Abstract
Measurements of affinity and efficacy are fundamental for work on agonists both in drug discovery and in basic studies on receptors. In this review I wish to consider methods for measuring affinity and efficacy at G protein coupled receptors (GPCRs). Agonist affinity may be estimated in terms of the dissociation constant for agonist binding to a receptor using ligand binding or functional assays. It has, however, been suggested that measurements of affinity are always contaminated by efficacy so that it is impossible to separate the two parameters. Here I show that for many GPCRs, if receptor/G protein coupling is suppressed, experimental measurements of agonist affinity using ligand binding (Kobs) provide quite accurate measures of the agonist microscopic dissociation constant (KA). Also in pharmacological functional studies, good estimates of agonist dissociation constants are possible. Efficacy can be quantitated in several ways based on functional data (maximal effect of the agonist (Emax), ratio of agonist dissociation constant to concentration of agonist giving half maximal effect in functional assay (Kobs/EC50), a combined parameter EmaxKobs/EC50). Here I show that EmaxKobs/EC50 provides the best assessment of efficacy for a range of agonists across the full range of efficacy for full to partial agonists. Considerable evidence now suggests that ligand efficacy may be dependent on the pathway used to assess it. The efficacy of a ligand may, therefore, be multidimensional. It is still, however, necessary to have accurate measures of efficacy in different pathways.
Keywords: agonist binding, agonist affinity, agonist efficacy, G protein-coupled receptors
Introduction
The actions of ligands at receptors depend on two fundamental events. First, the ligand must bind to the receptor; it is said to have affinity for the receptor. Second, the ligand may have effects on the receptor and its associated signalling systems. This second attribute has been termed efficacy. Agonists are said to have positive efficacy, inverse agonists are said to have negative efficacy and neutral antagonists have zero efficacy. Accurate measurement of affinity and efficacy for ligands is very important for drug discovery and for basic biology.
In drug discovery, these measurements guide the efforts of medicinal chemists. In the past, this has mainly been in terms of affinity measurements but, more recently, measurements of ligand efficacy have become important (Williams and Sewing, 2005). In basic biology studies of receptor structure and function, measurements of affinity and efficacy underpin analyses of ligand/receptor interaction in both SAR and mutagenesis analyses. If these measurements are inaccurate or inadequate, then this has great importance and, in this review, I wish to consider different ways in which affinity and efficacy can be measured. I shall consider this problem for agonists at G protein-coupled receptors (GPCRs). GPCRs are of great importance for drug action constituting more than 30% of current drug targets.
Defining the affinity of agonists at their receptors
In principle, it should be possible to obtain estimates of agonist affinity in terms of dissociation constants for the binding of ligands to their receptors. Agonist dissociation constants can be estimated using in vitro techniques such as ligand-binding assays, providing the assay conditions are carefully controlled (see below). Alternatively, dissociation constants can be estimated using the receptor inactivation method of Furchgott (Furchgott, 1966; Furchgott and Bursztyn, 1967) or the comparative method of Barlow et al. (1967) in functional assays. Agonist dissociation constants are used in structure/activity studies for drug design, in providing estimates of agonist efficacy (see below) or in mutagenesis studies to examine how ligands and receptors interact.
It has, however, been stated that all estimates of agonist affinity are fundamentally flawed (Colquhoun, 1998). According to this view, affinity and efficacy are intrinsically linked for agonists, so it is impossible to obtain a value for the dissociation constant for an agonist that does not include some component of efficacy. In consequence, it is difficult to interpret the effects of any manipulation that appears to alter the dissociation constant for an agonist as it could be an effect on agonist binding or efficacy. This issue is very important for theoretical and applied pharmacology as several of the key theories of drug action assume that affinity and efficacy are separate quantities and agonist dissociation constant data are widely used in drug discovery.
This is such a fundamental issue for pharmacology that it is worthwhile restating it in a different way. What we would like to measure here is the microscopic dissociation constant for binding of agonist to receptor (KA). This is a measure of the affinity of the agonist for the ground state of the receptor. We can try to measure KA using techniques such as ligand binding. Because agonists activate receptors, however, it is unlikely that the binding of an agonist to a receptor does not cause some change in the conformation of the receptor associated with the activation process. Thus, we end up measuring an observed macroscopic dissociation constant (Kobs) that includes KA and some aspects of receptor activation. In the rest of this review, I shall use Kobs to refer to experimentally determined dissociation constants, whereas in theoretical treatments and simulations, I shall refer to KA as the agonist dissociation constant. In this section, I consider how much KA and Kobs differ. For the GPCRs, I will show that assay conditions can be adjusted to reduce the contribution of the activation process so that KA and Kobs do not differ very much. Measurements of Kobs are, therefore, mostly good estimates of KA.
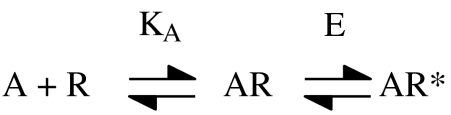
Let us look first at some of the background that lead to the realization that affinity and efficacy are linked quantities. Here many of the arguments come from the study of ion channel-linked receptors such as the nicotinic acetylcholine receptor. Del Castillo and Katz (1957) proposed a model to account for the properties of this receptor based on ligand-induced isomerization of the receptor between vacant and agonist-occupied states (Figure 1). In this model, the activation of the receptor depends on the binding of the agonist to the active state (R*) more strongly than to the inactive state (R). Thus, binding and activation (efficacy) are inextricably linked. This means that in an agonist-binding experiment, both AR and AR* will be measured and Kobs will differ from KA (Figure 1). This means that it is impossible to use Kobs data to make inferences about the binding of agonists to the ground state of the receptor in structure/activity or in mutagenesis studies. Any effects of changes in agonist structure or of mutations could be interpreted in terms of effects on either affinity (KA) or efficacy (E) and the two are difficult to separate.
Figure 1.
The Del Castillo and Katz model for activation of ion channel-linked receptors. In this model, the receptor exists in ground (R) and active (R*) states, with the R* state having a higher affinity for the agonist. KA is the dissociation constant for binding of agonist to the ground state of the receptor and E is the equilibrium constant for the AR/AR* transition (E=[AR*]/[AR]). If ligand-binding studies are applied to this scheme, the experimentally determined dissociation constant of an agonist (Kobs) is given by: Kobs=KA/1+E.
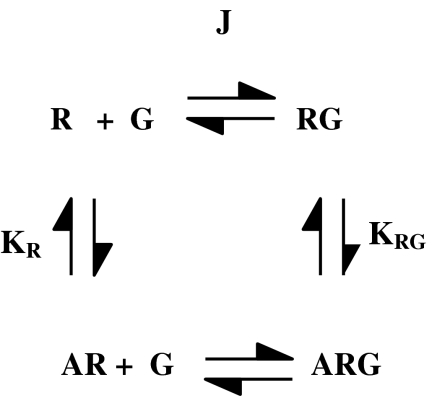
This problem clearly applies for the ion channel-linked receptors but what about GPCRs? It would be very useful for a variety of applications to be able to make measurements of dissociation constants for agonists at GPCRs that approximate ground-state affinities (KA). What sets the GPCRs apart from the ion channel-linked receptors, however, is the need for coupling of the receptor to a second protein, the G protein, to achieve activity in most assay read-outs. Thus, the receptor in its active state will be the ternary complex of agonist/receptor/G protein (Figure 2) (De Lean et al., 1980). If measurements of dissociation constants include this ternary complex, then there will be a strong linkage between affinity and efficacy and Kobs will not be a good estimate of KA (Figure 2).
Figure 2.
The ternary complex model for agonist/receptor/G protein coupling. In this model, the receptor exists in uncoupled (R) and G protein-coupled (RG) states and the agonist binds with higher affinity to the RG state. Equilibrium association constants (KR, KRG) for agonist binding are shown and J is defined as [RG]/([R][G]). We can define a cooperativity factor α for the effect of G protein coupling on agonist binding so that KRG=αKR. If ligand-binding studies are applied to this scheme with excess G protein over receptor, the experimentally determined dissociation constant of an agonist (Kobs) is given by: Kobs=KA ((1+JG)/(1+αJG)).
