metformin.
Figure 1.
Arterial plasma concentration–time profiles of DA-8159 (a), DA-8164 (b) and metformin (c) after single i.v. administration of DA-8159 at a dose of 30 mg kg–1, metformin at a dose of 100 mg kg–1 or both drugs simultaneously to rats. Data are presented as mean±s.d.
Table 1.
Pharmacokinetic parameters of DA-8159, DA-8164 and metformin after single i.v. administration of DA-8159 at a dose of 30 mg kg−1, metformin at a dose of 100 mg kg−1 and both drugs to rats
| Parameter |
DA-8159 |
Parameter |
Metformin |
||
|---|---|---|---|---|---|
| Without metformin | With metformin | Without DA-8159 | With DA-8159 | ||
| Body weight (g) | 286±8.82 | 284±7.82 | Body weight (g) | 284±12.4 | 284±7.82 |
| DA-8159 | Metformin | ||||
| AUC (μg min ml−1) | 504±71.2 | 611±69.0** | AUC (μg min ml−1) | 6130±877 | 8210±1590** |
| Terminal t1/2 (min) | 208±36.5 | 194±47.1 | Terminal t1/2 (min) | 176±61.5 | 237±77.3 |
| Vss (ml kg−1) | 4820±1650 | 5600±1410 | Vss (ml kg−1) | 539±243 | 454±71.6 |
| CL (ml min−1 kg−1) | 60.7±9.41 | 49.7±6.06** | CL (ml min−1 kg−1) | 16.8±3.41 | 12.5±1.94** |
| CLR (ml min−1 kg−1) | 3.97±1.24 | 1.79±1.15** | CLR (ml min−1 kg−1) | 9.46±2.54 | 6.56±1.40** |
| CLNR (ml min−1 kg−1) | 56.8±9.78 | 47.9±5.58* | CLNR (ml min−1 kg−1) | 7.30±1.13 | 5.98±0.643** |
| Ae0–24 h (% of DA-8159 dose) | 6.75±2.64 | 3.55±2.04* | Ae0–24 h (% of metformin dose) | 56.1±4.33 | 51.9±3.73* |
| GI24 h (% of DA-8159 dose) | 1.87±0.848 | 2.51±1.37 | GI24 h (% of metformin dose) | 1.00±1.05 | 0.816±0.600 |
| DA-8164 | |||||
| AUC (μg min ml−1) | 72.2±28.7 | 48.9±12.9* | |||
| Terminal t1/2 (min) | 194±57.4 | 205±72.5 | |||
| Cmax (μg ml−1) | 0.230±0.0655 | 0.211±0.0974 | |||
| Tmax (min) | 30 (5–240) | 5 (5–30) | |||
| Ae0–24 h (% of DA-8159 dose) | 0.160±0.0961 | 0.142±0.0511 | |||
| GI24 h (% of DA-8159 dose) | 0.665±0.197 | 0.772±0.274 | |||
Data are expressed as mean±s.d. (each group, n=9). Significantly different from without metformin or without DA-8159, *P<0.05 and **P<0.01.
After i.v. administration of DA-8159 with or without metformin, formation of DA-8164 was rapid; DA-8164 was detected in plasma from the first blood sampling time (1 min) for both with and without metformin (Figure 1b), and rapidly reached a Tmax for DA-8164: 5 and 30 min for with and without metformin, respectively. After i.v. administration of both drugs, the AUC of DA-8164 was significantly smaller (32.3% decrease) compared with that without metformin (DA-8159 alone). Other pharmacokinetic parameters of DA-8164 listed in Table 1 were not significantly different between the two groups of rats.
Pharmacokinetics of metformin after i.v. administration of metformin with or without DA-8159 to rats
The mean arterial plasma concentration–time profiles for metformin after i.v. administration of metformin, with or without DA-8159, are shown in Figure 1c and the relevant pharmacokinetic parameters are listed in Table 1. After i.v. administration of both drugs, the changes in pharmacokinetic parameters of metformin compared with those without DA-8159 (metformin alone) are as follows: the AUC was significantly greater (33.9% increase); CL, CLR, and CLNR were significantly slower (25.6, 30.7 and 18.1% decrease, respectively); Ae0–24 h was significantly smaller (7.49% decrease).
Pharmacokinetics of DA-8159 and DA-8164 after p.o. administration of DA-8159 with or without metformin to rats
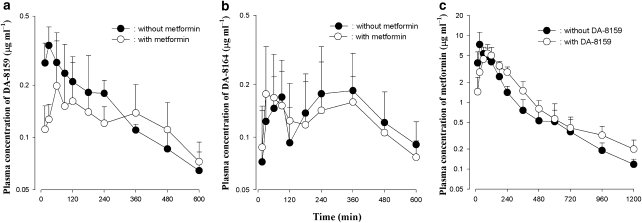
The mean arterial plasma concentration–time profiles of DA-8159 and DA-8164 after the p.o. administration of DA-8159 with or without metformin are shown in Figures 2a and b, respectively, and the relevant pharmacokinetic parameters are listed in Table 2. After p.o. administration of DA-8159 with or without metformin, absorption of DA-8159 was rapid: DA-8159 was detected in plasma from the first blood sampling time (15 min) for both with and without metformin groups (Figure 2a). Pharmacokinetic parameters of both DA-8159 and DA-8164, listed in Table 2, were similar (not significantly different) in the groups with and without metformin, except that the Tmax was significantly shorter in the DA-8159 (400% decrease) with metformin group than that of the DA-8159 alone group.
