Abstract
Background and purpose:
TRPC5 is a mammalian homologue of the Drosophila Transient Receptor Potential (TRP) channel and has expression and functions in the cardiovascular and nervous systems. It forms a calcium-permeable cation channel that can be activated by a variety of signals including carbachol (acting at muscarinic receptors), lanthanides (e.g. Gd3+) and phospholipids (e.g. lysophosphatidylcholine: LPC). Here we report the effects of inhalational (halothane and chloroform) and intravenous (propofol) general anaesthetics upon TRPC5.
Experimental approach:
Human TRPC5 channels were expressed in HEK 293 cells and studied using fura-2 and patch-clamp recording to measure intracellular calcium and membrane currents respectively at room temperature. Human TRPM2 channels were studied for comparison.
Key results:
TRPC5 activation by carbachol, Gd3+ or LPC was inhibited by halothane and chloroform at ⩾0.1 and 0.2 mM respectively. Neither agent inhibited TRPM2. Propofol had an initial stimulatory effect on TRPC5 (evident in patch-clamp recordings only) and an inhibitory effect at ⩾10 μM. TRPM2 was not affected by propofol. Propofol inhibited activation of TRPC5 by Gd3+ but not LPC, suggesting the effect was not directly on the channel. Propofol's anti-oxidant property was not necessary for its inhibitory effect because di-isopropyl benzene, a propofol analogue that lacks the hydroxyl group, also inhibited TRPC5.
Conclusions and implications:
The data show the sensitivity of TRPC5 channel to general anaesthetics and suggest that some of the effects could have clinical relevance. The effects may be explained in part by the sensitivity of the channel to biophysical properties of the lipid bilayer.
Keywords: calcium channel, cation channel, transient receptor potential, general anaesthetics
Introduction
Inhalational (volatile) and intravenous general anaesthetics induce safe and reversible loss of consciousness, enabling surgical procedures to be carried out (Franks and Lieb, 1994; Franks, 2006). Inhalational anaesthetics include the halogenated hydrocarbons, halothane and chloroform. The primary intravenous anaesthetic is propofol, a widely used agent approved for use in over 50 countries and receiving increasing attention because of the cardio-protection, evident with its use.
Whether the halogenated hydrocarbons act by perturbing the lipid bilayer or by direct binding to proteins has been actively debated for many years (Franks, 2006). Nevertheless, it is widely thought that changes in ion transport play pivotal roles. The excitatory NMDA receptor channels are suppressed, whereas inhibitory GABAA and glycine receptor channels are stimulated. Binding pockets in transmembrane-spanning segments are suggested to underlie these effects (Huidobro-Toro et al., 1987; Kash et al., 2003; Franks, 2006; Ogata et al., 2006). The anaesthetics inhibit voltage-dependent Ca2+ and Na+ channels, Na+–Ca2+ exchange, and Ca2+ release (Haydon and Urban, 1983; Mongo and Vassort, 1990; Bru-Mercier et al., 2005), whereas they activate twin-pore domain K+ channels (for example, TREK1) leading to membrane hyperpolarization and closure of voltage-dependent Ca2+ and Na+ channels (Patel et al., 1999; Terrenoire et al., 2001; Franks, 2006). Propofol potentiates GABAA receptor-mediated responses (Hales and Lambert, 1991; Franks, 2006). Anaesthetics also affect the redox potential; halogenated hydrocarbons may generate reactive oxygen species, whereas propofol is an antioxidant (Franks and Lieb, 1994; Rigobello et al., 2004).
Canonical transient receptor potential 5 gene (TRPC5) is one of 28 genes encoding cation channels in mammals with homology to the Drosophila gene encoding TRP (Beech, 2007; Nilius et al., 2007). The canonical (C) subclassification is based on the amino acid sequence similarity of the seven members. Another melastatin (M) subfamily includes transient receptor potential melastatin 2 (TRPM2), which is one of eight channels. Despite belonging to different subfamilies, TRPC5 and TRPM2 share the property of sensing redox potential; both may be activated by hydrogen peroxide (H2O2) or nitric oxide (McHugh et al., 2003; Miller, 2006; Yoshida et al., 2006). All TRP channels are thought to arise from homo- or hetero-tetrameric arrangements of TRP proteins around a central ion pore, each protein having six transmembrane-spanning segments. Most of the TRP channels (including TRPC5 and TRPM2) are permeable to Ca2+ as well as monovalent cations such as Na+. TRPC5 forms heteromultimeric channels, for example, with TRPC1, whereas TRPM2 is known only to form homomeric channels.