For the GPCRs, however, we have the possibility of inhibiting R/G coupling using approaches such as addition of excess guanine nucleotide, or the use of pertussis toxin (for Gi/o-linked responses) (De Lean et al., 1980). Under these conditions, it has been proposed that R and G interact with much reduced affinity (guanine nucleotide) or are uncoupled (pertussis toxin) so that ligand affinities approach those for the free receptor uncoupled from G proteins. This can result in a reduction in agonist affinity of up to 100-fold. In principle, this allows a separation of affinity and efficacy for the GPCRs. Measurements of Kobs under conditions where receptor/G protein coupling is suppressed should reduce the component of efficacy, for example, in ligand-binding assays in the presence of GTP.
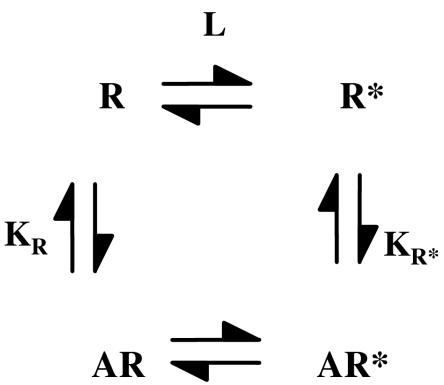
There is, however, a further potential problem, in that it can be argued that when an agonist binds to a GPCR, even to the ground state in the absence of G protein coupling, it must change the conformation of the receptor in a manner related to its efficacy as outlined earlier (Colquhoun, 1998; Strange, 1999). The more efficacious the agonist, the greater will this conformational change be in principle. This means that determinations of agonist dissociation constants in ligand-binding assays in the absence of G protein coupling may not be to the ground state but to a partially activated state and this will include a component of efficacy (Colquhoun, 1998; Strange, 1999; Trzeciakowski, 1999b; Ehlert, 2001; Rang, 2006). As a result, values of Kobs may include a component of ligand efficacy; this component will be greater for the full agonists and Kobs and KA may differ (Figure 3). This may be described in terms of inactive (R) and partially active (R*) forms of the receptor in the absence of G protein coupling as in Figure 3, and addition of this equilibrium to the ternary complex model (Figure 2) results in the extended ternary complex model (Samama et al., 1993). According to this analysis using R/R* states, affinity and efficacy are linked quantities and if there is appreciable stabilization of the R* state of a GPCR by the agonist in the absence of G protein coupling, then this will be a confounding factor in the use of affinity as a parameter independent of efficacy. Indeed, Leff (1995) modelled GPCR activity in terms of a two-state model with R and R* states, analogous to the model of Del Castillo and Katz (1957). In this model for GPCRs, affinity and efficacy are intrinsically linked. The Leff two-state model did not, however, consider the effects of G protein coupling and so may be seen as incomplete (Strange, 1998).
Figure 3.
Partial activation of a receptor by an agonist. The agonist stabilizes a partially active form of the receptor (R*) so that the measured agonist affinity is not equal to the ground-state affinity. Equilibrium association constants for agonist binding (KR, KR*) are shown and L is defined as [R*]/[R]. We can define a factor β for the effect of the R/R* transition on agonist binding so that KR*=βKR. If ligand-binding studies are applied to this scheme, the experimentally determined dissociation constant of an agonist (Kobs) is given by: Kobs=KA ((1+L)/(1+βL)).
It is unclear, therefore, to what extent there is stabilization of R* upon agonist binding to GPCRs and how much this affects affinity estimates for ground states. Also, it is unclear where any intermediates such as R* lie on the pathway between R and R*G.
How much does efficacy contaminate affinity for agonists at GPCRs?
Let us now consider the extent to which experimental determinations of agonist affinity (Kobs) differ from the microscopic dissociation constant for agonist binding to receptor (KA). To consider this question, I want to consider various pieces of evidence.
Manipulations that prevent receptor activation
One of the most conserved residues in GPCRs is an aspartic acid about two-thirds of the way down TMII (residue 2.50). It has been suggested that this residue is important for maintaining an active receptor conformation (Urizar et al., 2005; Smit et al., 2007). Mutation of this residue to Asn or Ala has been shown to prevent or reduce receptor activation and G protein coupling for many GPCRs. Thus, receptors with this mutation could be considered unable to assume the active state, that is, they cannot assume the R* conformation.
This mutation, therefore, potentially gives a tool for determining the extent of stabilization of R* by agonist in the absence of G protein coupling. To use this mutant receptor to address this question, it is necessary to compare agonist affinities for the G protein-uncoupled state in the native and mutant receptors. In Table 1, I have summarized the effects of this mutation on a variety of receptors. For the most part, the characterization of the mutant receptors is not extensive and often uses only one full agonist. The data show, however, that for the monoamine receptors tested this mutation prevents receptor activation and G protein coupling but has only a small effect (<3-fold) on the binding of a full agonist to the receptor concerned in the absence of G protein coupling. On the basis of the effects of this mutation, we may conclude that for many GPCRs there is little stabilization of the R* state in the absence of G protein coupling, and effects of this process can contribute no more than three-fold to estimates of agonist affinity. This is for full agonists and for partial agonists the effect will be less. A notable exception here is the β2-adrenergic receptor, where the effect is greater so that for this receptor values for Kobs will differ from the ground-state affinity (KA). For two of the peptide receptors in Table 1 (δ-opioid, SSTR2), mutation of this aspartate does not eliminate signalling so that for these receptors this mutant is not informative. In the case of receptors such as the β2-adrenergic receptor, estimates of KA may be obtained by combining measurements of agonist dissociation constants for TMII Asp mutants and constitutively active mutants (Strange, 2000).
Table 1.
Effect of mutation of aspartic acid residue 2.50 in the second transmembrane-spanning region of GPCRs
| Receptor | G protein coupling | Agonist signalling | Agonist affinity | Na+ sensitivity of agonist binding | Comments | Reference |
|---|---|---|---|---|---|---|
| α2-adrenergic | ↓ | ↓ | ⇔ Some increase | Lost | Horstman et al. (1990); Wang et al. (1991); Ceresa and Limbird (1994) | |
| β2-adrenergic | ↓ | ↓ | ↓↓ | — | Agonist affinity reduced (10-fold (isoprenaline), 70-fold (noradrenaline) | Strader et al. (1987, 1988); Chung et al. (1988); Liapakis et al. (2004) |
| D2 dopamine | ↓ | ↓ | ↓ | Lost | Agonist affinity reduced ∼3-fold | Neve et al. (1991) |
| 5-HT1A | ↓ | ↓ | ⇔ | — | JT Alder and PG Strange, unpublished data | |
| 5-HT2A | ↓ | ↓ | ⇔ | — | Wang et al. (1993) | |
| m1 muscarinic | — | ↓ | ⇔ Some increased | — | Fraser et al. (1989) | |
| TRH | — | ↓ | ↓ | — | Perlman et al. (1992) | |
| Tachykinin NK2 | — | ↓ | ⇔ | — | D79N mutant shows unchanged agonist affinity, whereas D79A mutant shows reduced affinity | Donnelly et al. (1999) |
| Neurotensin NTR1 | ⇔ | ↓ | ⇔ | ↓ | Martin et al. (1999) | |
| δ-opioid | ⇔ | ⇔ | ↓ | Lost | Kong et al. (1993b) | |
| Somatostatin (SSTR2) | ⇔ | ⇔ | ⇔ | Lost | Kong et al. (1993a) |
Abbreviation: GPCR, G protein-coupled receptor.