Figure 2.
Arterial plasma concentration–time profiles of DA-8159 (a), DA-8164 (b) and metformin (c) after single p.o. administration of DA-8159 at a dose of 30 mg kg–1, metformin at a dose of 100 mg kg–1 or both drugs simultaneously to rats. Data are presented as mean±s.d.
Table 2.
Pharmacokinetic parameters of DA-8159, DA-8164 and metformin after single p.o. administration of DA-8159 at a dose of 30 mg kg−1, metformin at a dose of 100 mg kg−1 and both drugs to rats
| Parameter |
DA-8159 |
Parameter |
Metformin |
||
|---|---|---|---|---|---|
| Without metformin | With metformin | Without DA-8159 | With DA-8159 | ||
| Body weight (g) | 226±42.7 | 242±17.9 | Body weight (g) | 243±7.56 | 242±17.9 |
| DA-8159 | Metformin | ||||
| AUC (μg min ml−1) | 107±15.1 | 104±14.5 | AUC (μg min ml−1) | 1400±174 | 1690±277* |
| Terminal t1/2 (min) | 216±50.4 | 262±62.7 | Terminal t1/2 (min) | 310±32.9 | 392±84.2* |
| CLR (ml min−1 kg−1) | 2.85±0.681 | 2.70±1.12 | CLR (ml min−1 kg−1) | 30.5±10.1 | 19.6±4.01* |
| Cmax (μg ml−1) | 0.355±0.0911 | 0.268±0.171 | Cmax (μg ml−1) | 7.92±3.41 | 5.74±1.69 |
| Tmax (min) | 30 (15–90) | 150 (60–480)* | Tmax (min) | 30 (30–90) | 90 (60–90)** |
| Ae0–24 h (% of DA-8159 dose) | 1.04±0.354 | 0.931±0.388 | Ae0–24 h (% of metformin dose) | 41.7±9.32 | 32.4±5.74* |
| GI24 h (% of DA-8159 dose) | 4.96±5.94 | 1.93±1.64 | GI24 h (% of metformin dose) | 1.51±0.583 | 0.967±0.654 |
| F (%) | 21.2 | 17.0 | F (%) | 23.5 | 20.6 |
| DA-8164 | |||||
| AUC (μg min ml−1) | 101±38.3 | 97.8±36.6 | |||
| Terminal t1/2 (min) | 204±38.1 | 254±90.1 | |||
| Cmax (μg ml−1) | 0.269±0.110 | 0.111±0.0382 | |||
| Tmax (min) | 300 (30–360) | 90 (30–360) | |||
| Ae0–24 h (% of DA-8159 dose) | 0.124±0.0471 | 0.524±0.232 | |||
| GI24 h (% of DA-8159 dose) | 0.276±0.235 | 0.421±0.232 | |||
Data are expressed as mean±s.d. (DA-8159 without metformin, n=6; DA-8159 with metformin and metformin with DA-8159, n=8; metformin without DA-8159, n=7). Significantly different from without metformin or without DA-8159, *P<0.05 and **P<0.01.
Pharmacokinetics of metformin after p.o. administration of metformin with or without DA-8159 to rats
The mean arterial plasma concentration–time profiles of metformin after the p.o. administration of metformin with or without DA-8159 are shown in Figure 2c, and relevant pharmacokinetic parameters are also listed in Table 2. After p.o. administration of metformin with or without DA-8159, absorption of metformin was also rapid: metformin was detected in plasma from the first blood sampling time (15 min) in both with and without DA-8159 groups (Figure 2c). After p.o. administration of both drugs, changes in pharmacokinetic parameters of metformin compared with those of without DA-8159 (metformin alone) are as follows: the AUC was significantly greater (20.7% increase), terminal t1/2 was significantly longer (26.5% increase), CLR was significantly slower (35.7% increase), Tmax was significantly longer (200% increase) and Ae0–24 h was significantly smaller (22.3% decrease) than that of the metformin alone group.