Canonical transient receptor potential 5 (TRPC5) seems most abundant in the brain but it is also present in smooth muscle and endothelial cells of blood vessels and in the heart, with upregulation of expression suggested in a model of cardiac myocyte hypertrophy and human heart failure (Philipp et al., 1998; Greka et al., 2003; Seth et al., 2004; Bush et al., 2006; Xu et al., 2006; Yoshida et al., 2006). Dominant negative mutant TRPC5 enhances neuronal growth cone extension but suppresses smooth muscle cell motility evoked by sphingosine-1 phosphate, suggesting roles of TRPC5 or TRPC5-associating proteins in cell process extension and motility (Hui et al., 2006; Xu et al., 2006). TRPC5 is notable for its polymodality—activation by a multiplicity of activators (Beech, 2007). It may be activated by receptor agonists (for example, acetylcholine or carbachol, or sphingosine-1 phosphate), store depletion, external lanthanides (for example, gadolinium ion, Gd3+), extracellular or intracellular LPC, H2O2 or acid (Schaefer et al., 2000; Zeng et al., 2004; Flemming et al., 2006; Yoshida et al., 2006; Beech, 2007; Semtner et al., 2007). Another polymodal channel, on which there is more information, is the TREK-1 potassium channel. Like TRPC5, TREK-1 is activated by lysophospholipids, an effect suggested to occur because of deformation of the lipid bilayer (Maingret et al., 2000; Honore, 2007). Because chemical requirements for activation of TRPC5 by lysophospholipids show similarities to those of TREK-1, TRPC5 might also sense physical properties of the bilayer. We were, therefore, prompted to explore whether TRPC5 has other properties similar to TREK-1. Here we report on the effects of selected general anaesthetics and make a comparison with TRPM2, which is not activated by LPC (Flemming et al., 2006).
Methods
Cells expressing TRPC5 or TRPM2
Human embryonic kidney cells 293 (T-Rex: Invitrogen, Paisley, UK) cells expressing human TRPC5 or TRPM2 have been described (McHugh et al., 2003; Zeng et al., 2004). They were maintained in Dulbecco's minimal essential medium (DMEM-F12, Invitrogen, Paisley, UK) supplemented with 10% fetal calf serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37 °C in a humidified incubator, gassed with 95% air and 5% CO2. Cells stably expressing tetracycline-regulated TRPC5 or TRPM2 were selected using 5 μg ml−1 blasticidin and 400 μg ml−1 zeocin. Expression was induced by 1 μg ml−1 tetracycline 24 h prior to experiments.
Intracellular calcium measurement on the FlexStation II
Cells were plated at 70–80% confluence on 96-well, poly-D-lysine black-walled, clear-bottomed plates (BD Bioscience, Oxford, UK) 24 h before experiments. Immediately prior to recording, cells were incubated for 1 h at 37 °C in standard bath solution (SBS; see below for composition) containing 2 μM fura-2 acetoxymethyl ester and then washed with SBS 2–3 times before adding SBS, which included 1 μM Gd3+ in TRPC5 recordings to minimize signals from endogenous channels (Zeng et al., 2004). The FlexStation II and SoftMax Pro 4.7.1 software were from Molecular Devices (Sunnyvale, CA, USA). Excitation light alternated between 340 and 380 nm, while emission was filtered at 510 nm. Experiments were at room temperature (21±3 °C). SBS contained (mM): 140 NaCl, 5 KCl, 1.2 MgCl2, 1.5 CaCl2, 8 glucose and 10 HEPES (titrated to pH 7.4 using NaOH). Osmolality was adjusted to 290 mOsm using mannitol.