Manipulations that promote receptor activation
The analysis of mutant receptors where the activation process is favoured (constitutively active mutants) can also yield some information about the extent of activation of the native receptor. This has been examined in detail for the α2A-adrenergic receptor (Wade et al., 2001), where it was estimated that for the native receptor, 0.04% of the α2A-receptor was present in the R* state in the absence of agonist. From these data, it may be calculated that in the presence of adrenaline, 0.7% of the receptor is in the R* state and the value of Kobs for adrenaline differs by less than two-fold from KA for this agonist. Similarly, analysis of mutants of the m1 muscarinic receptor has shown that Kobs for acetylcholine differs by less than two-fold from the ground-state affinity (KA) (Hulme and Lu, 1998). In both cases, these estimates support the data obtained using the TMII Asp mutation. In the latter study, it was also shown that the equilibrium constant associated with the AR*/AR*G transition was about 15-fold higher than that associated with the AR/AR* transition.
Effects of sodium ions
Sodium ions are very important for the regulating the effects of agonists on GPCRs. The binding of agonists to some GPCRs is Na+ dependent with affinities being reduced in the presence of Na+ ions (Neve, 1991). Interaction of receptor and G protein is sensitive to the level of Na+ in buffers (Costa et al., 1990; Williams et al., 1997; Lin et al., 2006). The conserved aspartic acid in TM II (residue 2.50, see above) has been proposed to be the site of interaction of the Na+ ion and, indeed, Na+ regulation of agonist affinity is generally lost in TM II Asp mutants (Table 1). One possibility is that the Na+ may be affecting the R/R* transition. Support for this idea comes from studies on a constitutively active mutant D2 dopamine receptor (Wilson et al., 2001). Agonist binding to this mutant was of higher affinity than for the native receptor in the absence of G protein coupling consistent with increased isomerization of the mutant towards R*. The increase in affinity could be suppressed by inclusion of Na+ in the buffers so that measurements of agonist affinity in the absence of G protein coupling will be for the R state if Na+ ions are included in buffers. It, therefore, seems that for the native receptor, effects of Na+ ions on agonist binding also reflect the R/R* transition and for dopamine, the effect of this transition on affinity is <2-fold in good agreement with data obtained using the TM II Asp mutant.
Use of mutations to probe the structure of AR* and AR*G
Another way to probe these issues is to examine the effects of mutations on the coupled and uncoupled forms of the receptor. If the interactions between agonist and receptor were similar in the two states but intensified upon activation, then we might expect similar effects of mutations in residues that contact the ligand in the ground and activated states.
For the m1 and m2 muscarinic receptors, mutation of the TM3Asp that is thought to provide the counter ion for the charged head group of ligands has varying effects on the binding of agonists to the G protein-uncoupled state and their ability to activate the receptor. It has been suggested that many agonists interact rather loosely with this residue in the ground state and may form other sets of interactions with the receptor. They then interact more strongly with this residue in the activated state, suggesting that there is only partial similarity between the ground and activated states (Hulme et al., 1995), emphasizing the distinction between ground-state and activated-binding configurations.
Mutation of Ty381 in TMVI of the m1 muscarinic receptor showed that the hydroxyl group of this residue interacted with the ester side chain of acetylcholine in both the ground and the activated state but that the aromatic ring had little interaction with acetylcholine in the ground state compared with a stronger one in the activated state (Ward et al., 1999). Again, this suggests a difference between the ligand-receptor interactions in the ground and activated states. In particular, there are new interactions made in the activated state that are absent in the ground state.
These studies show that there is only partial similarity between the agonist/receptor complex in the absence and presence of G protein coupling. This is consistent with little receptor activation in the G protein-uncoupled state, that is, little R*, and a large change in free energy for agonist/receptor interaction upon G protein coupling.
On the basis of the discussion above, we may conclude that for many GPCRs but not all, the free energy change that occurs when AR* couples to G is much larger than any changes in the G protein-uncoupled state (AR to AR*). In many cases, there may be a different set of interactions between agonist and receptor in the ground and activated states. For a full agonist, the R/R* transition contributes no more than two-fold to the observed affinity for several GPCRs, whereas the R*/R*G transition contributes up to 100-fold to the affinity. If the R*/R*G transition is suppressed, then only the minor effects of the R/R* transition remain. In some cases, if Na+ ions are included in assays this will suppress the R/R* transition. Measurements of agonist affinity for GPCRs in ligand-binding assays (Kobs) can, therefore, provide good estimates of ground-state affinities (KA), providing assay conditions are carefully controlled.
Methods for determining agonist dissociation constants: where should we be cautious?
In this section, I wish to consider different ways of determining dissociation constants for agonists and, in particular, how much the experimentally determined agonist dissociation constant (Kobs) differs from the microscopic agonist dissociation constant (KA).
Functional assays to determine agonist dissociation constants
Methods have been devised to determine values of dissociation constants for agonists based on functional assays in whole cell/tissue systems. For example, the partial alkylation method of Furchgott analyses concentration/response curves for agonists before and after partial inactivation of receptors with an irreversible antagonist (Furchgott, 1966; Furchgott and Bursztyn, 1967). The comparative method estimates the dissociation constant for a partial agonist by comparing concentration-response curves for a full and partial agonist (Barlow et al., 1967). Determination of dissociation constants may potentially be problematical when using such pharmacological methods where the affinity determined may include a component of the activation/efficacy process, that is, the experimentally determined Kobs may not be a good estimate of KA (Mackay, 1988, 1990; Black and Shankley, 1990; Leff et al., 1990a).
The problem has been addressed in theoretical studies where the ternary complex model or operational model were used to simulate data (Mackay, 1988; Black and Shankley, 1990; Leff et al., 1990b). It was suggested that only when R>>G would these pharmacological methods give accurate measures of KA. If R∼G or R<G, then erroneously high estimates of affinity may be obtained. This problem arises because, when the number of receptors does not exceed the number of G proteins, there is partitioning of receptor among activated states. It was also shown that the errors in KA determination were greater for the inactivation method than for the comparative method.
Several studies were performed to address this issue using functional experiments in native tissues. Two studies examined values of Kobs determined using the inactivation and comparative methods (Waud, 1969; Leff et al., 1990b). In neither study was any evidence obtained for the differences between Kobs and KA that had been predicted. Two studies examined the effects of progressive inactivation of receptors on KA estimates and again found no evidence for the predicted errors (Besse and Furchgott, 1976; Black and Shankley, 1990). In two studies, the inhibition of cAMP via m2 muscarinic acetylcholine receptors has been examined (Ehlert, 1987; McKinney et al., 1991) and Kobs determined using receptor inactivation (Furchgott and Bursztyn, 1967). Values of Kobs agreed well with dissociation constants determined using ligand binding under conditions where there was little G protein coupling, again suggesting little error in KA determination. Related studies on D1 dopamine, α2c-adrenergic receptors and 5-HT1A serotonin receptors provided affinity estimates for agonists in functional assays that agreed with values obtained from ligand binding where G protein coupling was suppressed (Sundaram et al., 1993; Mak et al., 1996; Stanton and Beer, 1997; Umland et al., 2001).
There are various explanations for why the predicted errors in KA determination are not seen experimentally. Receptors could be in excess of G proteins in the signalling systems. Alternatively, only low amounts of ARG intermediate are formed but there is substantial downstream amplification. Also, cells contain high concentrations of GTP (∼50 μM) (Otero, 1990; Jinnah et al., 1993) so that for GPCRs in whole cells, intermediate states such as ARG may form but only transiently as they are unstable in the presence of GTP. Some support for this idea comes from studies on agonist stimulation of [35S]GTPγS binding via the D2 dopamine and 5-HT1A serotonin receptors where increasing concentrations of GDP reduced agonist potency to values approaching the dissociation constant for the free receptor (McLoughlin and Strange, 2000; Roberts et al., 2004). These observations suggest that ARG formation will be suppressed in cells containing high levels of guanine nucleotides. In contrast, if functional assays are conducted with low GTP present, for example, in membrane preparations then ARG formation will be significant and affinities will be overestimated.