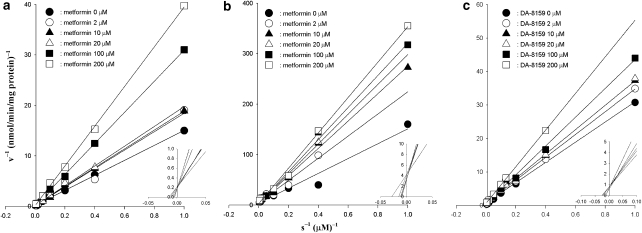
Competitive inhibition for the metabolism of DA-8159 and for the formation of DA-8164 by metformin, and for the disappearance of metformin by DA-8159 in rat hepatic microsomal fractions
Lineweaver and Burk (1934) for the disappearance of DA-8159 and for the formation of DA-8164, in the presence and absence of metformin, and for the disappearance of metformin, in the presence and absence of DA-8159, in hepatic microsome are shown in Figures 3a–c, respectively. A linear relationship between the inverse of the substrate (DA-8159 or metformin) concentrations and the inverse of the velocities of substrate (disappearance of DA-8159 and metformin and formation of DA-8164) was observed, indicating that the inhibition by each drug was competitive. Metformin competitively inhibited metabolism of DA-8159 and formation of DA-8164, and DA-8159 also competitively inhibited metabolism of metformin.
Figure 3.
Lineweaver–Burk plots showing inhibition of the disappearance of DA-8159 (a) and of the formation of DA-8164 (b) by metformin, and of the disappearance of metformin (c) by DA-8159 in rat hepatic microsomal fractions. The ‘s' represents the DA-8159 (a), DA-8164 (b) or metformin (c) concentration. ‘v' represents the velocity for the disappearance of DA-8159 (a), for the formation of DA-8164 (b) and for the disappearance of metformin (c).
Measurement of Vmax, Km, CLint, Ki and [I]/Ki for the disappearance of DA-8159 and for the formation of DA-8164 with or without metformin, and for the disappearance of metformin with or without DA-8159 in rat hepatic microsomal fractions
The Vmax, Km and CLint for the disappearance of DA-8159 and for the formation of DA-8164 in hepatic microsomal fractions in the with and without metformin groups are listed in Table 3. The Vmax for the disappearance of DA-8159 and for the formation of DA-8164 was comparable between the with and without metformin groups, suggesting that the Vmax for the disappearance (primarily metabolism) of DA-8159 and for the formation of DA-8164 was not changed by metformin in the liver. However, the Km for the disappearance of DA-8159 (51.3% increase; P=0.081) and for the formation of DA-8164 (68.3% increase; P<0.05) in the presence of metformin was higher than that in the absence of metformin. This suggests that the affinity of DA-8159 for the enzyme(s) in the liver (primarily hepatic CYP3A1/2) was decreased by metformin. As a result, the CLint for the disappearance of DA-8159 (48.2% decrease; P<0.01) and for the formation of DA-8164 (24.1% decrease; P=0.0670) were slower in the presence of metformin. This suggests that the metabolism of DA-8159 and formation of DA-8164 were inhibited by metformin in the liver.
Table 3.
Vmax, Km, CLint, Ki and [I]/Ki for the disappearance of DA-8159 and for the formation of DA-8164 with and without metformin, or for the disappearance of metformin with and without DA-8159 in hepatic microsomal fractions
| Parameter |
DA-8159 |
Parameter |
Metformin |
||
|---|---|---|---|---|---|
| Without metformin | With metformin | Without DA-8159 | With DA-8159 | ||
| Disappearance of DA-8159 | Disappearance of metformin | ||||
| Vmax (nmol min−1 mg protein−1) | 2.71±0.489 | 2.18±0.593 | Vmax (nmol min−1 mg protein−1) | 5.01±0.845 | 7.43±3.12 |
| Km (μM) | 115±36.4 | 174±43.1 | Km (μM) | 175±26.3 | 319±159 |
| CLint (μl min−1 mg protein−1) | 24.5±5.11 | 12.7±2.21** | CLint (μl min−1 mg protein−1) | 28.5±0.740 | 24.1±2.10** |
| Ki (μM) for the inhibition of metabolism of metformin | 2.30–47.7 | Ki (μM) for the inhibition of metabolism of DA-8159 (formation of DA-8164) | 21.6–120 (10.8–49.3) | ||
| [I]/Ki for the inhibition of metabolism of metformin | 0.407–8.43 | [I]/Ki for the inhibition of metabolism of DA-8159 (formation of DA-8164) | 0.503–2.79 (1.22–5.58) | ||
| Formation of DA-8164 | |||||
| Vmax (nmol min−1 mg protein−1) | 0.253±0.0832 | 0.334±0.0856 | |||
| Km (μM) | 83.2±31.4 | 140±16.1* | |||
| CLint (μl min−1 mg protein−1) | 3.11±0.590 | 2.36±0.330 | |||
Data are expressed as mean±s.d. (DA-8159 without and with metformin, n=4; metformin without and with DA-8159, n=5). Significantly different from without metformin or without DA-8159, *P<0.05 and **P<0.01.