Whole-cell patch-clamp recording
Voltage clamp was performed at room temperature with the whole-cell patch configuration. Signals were amplified with an Axopatch 200A patch clamp amplifier, controlled with pClamp software 6.0 (Axon Instruments, Foster City, CA, USA), sampled at 2 kHz and filtered at 1 kHz. Patch pipettes were made from borosilicate glass capillary tubing with an outside diameter of 1 mm (Clark Electromedical Instruments, Pangbourne, UK). After fire polishing and filling with pipette solution, the resistances were 3–5 MΩ. Patch pipette solution contained (mM): 135 CsCl, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2ATP and 0.1 NaGTP, titrated to pH 7.2 with CsOH. One of two ramp protocols was applied, which were as follows: from a holding potential of 0 mV the voltage was stepped, every 10 s, to −100 mV followed by a 1-s ramp to +100 mV (Ramp protocol 1); from a holding potential of −60 mV the voltage was stepped, every 5 s, to −100 mV followed by a 0.25-s ramp to +100 mV (Ramp protocol 2). Effects of anaesthetics were not different for the two protocols except that the reversal potential for the TRPC5-mediated currents tended to be more negative with Ramp protocol 2. The reason for this difference is unknown. When constructing a concentration inhibition curve, currents at the end of the anaesthetic application were normalized to the initial basal current, using an average of five current measurements for each data point.
Data analysis
Origin (Origin Lab Corporation, Northampton, CA, USA) software package was used for data analysis. Data are expressed as mean±s.e.m. and data sets were compared using paired two-tailed Student's t-tests. For the FlexStation recordings, the numbers of experiments are indicated as n/N, where n is the number of wells used in the 96-well plate and N is the number of independent experiments (that is, on different 96-well plates and separate batches of cells). For patch-clamp recordings, the number of independent experiments on individual cells is given as N. Statistical significance is indicated by probability P-values of *P<0.05 or **P<0.01.
Reagents and preparation of anaesthetics
Halothane (Fluthane) was from Astra-Zeneca (Macclesfield, UK) and chloroform from Fisher Scientific (Dublin, Ireland). Other reagents were purchased from Sigma-Genosys (Poole, UK). Gd3+ and carbachol (CCh) were prepared as 100 mM stocks in water and stored at room temperature. LPC was prepared as a 50 mM stock in 100% methanol and stored at −20 °C. 2-aminoethoxydiphenyl borate was prepared as a 375 mM stock in DMSO and stored at −20 °C. H2O2 was freshly prepared in SBS immediately prior to experiments. The chemical structures of the anaesthetics are shown in Supplementary Figure I. Halothane and chloroform were added to SBS from a freshly prepared DMSO stock immediately prior to experiments. In FlexStation experiments with propofol, cells were preincubated with propofol for 30 min. The concentration of DMSO was ⩽0.1% and remained constant throughout the experiments. Concentrations of halothane in the recording chambers of the FlexStation and patch-clamp system were measured directly using gas chromatography. Using chloroform as an internal standard, samples were extracted into carbon tetrachloride and run on a column packed with 10% SE-30 on Diatomite C-AW 60–80 mesh (Harrison et al., 1999). Halothane standards were used to calculate the concentrations in the samples. Chloroform concentrations in the samples were calculated assuming equivalence to halothane.
Results
Expression of TRPC5 or TRPM2 was induced by exposure of cells to tetracycline (Tet) prior to recording. Intracellular Ca2+ measurement using fura-2 indicator dye showed that responses to TRPC5 or TRPM2 activators occurred in induced (Tet+) but not noninduced (Tet−) cells, as described previously (McHugh et al., 2003; Zeng et al., 2004; Flemming et al., 2006) and are shown in Supplementary Figure II. Tet− cells had an initial transient response to CCh that resulted from TRPC5-independent Ca2+ release from intracellular stores.