Agonist-binding studies to determine agonist dissociation constants
Partitioning of receptors between ground and activated states can potentially be a problem in using ligand-binding assays to determine KA values. Agonists bind to G protein-coupled (RG) and -uncoupled (R) states with higher and lower affinities, respectively. Some determinations of agonist affinity using ligand binding have analysed the coupled state using radiolabelled agonist binding or combinations of R and RG in agonist/radiolabelled antagonist competition assays and, for accurate determination of KA, it is important to restrict affinities to the uncoupled ground state. In contrast to the functional studies above, accumulation of intermediate states in ligand-binding assays can be favoured as the assays often do not contain exogenous guanine nucleotides. In principle, effects of binding to RG may be eliminated by inclusion of GTP in assays (see above and Lin et al., 2006). If agonist-binding studies are performed in the presence of concentrations of GTP sufficient to suppress formation of the ARG state, this will largely eliminate problems in KA determinations. As discussed earlier, if Na+ ions are also included in assays this may suppress any contribution from the R/R* transition.
On the basis of the foregoing discussion, it seems that experimental estimates of agonist dissociation constants (Kobs) made using ligand binding will mostly be good approximations of KA providing these are for the G protein-uncoupled state. Measurements of dissociation constants made using pharmacological functional tests need to be approached with caution but when examined they do not seem to differ greatly from ground-state values (KA).
Defining the efficacy of agonists at their receptors
Defining the efficacy of a drug is an important part of its characterization, particularly in drug design. For some indications, agonists are required and in this case careful definition of the efficacy of a candidate drug is required. Many drugs are, however, antagonists, that is, compounds with zero efficacy, so that drug design depends on showing that the compounds indeed do have zero efficacy. Efficacy is a functional concept and so it is defined in terms of a function linked to the receptor concerned. Efficacy is then assessed by determining the activity in a suitable assay system linked to the receptor using a range of concentrations of the drug under test, that is, a concentration/response experiment. Figures 4 and 5 show some simulations of concentration/response curves and parameters derived from these. Three parameters are accessible from this concentration/response experiment. These are the Emax (maximal agonist effect), EC50 (concentration of drug that gives a half maximal effect) and the Hill coefficient (an indicator of the form of the relationship between agonist concentration and response). For many responses linked to GPCRs, the Hill coefficient is close to unity, somewhat simplifying the analysis of drug action.
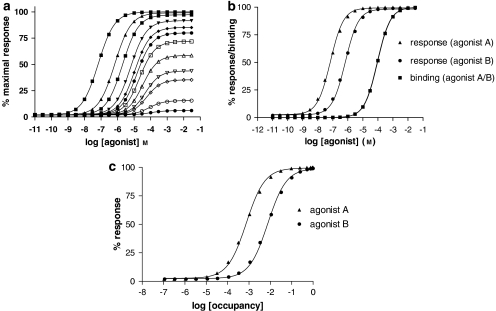
Figure 4.
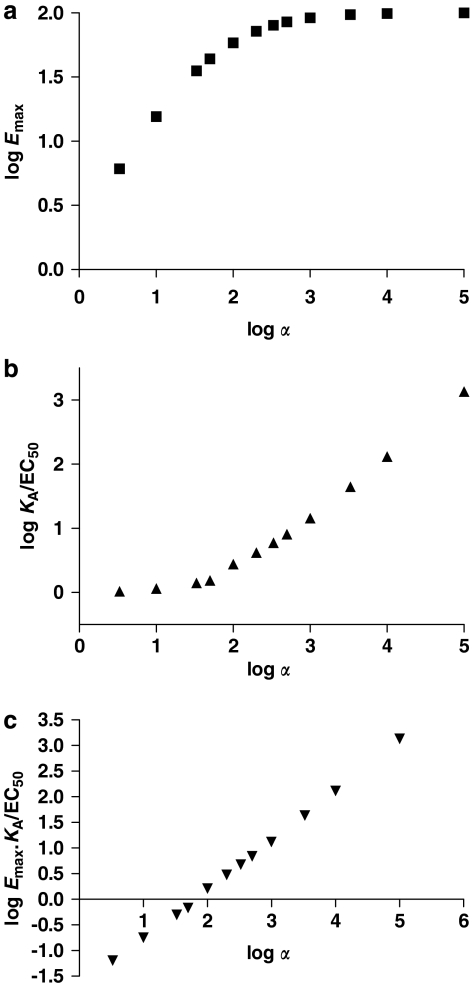
Methods for determination of agonist efficacy. (a) Shows a family of concentration/response curves for a range of agonists with different maximal agonist effects (Emax) in the system used to study their activity. The data were simulated using a ternary complex model (Figure 2) (Alder et al., 2003) with the following parameters: association constant for free receptor KR=104 (M−1); association constant for G protein-coupled receptor KRG (M−1)=109, 108, 3.33 × 107, 107, 5 × 106, 3.33 × 106, 2 × 106, 106, 5 × 105, 3.33 × 105, 105, 3.33 × 104; J=108 M−1; [R]=2 × 10−10 M, [G]=10−10 M. On the basis of these simulations values for Emax and EC50 were determined. Simulations of agonist-binding curves were also performed allowing the dissociation constant of the uncoupled state of the receptor (KA) to be determined (Alder et al., 2003). For agonists that do not give a maximal response, comparison of Emax values provides a measure of relative efficacy. (b) Shows concentration/response curves and binding curves for two agonists, each of which exhibits an Emax value of 100% in the test system. Both agonists have the same association constant for the uncoupled form of the receptor (KR=104 M−1) but different efficacies. The data were simulated using a ternary complex model (Alder et al., 2003) as in panel a with the following parameters: agonist A, KRG=109 M−1; agonist B, KRG=108 M−1. Response curves for agonists A and B are shown together with binding curves for the uncoupled ground-state receptor. Analysis of the simulated data gives KA/EC50 values of 1354 and 131 for agonists A and B, respectively. The ratio of these two values is 10.3 (see panel c). (c) Shows response/occupancy curves for two agonists. The data for responses are taken from the simulations in panel b. Percentage occupancy for each agonist concentration was calculated based on the dissociation constants of the uncoupled states of the receptor. The inverse occupancy ratio at 50% response for the two agonists is 10.2 in good agreement with the ratio of KA/EC50 values. KA, microscopic agonist dissociation constant.
Figure 5.
Relation between agonist efficacy and different parameters used experimentally to quantify efficacy. The set of simulated agonist concentration/response curves in Figure 4a was used together with simulated agonist-binding curves (Alder et al., 2003) to derive values for maximal agonist effect (Emax), KA/EC50 and EmaxKA/EC50. The ratio of KRG/KR was defined as α the efficacy in the ternary complex model. Values of KA correspond to the dissociation constant of the uncoupled form of the receptor derived as in Alder et al. (2003). The relationships between α and Emax, KA/EC50 and EmaxKA/EC50 are shown, respectively, in panels a, b and c. In panel a, Emax is expressed as a percentage; in panel c, Emax is expressed as a fraction. KA, microscopic agonist dissociation constant.