The Vmax, Km and CLint for the disappearance of metformin in the absence and presence of DA-8159 in hepatic microsomal fractions are also listed in Table 3. The Vmax for the disappearance of metformin was similar in the absence and presence of DA-8159, suggesting that the Vmax for the disappearance (primarily metabolism) of metformin was not altered by DA-8159 in the liver. However, the Km for the disappearance of metformin in the presence of DA-8159 was higher (82.3% increase; P=0.0817) than that of metformin alone, suggesting that the affinity of metformin for the enzyme(s) was decreased by DA-8159. As a result, the CLint for the disappearance of metformin was significantly slower (15.4% decrease) in the presence of DA-8159, suggesting that metabolism of metformin was inhibited by DA-8159 in the liver. The above data indicate that inhibition for the metabolism of metformin by DA-8159 and for the metabolism of DA-8159 and for the formation of DA-8164 by metformin is competitive in the liver.
The apparent Ki values of metformin for the inhibition of metabolism of DA-8159 and formation of DA-8164 in hepatic microsomal fractions were 21.6–120 and 10.8–49.3 μM, respectively. The corresponding value of DA-8159 for the inhibition of metabolism of metformin in hepatic microsomal fraction was 2.30–47.7 μM. The ratios of [I]/Ki of metformin for the inhibition of metabolism of DA-8159 and formation of DA-8164 in the liver were 0.503–2.79 and 1.22–5.58, respectively. The corresponding value of DA-8159 for the inhibition of metabolism of metformin was 0.407–8.43.
Measurement of Vmax, Km, CLint, Ki and [I]/Ki for the disappearance of DA-8159 and for the formation of DA-8164 with or without metformin, and for the disappearance of metformin with or without DA-8159 in rat intestinal microsomal fractions
The Vmax, Km and CLint for the disappearance of DA-8159 and for the formation of DA-8164 in the absence and presence of metformin, and for the disappearance of metformin in the absence and presence of DA-8159 in the intestinal microsomal fractions are listed in Table 4. The Vmax, Km and CLint for the disappearance of DA-8159 and for the formation of DA-8164 in the absence and presence of metformin, and for the disappearance of metformin with and without DA-8159 were comparable. The above data suggest that the Vmax for the disappearance of DA-8159 and metformin, for the formation of DA-8164, the affinity of the enzyme(s) for DA-8159 and metformin, and metabolism of DA-8159 and metformin and formation of DA-8164, were not inhibited by either drug in the intestinal microsomal fractions.
Table 4.
Vmax, Km, CLint, Ki and [I]/Ki for the disappearance of DA-8159 and for the formation of DA-8164 with and without metformin, or the disappearance of metformin with and without DA-8159 in intestinal microsomal fractions
| Parameter | DA-8159 | Parameter | Metformin | ||
|---|---|---|---|---|---|
| Without metformin | With metformin | Without DA-8159 | With DA-8159 | ||
| Disappearance of DA-8159 | Disappearance of metformin | ||||
| Vmax (nmol min–1 mg protein–1) | 81.6±24.9 | 96.1±21.9 | Vmax (nmol min–1 mg protein–1) | 107±43.5 | 134±36.9 |
| Km (μM) | 0.166±0.0252 | 0.226±0.0940 | Km (μM) | 0.284±0.101 | 0.347±0.159 |
| CLint (μl min–1 mg protein–1) | 2.14±0.565 | 2.30±0.527 | CLint (μl min–1 mg protein–1) | 2.84±1.11 | 2.59±1.01 |
| Ki (μM) for the inhibition of metabolism of metformin | 11.2–32.6 | Ki (μM) for the inhibition of metabolism of DA-8159 (formation of DA-8164) | 50.2–86.2 (11.0–35.9) | ||
| [I]/Ki for the inhibition of metabolism of metformin | 0.199–0.346 | [I]/Ki for the inhibition of metabolism of DA-8159 (formation of DA-8164) | 0.0700–0.120 (0.168–0.548) | ||
| Formation of DA-8164 | |||||
| Vmax (nmol min–1 mg protein–1) | 52.0±26.1 | 46.6±11.1 | |||
| Km (μM) | 0.0550±0.0160 | 0.0693±0.0187 | |||
| CLint (μl min–1 mg protein–1) | 1.19±0.364 | 1.54±0.562 | |||
Data are expressed as mean±s.d. (DA-8159 without metformin, DA-8159 with metformin, n=4; metformin without and with DA-8159, n=5).
The apparent Ki values of metformin for the inhibition of metabolism of DA-8159 and of formation of DA-8164 in intestinal microsomal fractions were 50.2–86.2 and 11.0–35.9 μM, respectively. The corresponding value of DA-8159 for the inhibition of metabolism of metformin was 11.2–32.6 μM. The values of [I]/Ki of metformin for the inhibition of metabolism of DA-8159 and of formation of DA-8164 in the intestine were 0.0700–0.120 and 0.168–0.548, respectively. The corresponding value of DA-8159 for the inhibition of metabolism of metformin was 0.199–0.346.