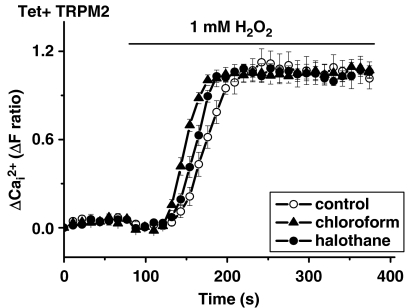
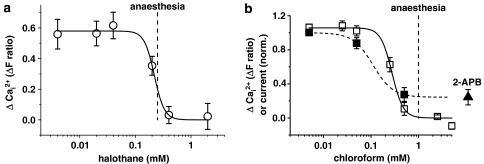
Preincubation of cells with 0.17 mM halothane or 0.37 mM chloroform inhibited TRPC5 signals evoked by Gd3+, LPC or CCh (Figure 1). As a test of selectivity we explored the activity of a related channel, TRPM2, which was activated by H2O2. Neither halothane nor chloroform had any significant effect on TRPM2 (Figure 2). Chloroform was also directly applied in the absence of H2O2 and did not activate TRPM2 (data not shown). As a further test of the capacity of the anaesthetics to inhibit TRPC5, we made patch-clamp recordings from single cells. Halothane or chloroform reversibly inhibited currents through TRPC5 channels (Figure 3), which showed the characteristic curvilinear current–voltage relationships (I–Vs) of TRPC5 (Zeng et al., 2004; Flemming et al., 2006). Halothane and chloroform produced concentration-dependent inhibition and were effective at concentrations (IC50 values ∼0.2 mM) that are used to produce clinical anaesthesia (Figure 4).
Figure 1.
Inhibitory effects of halothane and chloroform on TRPC5 (canonical transient receptor potential 5)-mediated Ca2+ entry. All cells were induced to express TRPC5 (Tet+) and intracellular Ca2+ was measured using fura-2. TRPC5 was activated by (a, d) 100 μM Gd3+, (b, e) 10 μM LPC or (c, f) 100 μM CCh. Responses were compared in the presence of vehicle and 0.17 mM halothane (a–c) or 0.37 mM chloroform (d–f). Each experiment is n=12 and representative of 3 (N) independent experiments.
Figure 2.
Insensitivity of TRPM2 to halothane and chloroform. All cells were induced to express TRPM2 (Tet+) and intracellular Ca2+ was measured using fura-2. Bath-applied 1 mM H2O2 activated TRPM2. Halothane and chloroform were present at 0.17 mM and 0.37 mM respectively. Each experiment is n=11 and representative of 3 (N) independent experiments.
Figure 3.
Inhibitory effects of halothane and chloroform on ionic current through TRPC5. Whole-cell patch-clamp recordings are shown. All cells were expressing TRPC5 (Tet+). Ramp protocol 1 was used. Gd3+, halothane and chloroform were present at 0.02, 0.17 and 0.37 mM, respectively. The current–voltage relationships (I–Vs) of (b) and (d) relate to the time-series plots of (a) and (c). All data are representative of 5 (N) independent experiments. The arrows in (a) and (c) indicate the times at which the I–V ramps in (b) and (d) were sampled.
Figure 4.
Concentration-inhibition curves for halothane and chloroform. Data are from cells expressing TRPC5 (Tet+) and are (a) of Ca2+ measurements in the presence of halothane (n/N=18/3). In (b), both Ca2+ measurements and whole-cell patch clamp recording of current at +80 mV in the presence of chloroform are shown (n/N=18/3 for Ca2+; N=5 for current; estimated concentrations of chloroform). In (b), the last data point (black triangle) is for 75 μM 2-aminoethoxydiphenyl borate and was used as a reference point at which all TRPC5 current was assumed to be blocked. The fitted curves are the Hill equation, which gave IC50 values of 0.22 mM (a), 0.28 mM (b, Ca2+) and 0.11 mM (b, current). The vertical dashed lines indicate EC50 values for general anaesthesia in mammals (Franks and Lieb, 1994).
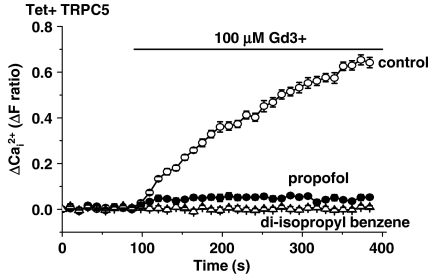
Ca2+ measurements revealed a strong inhibitory effect of 100 μM propofol on Gd3+-evoked TRPC5 activation (Figure 5a). TRPM2 was unaffected (Figure 5b). The effect of propofol on Gd3+-activated TRPC5 was concentration-dependent, being first evident at about 10 μM propofol (Figure 5c). Concentrations of propofol associated with clinical anaesthesia and toxicity are indicated for comparison (Figure 5c). In sharp contrast, LPC-activated TRPC5 was unaffected by 100 μM propofol (Figure 5d).