Emax as a measure of efficacy
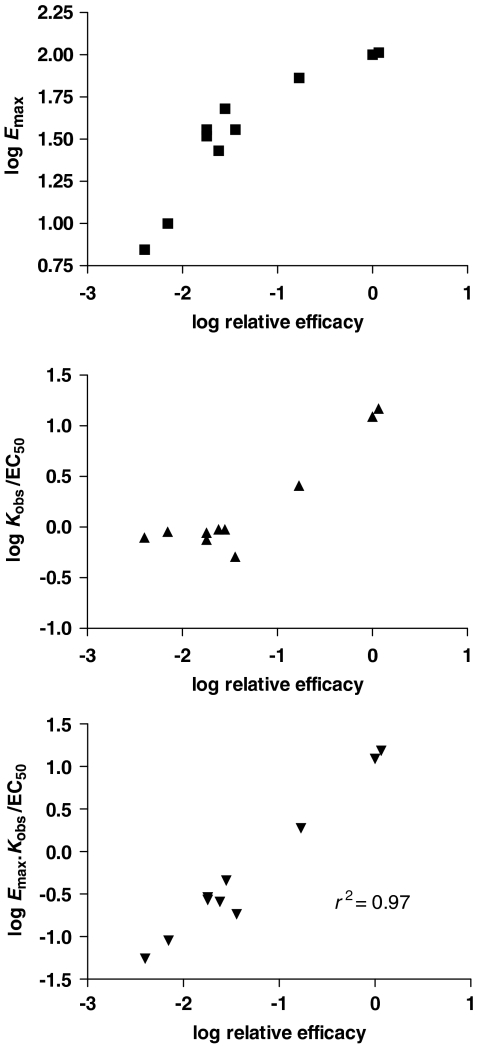
The Emax is the parameter used most frequently to assess efficacy in compounds. It is given by the upper asymptote of the concentration/response curve and is, therefore, relatively easy to determine. If this parameter is determined for a range of agonists at one receptor then the different Emax values should provide us with measures of the efficacy for the drugs relative to one another (Figure 5). It is expected that some drugs will produce a similar, ‘maximal' response in the system and these compounds may be defined operationally as full agonists. The other compounds then are partial agonists. This determination of Emax values provides one very convenient evaluation of the relative efficacy of drugs; indeed, there is a linear relationship between log Emax and log (relative efficacy) for partial agonists in the simulation based on the ternary complex model (Figures 4 and 5). The use of Emax as an efficacy parameter fails, however, when we come to the full agonists, for which the system will limit their response. The system response limit imposes an artificial ceiling on the measures of relative efficacy and limits the usefulness of Emax for assessment of efficacy. Indeed, it will be impossible to extend the scale of agonism beyond the maximal system response and based on determination of Emax it will not be possible to determine differences in efficacy for the compounds that give a maximal response even though they may actually have different values of intrinsic efficacy (Figures 4 and 5). This may lead to an underestimation of the relative efficacy of some full agonists and a corresponding overestimation of the relative efficacy of some partial agonists. Emax, therefore, provides some discrimination of relative efficacy for the partial agonists but not for the full agonists.
Maximal agonist effect can, therefore, be used for assessment of relative efficacy but only for compounds giving submaximal responses in the system. Comparison of the responses given by two agonists then gives a measure of their relative efficacies. Emax performs better as a measure of relative efficacy in response systems close to the receptor where few compounds will reach the maximal system response. In highly amplified systems, typically systems downstream of the receptor, many agonists will elicit similar maximal activation. If this is a recombinant system then it may be possible to reduce responses either by reducing receptor expression or by removing a proportion of the receptors with an alkylating agent.
Alternatively, a drug discovery programme may be aimed at antagonists. In this case the goal of zero efficacy can be assessed using Emax but this will also depend on the assay system. In some assay systems, it is difficult to distinguish antagonists and low efficacy partial agonists, and a well-amplified system may be better to make this discrimination.
The Kobs/EC50 ratio
A second measure of relative efficacy, which can also be used for the compounds that produce maximal responses in the system, is offered by considering the EC50 for agonists. Here we can look at the concentration/response curve relative to the concentrations of the drug that bind to the receptor. The more efficacious the drug, the more the concentration/response curve for signalling should be shifted away from that for binding. The EC50 will be lower than the KA and this may be expressed conveniently as the KA/EC50 ratio or amplification ratio (Furchgott, 1966; Black and Leff, 1983; Gardner et al., 1997) (Figure 4). Theoretical studies suggest that KA/EC50 should be related to efficacy (Black and Leff, 1983; Alder et al., 2003) and even for partial agonists, there will be a small shift of the response curve away from the binding curve, that the KA/EC50 ratio will be greater than one. In the Operational model (Black and Leff, 1983), a fundamental efficacy parameter τ is defined and termed the operational efficacy. The KA/EC50 ratio is equivalent to τ+1 emphasizing its importance as an efficacy parameter. On the basis of the discussion of agonist affinity and the similarity of KA and Kobs above, the Kobs/EC50 ratio will approximate the KA/EC50 ratio in many cases.
The Kobs/EC50 ratio, therefore, provides a second, experimentally accessible measure of efficacy, although it has not been used as much as Emax as it requires additionally a determination of the dissociation constant for binding of the drugs to the receptors (Kobs). Values for Kobs may be obtained using ligand-binding techniques or using pharmacological functional methods (see above). In principle, Kobs/EC50 data should provide a continuous measure of efficacy for a set of agonists but in practice, it becomes difficult to get accurate values of Kobs/EC50 for low efficacy agonists, where Kobs/EC50 is close to unity. Indeed, when simulations of agonist concentration/response curves are performed and values of KA/EC50 are examined in relation to efficacy, the simulations show that there is a good relationship at high values of efficacy but for low values of efficacy, the KA/EC50 ratio approaches unity (Figure 5). The KA/EC50 ratio and hence the Kobs/EC50 ratio, therefore, perform better for higher efficacy agonists in contrast to Emax, which performs better for partial agonists.
It should be noted that the KA/EC50 ratio is a measure of the difference in affinity of the agonist for the ground and activated states of the receptor and, therefore, is related to the free energy difference between the ground and activated states. On the basis of the discussion above, for several GPCRs, values of Kobs are reasonably accurate measures of ground-state affinity (KA), so that Kobs/EC50 and KA/EC50 are similar, but this may not be the case for all GPCRs. It should also be noted that determinations of Kobs should be performed under the same conditions as the EC50 determinations.
The EmaxKobs/EC50 parameter
These two measures of efficacy can be combined to provide another measure of relative efficacy for two drugs by determining the product of Emax and Kobs/EC50 (Ehlert et al., 1999; Trzeciakowski, 1999a). Relative efficacy for two drugs is then [EmaxKobs/EC50]1/[EmaxKobs/EC50]2, which for full agonists reduces to a comparison of the Kobs/EC50 ratios. Because of the similarity between Kobs and KA in many cases (see above), the EmaxKobs/EC50 parameter approximates Emax·KA/EC50. In terms of the Operational model, Emax·KA/EC50 is proportional to the operational efficacy, τ so that EmaxKobs/EC50 will also reflect efficacy. This parameter, EmaxKobs/EC50, has been used once experimentally for evaluation of efficacy (Avalos et al., 2001) and should provide a continuous measure of efficacy across the full range of efficacy. The EmaxKobs/EC50 parameter should spread out the efficacy data for the partial agonists (Emax<1) that would give Kobs/EC50 values close to unity as well as spreading out the efficacy data for the full agonists that give the same Emax. This is seen clearly in the simulations of data in Figure 5, where this combined parameter provides a striking relationship with the relative efficacy across the full efficacy range. The combined parameter also performs well with real data, for example, the set of data obtained for the contraction of rat aortic rings via α1D-adrenoceptors (Figure 6) (Ruffolo et al., 1979b). The EmaxKobs/EC50 parameter shows a linear relationship with the functional relative efficacy data across the full efficacy range, whereas neither the Emax nor the Kobs/EC50 parameter provides this linear relationship across the full efficacy range. A similar agreement is seen for other data taken from the literature (Ruffolo et al., 1979a; Ruffolo and Waddell, 1982, 1983) supporting the use of this combined parameter (data not shown). It seems that this little-used parameter provides a very useful, experimentally accessible measure of agonist efficacy and can be used to set up scales of efficacy in an assay system. It should also be noted that the agreement between analyses of theoretical and real data here supports the application of the ternary complex model as well as validating the estimates of Kobs used, that is, Kobs is a good estimate of KA.
Figure 6.