Liver and/or intestinal concentrations of DA-8159 and metformin after i.v. and p.o. administration of both drugs
After i.v. and p.o. administration of DA-8159 and metformin, concentrations and tissue-to-plasma ratios of each drug in the liver and/or intestine are listed in Table 5. Rat liver and intestine showed a good affinity for DA-8159; the tissue-to-plasma ratios were greater than unity. However, intestine-to-plasma ratios of metformin after p.o. administration were lower than unity except at 1 min.
Table 5.
Concentrations (μM) of DA-8159 and metformin in the liver (i.v. and p.o. studies) and intestine (p.o. study) after simultaneous single i.v. and p.o. administration of DA-8159 (30 mg kg–1) and metformin (100 mg kg–1) to rats
| Time (min) |
i.v. |
Time (min) |
p.o. |
||||
|---|---|---|---|---|---|---|---|
|
Liver |
Liver |
Intestine |
|||||
| DA-8159 (n=4) | Metformin (n=4) | DA-8159 (n=4) | Metformin (n=4) | DA-8159 (n=3) | Metformin (n=3) | ||
| 1 | 145±64.5 | 216±37.9 | 5 | 16.0±4.84 | 3.22±1.23 | 32.7±7.83 | 41.2±1.37 |
| (2.67±2.14)a | (0.0853±0.0259) | (37.7±12.9) | (1.16±0.466) | (51.1±4.67) | (6.24±1.17) | ||
| 15 | 123±46.7 | 385±60.1 | 15 | 25.7±9.63 | 8.60±1.41 | 33.6±6.86 | 34.9±1.67 |
| (15.8±4.20) | (2.44±2.52) | (63.8±22.7) | (0.831±0.367) | (51.5±34.0) | (0.772±0.0490) | ||
| 30 | 60.6±18.8 | 317±40.0 | 30 | 56.9±23.3 | 14.4±1.29 | 4.44±1.42 | 10.5±1.39 |
| (20.2±6.46) | (3.06±0.247) | (68.6±10.5) | (0.774±0.417) | (5.23±0.909) | (0.136±0.0215) | ||
| 60 | 23.4±8.62 | 79.6±18.2 | 60 | 22.8±12.1 | 20.8±5.80 | 5.48±1.48 | 8.67±4.87 |
| (10.8±3.10) | (1.80±0.210) | (34.5±12.1) | (1.33±0.933) | (7.72±5.86) | (0.0606±0.0348) | ||
| 120 | 11.0±3.33 | 25.0±14.2 | 120 | 20.1±4.08 | 13.5±5.24 | 3.86±1.39 | 8.56±0.586 |
| (11.8±1.89) | (2.30±0.709) | (59.9±11.8) | (1.49±0.574) | (3.26±1.92) | (0.0679±0.00386) | ||
| 180 | 10.5±1.69 | 9.69±3.53 | 180 | 18.1±4.02 | 6.58±2.41 | 2.85±1.31 | 3.81±0.245 |
| (12.7±3.46) | (2.31±0.543) | (53.7±17.4) | (1.31±0.518) | (2.35±0.432) | (0.0812±0.0247) | ||
| 240 | 9.95±0.538 | 6.48±0.861 | 240 | 21.4±2.95 | 5.60±0 0.428 | 1.76±0.459 | 2.89±0.182 |
| (13.8±5.63) | (2.05±0.984) | (53.8±15.5) | (1.25±0.442) | (2.12±0.991) | (0.0533±0.00448) | ||
Data are expressed as mean±s.d. (each group, n=4).
The values in parentheses represent the tissue-to-plasma (T/P) ratios.
Discussion
Shim et al. (2003) and Choi et al. (2006) obtained dose-proportional AUC of DA-8159 and metformin, respectively, after i.v. or p.o. administration to rats. Thus, 30 mg kg–1 DA-8159 and 100 mg kg–1 metformin, which are within the range of dose-proportional AUC of each drug, were the concentrations used in the present study.
Following i.v. administration of both drugs together, a significantly greater AUC for both DA-8159 and metformin was observed which could be due to the significantly slower CL than that of each drug alone (Table 1). The slower CL of both drugs was attributable to a decrease in both the CLNR and CLR than that of each drug alone (Table 1). The slower CLNR of both drugs (Table 1) is probably due to competitive inhibition for the metabolism of each drug by each other via hepatic CYP3A1/2. Shim et al. (2003) and Choi et al. (2006) deduced that changes in the CLNR of DA-8159 and metformin, respectively, were due to changes in metabolism of each drug in rats. Shim et al. (2003) and Choi et al. (2006) also found that the hepatic first-pass effects of DA-8159 and metformin were 23.0 and 27.1%, respectively, in rats. Because both drugs are low hepatic extraction ratio drugs, their hepatic clearances depend more on the CLint for the disappearance of each drug rather than on the hepatic blood flow rate (Wilkinson and Shand, 1975). The significantly slower CLNR of DA-8159 and metformin than that of either drug alone (Table 1) is consistent with the significantly slower in vitro hepatic CLint for the disappearance of DA-8159 by metformin and for the disappearance of metformin by DA-8159, respectively (Table 3).