Figure 5.
Agonist-dependent inhibition of TRPC5 by propofol. Data were produced by Ca2+ measurement from cells expressing TRPC5 (a, c and d) or TRPM2 (b). Propofol was applied at 100 μM except in (c) when a range of concentrations was used. Each experiment is n⩾12 and representative of 3 (N) independent experiments. The vertical lines in (c) indicate the EC50 value for general anaesthesia in mammals (Franks and Lieb, 1994) and the upper concentration limit that may cause toxicity (Al-Jahdari et al., 2006). The fitted curve in (c) is the Hill equation with an IC50 of 84.5 μM.
As propofol is an antioxidant and TRPC5 may be stimulated by reactive oxygen species, we considered whether the inhibitory effect of propofol on Gd3+-activated TRPC5 might arise because endogenous reactive species were neutralized by propofol. We, therefore, investigated di-isopropyl benzene, which is chemically identical to propofol except that it lacks the hydroxyl group of propofol's benzene ring (Supplementary Figure I). We observed that di-isopropyl benzene is an effective inhibitor of TRPC5, showing that the hydroxyl group is not necessary for inhibition of TRPC5 (Figure 6).
Figure 6.
Inhibition of TRPC5 by di-isopropyl benzene. Data were produced by Ca2+ measurement from cells expressing TRPC5. Di-isopropyl benzene or propofol was applied at 100 μM. The experiment is n=12 and representative of 3 (N) independent experiments.
In Ca2+ measurement experiments, we did not observe any effect of propofol if it was applied directly on its own (data not shown). However, in patch-clamp recordings there was initial stimulation of TRPC5 by 100 μM propofol, with theI–V showing the TRPC5 signature (Figures 7a and b). A lower concentration of propofol (10 μM) was without effect (N=6; data not shown). Longer exposure to 100 μM propofol revealed fade of the stimulatory effect and inhibition of the ability of Gd3+ to activate the channel (Figures 7a and c). Washout of propofol led to fast recovery from activation, followed by slow and profound recovery from inhibition (Figures 7a and c).
Figure 7.
Complex effects of propofol on ionic current through TRPC5. All recordings were by whole-cell patch-clamp from cells expressing TRPC5. Propofol was applied at 100 μM and voltage protocols were 2 (a, c) and 1 (b). (a) Time-series plot showing the effect of propofol in the absence of Gd3+, the effect of Gd3+ in the presence of propofol, and then the effect of washing out propofol while maintaining Gd3+ (typical of N=7). The arrow marked (i) indicates the time at which propofol-induced I–Vs were sampled. Arrows marked (ii) and (iii) indicate the 1st and 2nd times at which recovery from block by propofol was quantified in (c). (b) I–V induced by propofol in the absence of Gd3+ (typical of N=5). (c) Summary data for the type of experiment illustrated in (a) and for current measured at +80 mV. Not all experiments were of the full duration shown in (a) and so N=5–10. Data are fold change (Δ) in current, where 1.0 indicates no change (as indicated by the control white bar). Shown are: peak and sustained propofol responses in the absence of Gd3+; Gd3+ responses in the absence and presence of propofol; and recovery of current amplitudes on wash-out of propofol (but not Gd3+), showing an initial loss (1st) of current and then a marked increase (2nd).
Discussion
Our data show that TRPC5 can be added to the list of ion channels modulated by general anaesthetics. It is the first example of a TRP channel affected by such drugs. Its sensitivity contrasts, however, with that of TRPM2, which was found to be resistant to the anaesthetics studied. Although the effects of halothane and chloroform on TRPC5 are simple (that is, there is general inhibition of all activity), the effect of the important intravenous anaesthetic, propofol, is complex, showing stimulatory, inhibitory and agonist-dependent features. Some of the effects may have relevance to clinical anaesthesia.