Relation between agonist efficacy and different parameters used experimentally to quantify efficacy: analysis of real data. The data for a set of imidazolines for contraction of rat aortic rings via α1D-adrenoceptors (Ruffolo et al., 1979b) have been analysed in terms of the different parameters shown in Figure 5. These functional data are very complete in that a large set of agonists of differing efficacies was used and maximal agonist effect (Emax), Kobs and EC50 were reported. Additionally, functional relative efficacy estimates were obtained using occupancy/response curves. Further data from the same lab examining contraction of aorta from different species (Ruffolo et al., 1979a; Ruffolo and Waddell, 1982, 1983) provide additional support for these results. In each of the studies, there is a good correlation between log EmaxKobs/EC50 and the log functional efficacy (P<0.05). Similar relationships are seen for the set of data for the inhibition of cAMP production via m2 muscarinic receptors (McKinney et al., 1991). If these are analysed in this manner, EmaxKobs/EC50 provides a good estimate of the functional efficacy. Kobs, experimentally determined agonist dissociation constant.
It should be noted that there is an error in applying the EmaxKobs/EC50 parameter if the Hill coefficient of the concentration/response curves for partial agonists are not equal to one. This has been covered in detail elsewhere (Ehlert et al., 1999; Ehlert, 2001).
It has also been suggested that a parameter (Emax/EC50 expressed relative to that for a reference agonist) termed the intrinsic relative activity, RAi, may be used to compare the activities of agonists acting at the same receptor (Ehlert et al., 1999; Griffin et al., 2007). RAi values for different responses mediated by the same receptor should be similar. If they are not then this may provide evidence of different receptors or pathway-dependent efficacy (see below). The RAi is presumably also analogous to the specificity constant (kcat/KM) used for enzymes (Fersht, 1999).
The inverse occupancy ratio
Concentration/response data for different agonists have been used to provide relative efficacy values for different drugs in another way. The concentration/response curves are converted into occupancy/response curves and for two drugs, the inverse ratio of occupancies required to produce the same response provides a measure of the relative efficacy of the two drugs (Figure 4). Again this method requires determination of the affinity of the drugs for the receptor. This method is related to the amplification ratio method as the inverse occupancy at 50% response is given by (1+Kobs/EC50).(1+Kobs/EC50) approximates to (1+KA/EC50), which in the Operational model corresponds to τ+2. One advantage of this method is that it may be used with incomplete concentration/response curves.
The multidimensional nature of efficacy
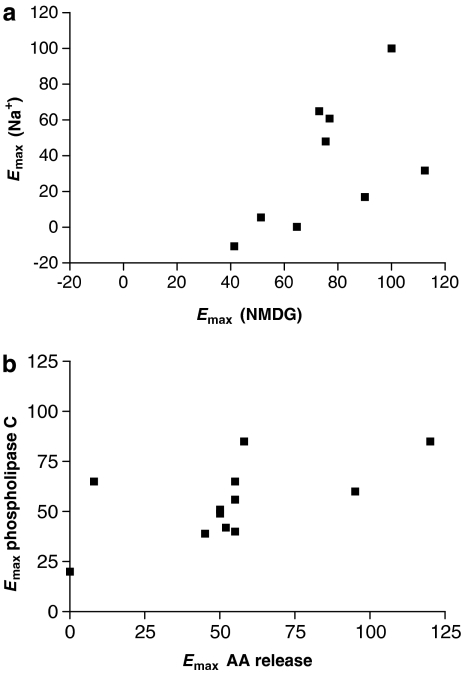
The methods described above allow ligand efficacy to be quantitated in a chosen assay read-out. It is becoming apparent, however, that the relative efficacy of compounds may depend on the read-out chosen for the assay or the conditions used. There are in fact many examples of differences in pharmacological profile for one receptor when different assay read-outs or conditions are used (Kenakin, 2005). We can see this variation in relative efficacy in two examples. In the first example, the relative efficacies of a range of agonists at the D2 dopamine receptor were determined using [35S]GTPγS binding in the presence of Na+ ions or in their absence with NMDG as a cation substitute (Lin et al., 2006). If the data are plotted together as an x/y plot (Figure 7) it is clear that there is a nonlinear relationship between the two sets of data and although the rank order is unchanged, some compounds are more affected by the removal of Na+ than others. This is presumably hinting at mechanistic differences.
Figure 7.
The multidimensional nature of efficacy. Data for the maximal agonist effect (Emax) values for agonists in different assays are plotted as an x/y plot. In (a), data for stimulation of [35S]GTPγS binding by agonists at the D2 dopamine receptor in the presence of Na+ and in the absence of Na+ (with N-methyl-D-glucamine (NMDG) substitution) are given as Emax relative to dopamine (Lin et al., 2006). In (b), data for stimulation of phospholipase C and arachidonic acid (AA) release via agonists at the serotonin 5-HT2C receptor (Moya et al., 2007) are given as Emax relative to 5-hydroxytryptamine.
In the second example, the relative efficacies of a range of compounds for stimulation of two assay read-outs (phospholipase C and arachidonic acid release) via the serotonin 5-HT2C receptor expressed in CHO cells (Moya et al., 2007) are shown, again plotted as an x/y plot. This plot highlights the pathway dependence of efficacy. In particular, the nonlinear relationship between efficacies in the two assay read-outs is clear from this plot, highlighting the differential efficacy profiles in the two assays.
These two-dimensional plots provide excellent representations of differences in efficacy in different pathways and the multidimensional nature of efficacy. In future it may be desirable to represent more than two dimensions of efficacy for compounds acting at one receptor.
Conclusion
On the basis of the discussion above it seems that affinity can be measured for agonists at several G protein-coupled receptors with some accuracy. Values for agonist dissociation constants measured experimentally (Kobs) may deviate from the true ground-state affinity for full agonists (KA), although these differences are mostly not great providing assay conditions are carefully controlled. In consequence, experimentally determined affinity values may be used to guide drug discovery and may be used in efficacy determinations.
I have also described several ways to determine efficacy for agonists and some of these allow scales of efficacy to be constructed. One problem here is the potential dependence of efficacy on the assay read-out. There are many examples of differences in pharmacological profile for one receptor when different assay read-outs are used (Kenakin, 2005). It may be necessary, therefore, to construct efficacy scales for each assay read-out but this only emphasizes the importance of having accurate and easily quantifiable measures of efficacy.
Acknowledgments
I thank Arthur Christopoulos for stimulating discussions and for critical reading of this paper.
Abbreviations
- Emax
maximal agonist effect
- GPCR
G protein-coupled receptor
- KA
microscopic agonist dissociation constant
- Kobs
experimentally determined agonist dissociation constant
Conflict of interest
The authors state no conflict of interest.