The significantly greater AUC of both DA-8159 and metformin, after i.v. administration together, accords with the apparent Ki and concentrations of each drug found in the liver. The liver concentrations of metformin (for up to 120 min; Table 5) were higher than or in the ranges of the apparent Ki of metformin for the inhibition of metabolism of DA-8159 and formation of DA-8164 (Table 3). The liver concentrations of DA-8159 (for up to 240 min; Table 5) were also higher than or in the ranges of apparent Ki values of DA-8159 for the inhibition of metabolism of metformin (Table 3). After i.v. administration of both drugs simultaneously, the contribution of the AUC0–120 min to the total AUC of DA-8159 (AUC0–120 min/AUC) and of metformin (AUC0–240 min/AUC) was substantial, 79.2 and 97.8% for DA-8159 and metformin, respectively. The above data indicate that metformin and DA-8159 significantly inhibit the metabolism of DA-8159 (formation of DA-8164) and metformin, respectively. The significantly smaller AUC of DA-8164 (Table 1) may be due to the inhibition of metabolism of DA-8159, to form DA-8164, by metformin. Also the ratios of [I]/Ki of metformin (Table 3) for the inhibition of metabolism of DA-8159 and for the formation of DA-8164, and of DA-8159 for the inhibition of metabolism of metformin, indicate that the metformin–DA-8159 interaction is mainly due to inhibition of CYP3A1/2 in the liver (Bechmann and Lewis, 2005).
The significantly slower CLR of each drug after their i.v. administration together could have been due to the significantly smaller 24-h urinary excretion (Ae0–24 h) and significantly greater AUC of each drug than those seen after administration of each drug alone (Table 1). The smaller Ae0–24 h of each drug (Table 1) was not likely to be due to inhibition of OCT2 by each drug as Kimura et al. (2005) showed that whereas metformin is a substrate for renal OCT2, DA-8159 is not. In a preliminary experiment involving LLC-PK1 cells expressing rOCT2 (Sung et al., 2005), the cellular uptake of [3H]-1-methyl-4-phenylpyridinium (MPP; specific activity of 83.5 Ci mmol−1), a standard substrate for renal OCT2 (Sung et al., 2005), was not affected by addition of DA-8159 for up to 1 mM, suggesting that DA8159 is not transported by a common transport mechanism with MPP (for example, OCT2).
After p.o. administration of both drugs, the AUC of metformin was significantly greater than that in the absence of DA-8159 (metformin alone) (Table 2). This is unlikely to be due to DA-8159 increasing the absorption of metformin from the gastrointestinal tract. The mean ‘true' fraction of the p.o. dose of metformin unabsorbed (‘Funabs'), based on an equation reported by Lee and Chiou (1983), was estimated to be 0.799 and 1.28% in the presence and absence of DA-8159, respectively. Thus, more than 98% of the p.o. dose of metformin was absorbed in both the with and without DA-8159 groups. The greater AUC of metformin after p.o. administration of both drugs (Table 2) could have been due to a competitive inhibition of hepatic metabolism of metformin by DA-8159. This hypothesis is supported by the significantly slower hepatic CLint of metformin by DA-8159 (Table 3) and by apparent Ki values and concentrations of DA-8159 found in the liver. The concentrations of DA-8159 in the liver after p.o. administration of both drugs (for up to 240 min; Table 5) were in the ranges of apparent Ki values of DA-8159 (Table 3). The AUC0–240 min/AUC of DA-8159 after p.o. administration of both drugs was considerable, 52.0%. Also the ratios of [I]/Ki of DA-8159 for the inhibition of hepatic metabolism of metformin (Table 3) indicate that the significantly greater AUC of metformin could have been due to inhibition of hepatic CYP3A1/2 by DA-8159 (Bechmann and Lewis, 2005). The above data indicate that DA-8159 significantly inhibits the hepatic metabolism of metformin after p.o. administration. However, DA-8159 has little effect on the intestinal metabolism of metformin and this does not appear to contribute to the greater AUC of metformin after p.o. administration of both drugs; the intestinal CLint values for the disappearance of metformin in the absence and presence of DA-8159 were similar (Table 4). Also, the intestinal concentrations of DA-8159 (Table 5) were in the ranges of the apparent Ki of DA-8159 (Table 4) for only 15 min and the AUC0–15 min/AUC of DA-8159 after p.o. administration of both drugs was almost negligible, only 1.86%. In addition, the ratios of [I]/Ki of DA-8159 for the inhibition of metabolism of metformin in the intestine (Table 4) indicate that the metformin–DA-8159 interaction was not due to intestinal CYP3A1/2 inhibition (Bechmann and Lewis, 2005). Therefore, the significantly greater AUC of metformin after p.o. administration of both drugs (Table 2) is likely to be mainly due to inhibition of hepatic metabolism of metformin by DA-8159.