The concentrations of halothane that affected TRPC5 are strikingly similar to those that affect the twin-pore domain potassium channel TASK-1 (Sirois et al., 2000). Such concentrations are also similar to those associated with clinical anaesthesia (Franks and Lieb, 1994; Franks, 2006), although it should be recognized that our experiments were performed at room temperature and anaesthetic potency is inversely related to temperature. Because TRPC5 channels are nonselective cationic channels, they are mostly considered to be excitatory. Also, TRPC5 was originally cloned from a gene locus associated with mental retardation and is widely expressed in the brain, including in cortical and limbic structures (Philipp et al., 1998; Sossey-Alaoui et al., 1999; Fowler et al., 2007). Therefore, suppression of TRPC5 might reasonably be expected to have a general inhibitory effect on brain function and thus potentially contribute to clinical anaesthesia.
Although chloroform affected TRPC5 at concentrations that produce anaesthesia, this halogenated hydrocarbon is no longer used in clinical practice because of its toxicity, which includes a tendency to cause cardiac arrhythmia. Although the effect of chloroform on TRPC5 is of no relevance to medical practice, it is an important effect to be aware of because one of the reasons for our project was the observation that the effect of a lipid activator of TRPC5 varied depending on the solvent for the lipid—methanol or chloroform. The effect of the lipid was absent with the chloroform solution, showing the vehicle (chloroform) to be blocking the channels.
Whether the effects of propofol on TRPC5 are clinically relevant is debatable. Plasma concentrations of propofol may be quite high but it is a drug that is heavily bound to plasma proteins, and so the free propofol concentration is generally estimated to be <10 μM or, indeed, <1 μM (Franks and Lieb, 1994; Dawidowicz et al., 2003; Servin et al., 2003). At such concentrations, little effect on TRPC5 can be expected (Figure 5). It is also apparent that other mechanisms are more sensitive to propofol (Hales and Lambert, 1991). Nevertheless, there may be circumstances when the effects of propofol on TRPC5 have relevance in the clinic. For example, high concentrations may occur in the pulmonary circulation because of back-diffusion (He et al., 2000) and prolonged anaesthesia may result in propofol concentrations as high as 50 μM, which cause growth cone collapse and neurite retraction (Al-Jahdari et al., 2006). Intriguingly, TRPC5 has a role in growth cone morphology (Greka et al., 2003; Hui et al., 2006).
The inhibitory effect of propofol on TRPC5 must occur via a mechanism that is distinct from that underlying the inhibitory effects of chloroform or halothane because propofol failed to affect TRPC5 activated by LPC. Therefore, the inhibitory effect of propofol does not reflect general suppression of the channel but depends on the activation mechanism of TRPC5. Such a result could be explained if LPC acts as a relatively direct agonist of TRPC5 (Flemming et al., 2006) and propofol acts indirectly on a pathway that leads to TRPC5 activation, perhaps upstream of LPC. However, this would be puzzling in view of the Gd3+ result because Gd3+ is considered to act directly on the channel (Jung et al., 2003). To explain this situation and be consistent with previous suggestions for TRPC5 activation, we could speculate that Gd3+ acts by stabilization of the open state of the channel, potentiating effects of other agonists that act through signal transduction pathways. If this is true, propofol may inhibit activation by Gd3+ because it inhibits constitutive channel activity evoked by a constitutively active signalling pathway that is inhibited by propofol.
In summary, we report striking effects of inhalational (halothane and chloroform) and intravenous (propofol) general anaesthetics on the function of TRPC5 channel and show that, by contrast, the TRPM2 channel is resistant. Halothane and chloroform are powerful inhibitors of TRPC5 and it would be worthwhile exploring effects of other types of inhalational anaesthetic, such as isoflurane, in future studies. Propofol also had profound effects on TRPC5 but these were complex; with stimulatory, inhibitory and agonist-dependent features being evident. The mechanisms of action of these anaesthetics on TRPC5 are unknown and they will not be straightforward to resolve. Given the sensitivity of TRPC5 to lipid (Flemming et al., 2006), it seems plausible that some effects of the anaesthetics reflect vulnerability of TRPC5 to distortion of the biophysical properties of the lipid bilayer. The full story will not be simple because there is evidence that ion channels have binding pockets that directly bind anaesthetics and the inhibitory effect of propofol depends on the agonist used to activate TRPC5.
External data objects
Acknowledgments
We thank the Egyptian Ministry of Higher Education and Wellcome Trust for financial support, and SM Harrison for helpful advice.