References
- Alder JT, Hacksell U, Strange PG. Analysis of molecular determinants of affinity and relative efficacy of a series of R- and S-2-(dipropylamino)tetralins at the 5-HT1A serotonin receptor. Br J Pharmacol. 2003;138:1129–1139. doi: 10.1038/sj.bjp.0705085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos M, Mak C, Randall PK, Trzeciakowski JP, Abell C, Kwan SW, et al. Nonlinear analysis of partial dopamine agonist effects on cAMP in C6 glioma cells. J Pharmacol Toxicol Methods. 2001;45:17–37. doi: 10.1016/s1056-8719(01)00118-6. [DOI] [PubMed] [Google Scholar]
- Barlow RB, Scott NC, Stephenson RP. The affinity and efficacy of onium salts on the frog rectus abdominis. Br J Pharmacol Chemother. 1967;31:188–196. doi: 10.1111/j.1476-5381.1967.tb01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse JC, Furchgott RF. Dissociation constants and relative efficacies of agonists acting on alpha adrenergic receptors in rabbit aorta. J Pharmacol Exp Ther. 1976;197:66–78. [PubMed] [Google Scholar]
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc London B Biol Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Black JW, Shankley NP. Interpretation of agonist affinity estimations: the question of distributed receptor states. Proc R Soc London B Biol Sci. 1990;240:503–518. doi: 10.1098/rspb.1990.0051. [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Limbird LE. Mutation of an aspartate residue highly conserved among G-protein-coupled receptors results in nonreciprocal disruption of alpha 2-adrenergic receptor-G-protein interactions. A negative charge at amino acid residue 79 forecasts alpha 2A-adrenergic receptor sensitivity to allosteric modulation by monovalent cations and fully effective receptor/G-protein coupling. J Biol Chem. 1994;269:29557–29564. [PubMed] [Google Scholar]
- Chung FZ, Wang CD, Potter PC, Venter JC, Fraser CM. Site-directed mutagenesis and continuous expression of human beta-adrenergic receptors. Identification of a conserved aspartate residue involved in agonist binding and receptor activation. J Biol Chem. 1988;263:4052–4055. [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure–activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Lang J, Gless C, Herz A. Spontaneous association between opioid receptors and GTP-binding regulatory proteins in native membranes: specific regulation by antagonists and sodium ions. Mol Pharmacol. 1990;37:383–394. [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc London B Biol Sci. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Donnelly D, Maudsley S, Gent JP, Moser RN, Hurrell CR, Findlay JB. Conserved polar residues in the transmembrane domain of the human tachykinin NK2 receptor: functional roles and structural implications. Biochem J. 1999;339 Part 1:55–61. [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ. Coupling of muscarinic receptors to adenylate cyclase in the rabbit myocardium: effects of receptor inactivation. J Pharmacol Exp Ther. 1987;240:23–30. [PubMed] [Google Scholar]
- Ehlert FJ.The ternary complex model Biomedical Applications of Computer Modelling 2001CRC Press LLC: Danvers; 21–85.In: Christopoulos A (ed). [Google Scholar]
- Ehlert FJ, Griffin MT, Sawyer GW, Bailon R. A simple method for estimation of agonist activity at receptor subtypes: comparison of native and cloned M3 muscarinic receptors in guinea pig ileum and transfected cells. J Pharmacol Exp Ther. 1999;289:981–992. [PubMed] [Google Scholar]
- Fersht A. Structure and Mechanism in Protein Science. W H Freeman: New York; 1999. [Google Scholar]
- Fraser CM, Wang CD, Robinson DA, Gocayne JD, Venter JC. Site-directed mutagenesis of m1 muscarinic acetylcholine receptors: conserved aspartic acids play important roles in receptor function. Mol Pharmacol. 1989;36:840–847. [PubMed] [Google Scholar]
- Furchgott RF.The use of beta-haloalkylamines in the determination of dissociation constants of receptor-agonist complexes Advances in Drug Research 1966Academic Press: New York; 21–55.In: Harper NJ, Simmonds AB (eds).vol. 3. [Google Scholar]
- Furchgott RF, Bursztyn P. Comparison of dissociation constants and of relative efficacies of selected agonists acting on parasympathetic receptors. Ann NY Acad Sci. 1967;144:882–898. [Google Scholar]
- Gardner BR, Hall DA, Strange PG. Agonist action at D2(short) dopamine receptors determined in ligand binding and functional assays. J Neurochem. 1997;69:2589–2598. doi: 10.1046/j.1471-4159.1997.69062589.x. [DOI] [PubMed] [Google Scholar]
- Griffin MT, Figueroa KW, Liller S, Ehlert FJ. Estimation of agonist activity at G protein-coupled receptors: analysis of M2 muscarinic receptor signaling through Gi/o,Gs, and G15. J Pharmacol Exp Ther. 2007;321:1193–1207. doi: 10.1124/jpet.107.120857. [DOI] [PubMed] [Google Scholar]
- Horstman DA, Brandon S, Wilson AL, Guyer CA, Cragoe EJ, Jr, Limbird LE. An aspartate conserved among G-protein receptors confers allosteric regulation of alpha 2-adrenergic receptors by sodium. J Biol Chem. 1990;265:21590–21595. [PubMed] [Google Scholar]
- Hulme EC, Curtis CA, Page KM, Jones PG. The role of charge interactions in muscarinic agonist binding, and receptor–response coupling. Life Sci. 1995;56:891–898. doi: 10.1016/0024-3205(95)00025-2. [DOI] [PubMed] [Google Scholar]
- Hulme EC, Lu ZL. Scanning mutagenesis of transmembrane domain 3 of the M1 muscarinic acetylcholine receptor. J Physiol Paris. 1998;92:269–274. doi: 10.1016/s0928-4257(98)80031-4. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Page T, Friedmann T. Brain purines in a genetic mouse model of Lesch–Nyhan disease. J Neurochem. 1993;60:2036–2045. doi: 10.1111/j.1471-4159.1993.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- Kong H, Raynor K, Yasuda K, Bell GI, Reisine T. Mutation of an aspartate at residue 89 in somatostatin receptor subtype 2 prevents Na+ regulation of agonist binding but does not alter receptor-G protein association. Mol Pharmacol. 1993a;44:380–384. [PubMed] [Google Scholar]
- Kong H, Raynor K, Yasuda K, Moe ST, Portoghese PS, Bell GI, et al. A single residue, aspartic acid 95, in the delta opioid receptor specifies selective high affinity agonist binding. J Biol Chem. 1993b;268:23055–23058. [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Leff P, Dougall IG, Harper DH, Dainty IA. Errors in agonist affinity estimation: do they and should they occur in isolated tissue experiments. Trends Pharmacol Sci. 1990a;11:64–67. doi: 10.1016/0165-6147(90)90319-4. [DOI] [PubMed] [Google Scholar]
- Leff P, Harper D, Dainty IA, Dougall IG. Pharmacological estimation of agonist affinity: detection of errors that may be caused by the operation of receptor isomerisation or ternary complex mechanisms. Br J Pharmacol. 1990b;101:55–60. doi: 10.1111/j.1476-5381.1990.tb12088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liapakis G, Chan WC, Papadokostaki M, Javitch JA. Synergistic contributions of the functional groups of epinephrine to its affinity and efficacy at the beta2 adrenergic receptor. Mol Pharmacol. 2004;65:1181–1190. doi: 10.1124/mol.65.5.1181. [DOI] [PubMed] [Google Scholar]
- Lin H, Saisch SG, Strange PG. Assays for enhanced activity of low efficacy partial agonists at the D(2) dopamine receptor. Br J Pharmacol. 2006;149:291–299. doi: 10.1038/sj.bjp.0706866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D. Continuous variation of agonist affinity constants. Trends Pharmacol Sci. 1988;9:156–157. doi: 10.1016/0165-6147(88)90026-0. [DOI] [PubMed] [Google Scholar]
- Mackay D. Agonist potency and apparent affinity: interpretation using classical and steady-state ternary-complex models. Trends Pharmacol Sci. 1990;11:17–22. doi: 10.1016/0165-6147(90)90036-8. [DOI] [PubMed] [Google Scholar]