In contrast to metformin, the AUCs of DA-8159 and DA-8164 were similar in the absence and presence of metformin after p.o. administration (Table 2), even though, after i.v. administration, the AUC of DA-8159 (DA-8164) was significantly greater (smaller) in the presence of metformin than in its absence (Table 1). It is unlikely that metformin decreases the absorption of DA-8159 from the gastrointestinal tract, as the estimated ‘Funabs' values were 1.50 and 4.56% in the presence and absence of metformin, respectively. Thus, more than 95% of the p.o. dose of DA-8159 was absorbed in both the absence and presence of metformin. Competitive inhibition by metformin of both hepatic and intestinal metabolism of DA-8159 had almost no effect on the disappearance of DA-8159 (for the formation of DA-8164). The ratios of [I]/Ki of metformin for the inhibition of metabolism of DA-8159 and formation of DA-8164 in the liver (Table 3) indicate that the concentration of metformin used was enough to inhibit the metabolism of DA-8159 via hepatic CYP3A1/2 (Bechmann and Lewis, 2005). However, the hepatic first-pass effect of DA-8159 after p.o. administration of the drug at a dose of 30 mg kg1 to rats was almost negligible; only 9.60% of the p.o. dose (Shim et al., 2003). Moreover, the liver concentrations of metformin at all times (Table 5) were considerably lower than the apparent Ki of metformin for the inhibition of metabolism of DA-8159 (Table 3) and formation of DA-8164, except at 30, 60 and 120 min (Table 3), indicating that the concentration of metformin was not sufficient to inhibit hepatic metabolism of metformin, possibly due to a lack of saturation of the hepatic enzymes involved in the metabolism of DA-8159. The concentrations of metformin in the intestine were higher than or equivalent to the apparent Ki for the inhibition of metabolism of DA-8159 (Table 4) and formation of DA-8164 (Table 4) only up to 15 min (Table 5); the AUC0–15 min/AUC of metformin was almost negligible, only 2.08%. Thus, the intestinal CLint values for the disappearance of DA-8159 and for the formation of DA-8164 in the intestine were similar in the absence and presence of metformin (Table 4). Also, based on the ratios of [I]/Ki (Table 4) metformin is a weak inhibitor of the metabolism of DA-8159 and the formation of DA-8164 in the intestine (Bechmann and Lewis, 2005). The above data support the similar AUC values of DA-8159 observed in the absence and presence of metformin after p.o. administration of the drugs (Table 2).
Conclusions
The significantly greater AUC of both DA-8159 and metformin observed after their simultaneous i.v. administration (Table 1) indicates competitive inhibition of the metabolism of each drug via hepatic CYP3A1/2. The significantly greater AUC of metformin in the presence of DA-8159 than in its absence, after p.o. administration, is also likely to be due to inhibition of the hepatic metabolism of metformin by DA-8159. On the other hand, the similar AUCs of DA-8159 and DA-8164 in the absence and presence of metformin, after p.o. administration, indicates that the dose of metformin used was not enough to inhibit the metabolism of DA-8159 and the formation of DA-8164 in either the liver or the intestine. As the pharmacokinetic interaction between metformin and DA-8159 has not yet been investigated in man, our results indicate that these studies should be done to determine whether changes in the p.o. dose of metformin are required when this drug is administered concomitantly with DA-8159.
Acknowledgments
This work was supported in part by a grant from the Seoul City Collaborative Project among the Industry, Academy and Research Institute, Korea.
Abbreviations
- Ae0–24 h
percentage of the dose excreted in the 24-h urine
- AUC
total area under the plasma concentration–time curve from time zero to time infinity
- Cmax
peak plasma concentration
- CL
time-averaged total body clearance
- CLint
intrinsic clearance
- CLNR
time-averaged nonrenal clearance
- CLR
time-averaged renal clearance
- F
extent of absolute oral bioavailability
- GI24 h
percentage of the dose recovered from the entire gastrointestinal tract (including its contents and faeces) at 24 h
- Ki
inhibition constant
- Km
Michaelis–Menten constant
- t1/2
half-life
- Tmax
time to reach Cmax
- Vmax
maximum velocity
- Vss
apparent volume of distribution at steady state
Conflict of interest
The authors state no conflict of interest.