Abbreviations
- CCh
carbachol
- LPC
lysophosphatidylcholine
- TRPC5
canonical transient receptor potential 5
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Al-Jahdari WS, Saito S, Nakano T, Goto F. Propofol induces growth cone collapse and neurite retraction in chick explant culture. Can J Anaesth. 2006;53:1078–1085. [PubMed] [Google Scholar]
- Beech DJ. Canonical transient receptor potential 5. Handb Exp Pharmacol. 2007;179:109–123. doi: 10.1007/978-3-540-34891-7_6. [DOI] [PubMed] [Google Scholar]
- Bru-Mercier G, Hopkins PM, Harrison SM. Halothane and sevoflurane inhibit Na+/K+ exchange current in rat ventricular myocytes. Br J Anaesth. 2005;95:305–309. doi: 10.1093/bja/aei185. [DOI] [PubMed] [Google Scholar]
- Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, et al. Canonical transient potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- Dawidowicz AL, Kalitynski R, Fijalkowska A. Free and bound propofol concentrations in human cerebrospinal fluid. Br J Clin Pharmacol. 2003;56:545–550. doi: 10.1046/j.1365-2125.2003.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, et al. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One. 2007;2:e573. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147:S72–S81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurons. Br J Pharmacol. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Robinson M, Davies LA, Hopkins PM, Boyett MR. Mechanisms underlying the inotropic action of halothane on intact rat ventricular myocytes. Br J Anaesth. 1999;82:609–621. doi: 10.1093/bja/82.4.609. [DOI] [PubMed] [Google Scholar]
- Haydon DA, Urban BW. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YL, Ueyama H, Tashiro C, Mashimo T, Yoshiya I. Pulmonary disposition of propofol in surgical patients. Anaesthesiology. 2000;93:986–991. doi: 10.1097/00000542-200010000-00019. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2p channels: focus on TREK1. Nat Rev Nerosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, et al. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol. 2006;572:165–172. doi: 10.1113/jphysiol.2005.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Bleck V, Allan AM, Harris RA. Neurochemical actions of anesthetic drugs on the gamma-aminobutyric acid receptor-chloride channel complex. J Pharmacol Exp Ther. 1987;242:963–969. [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Kash TL, Jenkins A, Harrison NL. Molecular volume determines the activity of the halogenated alkane bromoform at wild-type and mutant GABAA receptors. Brain Res. 2003;960:36–41. doi: 10.1016/s0006-8993(02)03748-4. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem. 2003;278:11002–11006. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- Mongo KG, Vassort G. Inhibition by alcohol, halothane and chloroform of the Ca2+ current in single frog ventricular cells. J Mol Cell Cardiol. 1990;22:939–953. doi: 10.1016/0022-2828(90)91034-5. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318:434–443. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, et al. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigobello MP, Stevanato R, Momo F, Fabris S, Scutari G, Boscolo R, et al. Evaluation of the antioxidant properties of propofol and its nitrosoderivative: comparison with homologue substituted phenols. Free Radic Res. 2004;38:315–321. doi: 10.1080/03079450310001652031. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- Servin FS, Bougeois B, Gomeni R, Mentre F, Farinotti R, Desmonts JM. Pharmacokinetics of propofol administered by target-controlled infusion to alcoholic patients. Anaesthesiology. 2003;99:576–585. doi: 10.1097/00000542-200309000-00012. [DOI] [PubMed] [Google Scholar]
- Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, et al. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodelling of the Ca2+ signaling mechanism in cardiac myocytes. Proc Natl Acad Sci USA. 2004;101:16683–16688. doi: 10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C, III, Bayliss DA. The Task-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossey-Alaoui K, Jones L, Abidi FE, Hartung AJ, Hane B, Schwartz CE, et al. Molecular cloning and characterization of TRPC5 (HTRPC5), the human homologue of a mouse brain receptor-activated capacitative Ca+2 entry channel. Genomics. 1999;60:330–340. doi: 10.1006/geno.1999.5924. [DOI] [PubMed] [Google Scholar]
- Terrenoire C, Lauritzen I, Lesage F, Romey G, Lazdunski M. A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circ Res. 2001;89:336–342. doi: 10.1161/hh1601.094979. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, et al. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, et al. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–750. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.