- Mak CK, Avalos M, Randall PK, Kwan SW, Abell CW, Neumeyer JL, et al. Improved models for pharmacological null experiments: calculation of drug efficacy at recombinant D1A dopamine receptors stably expressed in clonal cell lines. Neuropharmacology. 1996;35:549–570. doi: 10.1016/0028-3908(96)84625-9. [DOI] [PubMed] [Google Scholar]
- Martin S, Botto JM, Vincent JP, Mazella J. Pivotal role of an aspartate residue in sodium sensitivity and coupling to G proteins of neurotensin receptors. Mol Pharmacol. 1999;55:210–215. doi: 10.1124/mol.55.2.210. [DOI] [PubMed] [Google Scholar]
- McKinney M, Miller JH, Gibson VA, Nickelson L, Aksoy S. Interactions of agonists with M2 and M4 muscarinic receptor subtypes mediating cyclic AMP inhibition. Mol Pharmacol. 1991;40:1014–1022. [PubMed] [Google Scholar]
- McLoughlin DJ, Strange PG. Mechanisms of agonism and inverse agonism at serotonin 5-HT1A receptors. J Neurochem. 2000;74:347–357. doi: 10.1046/j.1471-4159.2000.0740347.x. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, et al. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Neve KA. Regulation of dopamine D2 receptors by sodium and pH. Mol Pharmacol. 1991;39:570–578. [PubMed] [Google Scholar]
- Neve KA, Cox BA, Henningsen RA, Spanoyannis A, Neve RL. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol. 1991;39:733–739. [PubMed] [Google Scholar]
- Otero AD. Transphosphorylation and G protein activation. Biochem Pharmacol. 1990;39:1399–1404. doi: 10.1016/0006-2952(90)90420-p. [DOI] [PubMed] [Google Scholar]
- Perlman JH, Nussenzveig DR, Osman R, Gershengorn MC. Thyrotropin-releasing hormone binding to the mouse pituitary receptor does not involve ionic interactions. A model for neutral peptide binding to G protein-coupled receptors. J Biol Chem. 1992;267:24413–24417. [PubMed] [Google Scholar]
- Rang HP. The receptor concept: pharmacology's big idea. Br J Pharmacol. 2006;147 Suppl 1:S9–S16. doi: 10.1038/sj.bjp.0706457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DJ, Lin H, Strange PG. Mechanisms of agonist action at D2 dopamine receptors. Mol Pharmacol. 2004;66:1573–1579. doi: 10.1124/mol.104.004077. [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Dillard RD, Yaden EL, Waddell JE. Receptor interactions of imidazolines. II. Affinities and efficacies of hydroxy-substituted tolazoline derivatives in rat aorta. J Pharmacol Exp Ther. 1979a;211:74–79. [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Rosing EL, Waddell JE. Receptor interactions of imidazolines. I. Affinity and efficacy for alpha adrenergic receptors in rat aorta. J Pharmacol Exp Ther. 1979b;209:429–436. [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Waddell JE. Receptor interactions of imidazolines: alpha-adrenoceptors of rat and rabbit aortae differentiated by relative potencies, affinities and efficacies of imidazoline agonists. Br J Pharmacol. 1982;77:169–176. doi: 10.1111/j.1476-5381.1982.tb09283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Waddell JE. Aromatic and benzylic hydroxyl substitution of imidazolines and phenethylamines: differences in activity at alpha-1 and alpha-2 adrenergic receptors. J Pharmacol Exp Ther. 1983;224:559–566. [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- Smit MJ, Vischer HF, Bakker RA, Jongejan A, Timmerman H, Pardo L, et al. Pharmacogenomic and structural analysis of constitutive g protein-coupled receptor activity. Annu Rev Pharmacol Toxicol. 2007;47:53–87. doi: 10.1146/annurev.pharmtox.47.120505.105126. [DOI] [PubMed] [Google Scholar]
- Stanton JA, Beer MS. Characterisation of a cloned human 5-HT1A receptor cell line using [35S]GTP gamma S binding. Eur J Pharmacol. 1997;320:267–275. doi: 10.1016/s0014-2999(96)00914-4. [DOI] [PubMed] [Google Scholar]
- Strader CD, Sigal IS, Candelore MR, Rands E, Hill WS, Dixon RA. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988;263:10267–10271. [PubMed] [Google Scholar]
- Strader CD, Sigal IS, Register RB, Candelore MR, Rands E, Dixon RA. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci USA. 1987;84:4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange PG. Three-state and two-state models. Trends Pharmacol Sci. 1998;19:85–86. doi: 10.1016/s0165-6147(98)01175-4. [DOI] [PubMed] [Google Scholar]
- Strange PG. G-protein coupled receptors: conformations and states. Biochem Pharmacol. 1999;58:1081–1088. doi: 10.1016/s0006-2952(99)00144-6. [DOI] [PubMed] [Google Scholar]
- Strange PG. Agonist binding to G-protein coupled receptors. Br J Pharmacol. 2000;129:820–821. doi: 10.1038/sj.bjp.0702991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram H, Newman-Tancredi A, Strange PG. Characterization of recombinant human serotonin 5HT1A receptors expressed in Chinese hamster ovary cells. [3H]spiperone discriminates between the G-protein-coupled and -uncoupled forms. Biochem Pharmacol. 1993;45:1003–1009. doi: 10.1016/0006-2952(93)90243-p. [DOI] [PubMed] [Google Scholar]
- Trzeciakowski JP. Stimulus amplification, efficacy, and the operational model. Part I—binary complex occupancy mechanisms. J Theor Biol. 1999a;198:329–346. doi: 10.1006/jtbi.1999.0919. [DOI] [PubMed] [Google Scholar]
- Trzeciakowski JP. Stimulus amplification, efficacy, and the operational model. Part II—ternary complex occupancy mechanisms. J Theor Biol. 1999b;198:347–374. doi: 10.1006/jtbi.1999.0920. [DOI] [PubMed] [Google Scholar]
- Umland SP, Wan Y, Shah H, Billah M, Egan RW, Hey JA. Receptor reserve analysis of the human alpha(2C)-adrenoceptor using. Eur J Pharmacol. 2001;411:211–221. doi: 10.1016/s0014-2999(00)00909-2. [DOI] [PubMed] [Google Scholar]
- Urizar E, Claeysen S, Deupi X, Govaerts C, Costagliola S, Vassart G, et al. An activation switch in the rhodopsin family of G protein-coupled receptors: the thyrotropin receptor. J Biol Chem. 2005;280:17135–17141. doi: 10.1074/jbc.M414678200. [DOI] [PubMed] [Google Scholar]
- Wade SM, Lan K, Moore DJ, Neubig RR. Inverse agonist activity at the alpha(2A)-adrenergic receptor. Mol Pharmacol. 2001;59:532–542. doi: 10.1124/mol.59.3.532. [DOI] [PubMed] [Google Scholar]
- Wang CD, Buck MA, Fraser CM. Site-directed mutagenesis of alpha 2A-adrenergic receptors: identification of amino acids involved in ligand binding and receptor activation by agonists. Mol Pharmacol. 1991;40:168–179. [PubMed] [Google Scholar]
- Wang CD, Gallaher TK, Shih JC. Site-directed mutagenesis of the serotonin 5-hydroxytrypamine2 receptor: identification of amino acids necessary for ligand binding and receptor activation. Mol Pharmacol. 1993;43:931–940. [PubMed] [Google Scholar]
- Ward SD, Curtis CA, Hulme EC. Alanine-scanning mutagenesis of transmembrane domain 6 of the M(1) muscarinic acetylcholine receptor suggests that Tyr381 plays key roles in receptor function. Mol Pharmacol. 1999;56:1031–1041. doi: 10.1124/mol.56.5.1031. [DOI] [PubMed] [Google Scholar]
- Waud DR. On the measurement of the affinity of partial agonists for receptors. J Pharmacol Exp Ther. 1969;170:117–122. [PubMed] [Google Scholar]
- Williams AJ, Michel AD, Feniuk W, Humphrey PP. Somatostatin5 receptor-mediated [35S]guanosine-5′-O-(3-thio)triphosphate binding: agonist potencies and the influence of sodium chloride on intrinsic activity. Mol Pharmacol. 1997;51:1060–1069. doi: 10.1124/mol.51.6.1060. [DOI] [PubMed] [Google Scholar]
- Williams C, Sewing A. G-protein coupled receptor assays: to measure affinity or efficacy that is the question. Comb Chem High Throughput Screen. 2005;8:285–292. doi: 10.2174/1386207054020778. [DOI] [PubMed] [Google Scholar]
- Wilson J, Lin H, Fu D, Javitch JA, Strange PG. Mechanisms of inverse agonism of antipsychotic drugs at the D(2) dopamine receptor: use of a mutant D(2) dopamine receptor that adopts the activated conformation. J Neurochem. 2001;77:493–504. doi: 10.1046/j.1471-4159.2001.00233.x. [DOI] [PubMed] [Google Scholar]