References
- Bechmann KA, Lewis JD. Predicting inhibitory drug–drug interactions and evaluating drug interaction reports using inhibition constants. Ann Pharmacother. 2005;39:1064–1072. doi: 10.1345/aph.1E508. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buvat J, Ahlen H, Schmitt H, Chan M, Kuepfer C, Varanese L. Efficacy and safety of two dosing regimens of tadalafil and patterns of sexual activity in men with diabetes mellitus and erectile dysfunction: scheduled use vs on-demand regimen evaluation (SERE) study in 14 European countries. J Sex Med. 2006;3:512–520. doi: 10.1111/j.1743-6109.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Ji HY, Lee H-Y, Kim DS, Kim WB, Lee HS. In vitro metabolism of a novel phosphodiesterase-5 inhibitor DA-8519 in rat liver preparations using liquid chromatography/electrospray mass spectrometry. Biomed Chromatogr. 2002;16:395–399. doi: 10.1002/bmc.173. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kim SG, Lee MG. Dose-independent pharmacokinetics of metformin in rats: hepatic and gastrointestinal first-pass effects. J Pharm Sci. 2006;95:2543–2552. doi: 10.1002/jps.20744. [DOI] [PubMed] [Google Scholar]
- Choi YH, Lee MG. Effects of enzyme inducers and inhibitors on the pharmacokinetics of metformin in rats: involvement of CYP2C11, 2D1, and 3A1/2 for the metabolism of metformin. Br J Pharmacol. 2006;149:424–430. doi: 10.1038/sj.bjp.0706875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby RG. Analysis of enzyme progress curves by nonlinear regression. Methods Enzymol. 1995;249:61–90. doi: 10.1016/0076-6879(95)49031-0. [DOI] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D. Pharmacokinetics 1982Marcel–Dekker: New York; 2nd edn. [Google Scholar]
- Hale TW, Kristensen JH, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabetologia. 2002;45:1509–1514. doi: 10.1007/s00125-002-0939-x. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee I, Kim SG, Ko SH, Lee MG, Kim SH. Effects of glucose supplementation on the pharmacokinetics of intravenous chlorzoxazone in rats with water deprivation for 72 h. Life Sci. 2006;79:2179–2186. doi: 10.1016/j.lfs.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Kim YC, Shim HJ, Lee JH, Kim SH, Kwon JW, Kim WB, et al. Effects of enzyme inducers and inhibitors on the pharmacokinetics of intravenous DA-8159, a new erectogenic, in rats. Biopharm Drug Dispos. 2005a;26:233–241. doi: 10.1002/bdd.453. [DOI] [PubMed] [Google Scholar]
- Kim YC, Yoo M, Lee MG. DA-8159, erectogenic. Drugs Future. 2005b;30:678–682. [Google Scholar]
- Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20:379–386. doi: 10.2133/dmpk.20.379. [DOI] [PubMed] [Google Scholar]
- Lee DY, Kim JY, Kim YC, Kwon JW, Kim WB, Lee MG. Dose-independent pharmacokinetics of torasemide after intravenous and oral administration to rats. Biopharm Drug Dispos. 2005;26:173–182. doi: 10.1002/bdd.447. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chiou WL. Evaluation of potential causes for the incomplete bioavailability of furosemide: gastric first-pass metabolism. J Pharmacokinet Biopharm. 1983;11:623–640. doi: 10.1007/BF01059061. [DOI] [PubMed] [Google Scholar]
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. [Google Scholar]
- Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007;19:129–138. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- Peng JZ, Remmel RP, Sawchuk RK. Inhibition of murine cytochrome P4501A by tacrine: in vitro studies. Drug Metab Dispos. 2004;32:805–812. doi: 10.1124/dmd.32.8.805. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- Segel I. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. Wiley: New York; 1975. pp. 100–206. [Google Scholar]
- Shim HJ, Kim YC, Lee JH, Park KJ, Kwon JW, Kim WB, et al. Species differences in the formation of DA-8164 after intravenous and/or oral administration of DA-8159, a new erectogenic, to mice, rats, rabbits, dogs and humans. Biopharm Drug Dispos. 2005;26:161–166. doi: 10.1002/bdd.444. [DOI] [PubMed] [Google Scholar]
- Shim HJ, Kim YC, Park KJ, Kim DS, Kwon JW, Kim WB, et al. Pharmacokinetics of DA-8159, a new erectogenic, after intravenous and oral administration to rats: hepatic and intestinal first-pass effects. J Pharm Sci. 2003;92:2185–2195. doi: 10.1002/jps.10482. [DOI] [PubMed] [Google Scholar]
- Shim HJ, Lee EJ, Jung YH, Kim SH, Kim SH, You M, et al. Determination of a new phosphodiesterase V inhibitor, DA-8159, in plasma and urine by high-performance liquid chromatography. J Pharm Biomed Anal. 2002;30:527–533. doi: 10.1016/s0731-7085(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Sung JH, Yu KH, Park JS, Tsuruo T, Kim DD, Shim CK, et al. Saturable distribution of tacrine into the striatal extracellular fluid of the rat: evidence of involvement of multiple organic cation transporters in the transport. Drug Metabolism Dispos. 2005;33:440–448. doi: 10.1124/dmd.104.002220. [DOI] [PubMed] [Google Scholar]
- Wilkinson GR, Shand DG. A physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]