Abstract
Background and purpose: We previously demonstrated that chronic hyperinsulinaemia induced by drinking high levels of fructose augments adrenergic nerve-mediated vasoconstriction and suppresses vasodilatation mediated by calcitonin gene-related peptide (CGRP)-containing (CGRPergic) vasodilator nerves. In this study, the effects of pioglitazone on vascular responses induced by stimulation of adrenergic nerves, CGRPergic nerves and vasoactive agents were investigated in pithed rats given 15% fructose solution to drink (FDR).
Experimental approach: To assess the effect of pioglitazone on the altered vascular responsiveness in the hyperinsulinaemic state in vivo, changes in vascular responses to spinal cord stimulation (SCS) and intravenous bolus injections of noradrenaline, angiotensin II and CGRP were evaluated in pithed control rats and FDR either untreated or treated with pioglitazone.
Key results: In the pithed FDR, vasoconstrictor responses to SCS and to injections of noradrenaline and angiotensin II were significantly greater than those of pithed control rats. In pithed FDR with artificially increased blood pressure and blockade of the autonomic ganglia, the vasodilator responses to SCS and CGRP injection were significantly smaller than those of pithed control rats. Oral administration of pioglitazone to FDR for two weeks markedly decreased plasma levels of insulin, triglycerides and blood glucose. In FDR pioglitazone diminished the augmented vasoconstrictor responses to SCS, noradrenaline and angiotensin II, and ameliorated the decrease in vasodilator responses to SCS.
Conclusions and implications: The present results suggest that pioglitazone improves not only insulin resistance, but also the dysfunctions in vascular control regulated by adrenergic and CGRPergic nerves in the hyperinsulinaemic state.
Keywords: chronic hyperinsulinaemia, pioglitazone, pithed rat, spinal cord stimulation, calcitonin gene-related peptide-containing nerve-mediated depressor, adrenergic nerve-mediated pressor
Introduction
Many clinical studies have indicated a possible relationship between hypertension and insulin resistance as patients with non-insulin-dependent diabetes mellitus frequently have hypertension (Skarfors et al., 1991; Haffner et al., 1992; Lissner et al., 1992). Also, Modan et al. (1985) suggested that there is a relationship between insulin levels and BP, and several groups have hypothesized that insulin resistance and/or hyperinsulinaemia contributes to the pathogenesis of hypertension (Landsberg, 1986; Reaven and Hoffman, 1987). Thus, insulin plays an important role in pathophysiological regulation of CVS. However, the association of insulin with high BP remains controversial.
In previous studies, we found that acute insulin infusion augments adrenergic nerve-mediated vasoconstriction and inhibits calcitonin gene-related peptide (CGRP)-containing (CGRPergic) nerve-mediated vasodilatation in pithed rats without a central vasoreflex (Takatori et al., 2003). More recently, we demonstrated that pithed rats with chronic hyperinsulinaemia, induced by giving a 15% fructose solution in drinking water for 10 weeks (fructose-drinking rats, FDR), have an augmented adrenergic nerve-mediated vasoconstriction and decreased CGRPergic nerve-mediated vasodilatation (Takatori et al., 2006). This suggests that insulin exerts neurogenic regulation of vascular tone.
Pioglitazone is a ligand for thiazolidinedione derivative and peroxisome proliferator-activated receptor-γ (PPARγ), and is used in the treatment of type 2 diabetes mellitus with insulin resistance. Numerous studies have suggested that pioglitazone increases sensitivity to insulin and inhibits the development of experimental hypertension (Zhang et al., 1994; Buchnan et al., 1995; Kaufman et al., 1995; Uchida et al., 1997; Verma et al., 1998; Grinsell et al., 2000). In addition, PPARγ has been shown to inhibit endothelial expression of adhesion molecules, inflammation and proliferation processes (Marx et al., 1998; Kato et al., 1999; Li et al., 2000; Pasceri et al., 2000). Therefore, active treatment with a PPARγ ligand is considered to be useful for insulin-resistant patients with complications such as hypertension and vascular disorders. However, the effect of pioglitazone on neurogenic regulation of vascular tone remains unclear.
Fructose has been shown to induce insulin resistance (Suzuki et al., 1997). Also, it has been found that a hyperinsulinaemic state with the added complication of hypertension is more likely to be induced by adding fructose to the drinking water rather than by adding it to the fed diet (Tobey et al., 1982). It has been suggested that insulin resistance induces hypertension as a result of a decrease in insulin-induced vasodilatation or by causing an imbalance between the pressor and depressor effects of insulin on the regulation of vascular tone (Ferrannini et al., 1997).
Thus, in the present study, we investigated the effects of pioglitazone on the vascular responses induced by spinal cord stimulation (SCS) and vasoactive agents in pithed rats with chronic hyperinsulinaemia. In addition, we examined whether insulin resistance is associated with the development of hypertension.
Methods
Animals
Thirty-two male Wistar rats weighing 350–420 g were used in this study. The animals were given food and water ad libitum. They were housed in the Animal Research Center of Okayama University at a controlled ambient temperature of 22±2 °C with 50±10% relative humidity and with a 12-h light/12-h dark cycle (lights on at 0800 hours). This study was carried out in accordance with the Guidelines for Animal Experiments at Okayama University Advanced Science Research Center, Japanese Government Animal Protection and Management Law (no. 105) and Japanese Government Notification on Feeding and Safekeeping of Animals (no. 6). Every effort was made to minimize the number of animals used and their suffering.
Fructose-drinking rats
At 6 weeks of age, the animals were randomly divided into four groups: a control group (n=6) and a control treatment group (n=6) that were given normal drinking water; a fructose group (n=8) and a fructose treatment group (n=12) that were given 15% fructose solution. The 15% fructose solution was given as drinking water from 6 to 16 weeks of age. Body weight, food and liquid intakes were measured at 1-week intervals between 6 and 16 weeks of age.
Treatment with pioglitazone and systolic BP and heart rate measurements
At 14 weeks of age, the FDR treatment group was orally administered pioglitazone at a dose of 2.5 (n=6) or 10 mg kg−1 day−1 (n=6; p.o.) for 2 weeks from 14 to 16 weeks of age. Similarly, the control treatment group was orally administered pioglitazone at a dose of 10 mg kg−1 day−1 (n=6). Pioglitazone was suspended in a 0.5% methylcellulose solution. Non-treated rats were administered 0.5% methylcellulose solution at a volume of 2 ml kg−1 day−1 for 2 weeks. Systolic BP (SBP) and heart rate (HR) were measured, in the conscious animals, with a tail-cuff plethysmograph (TK-370C; Unicom, Tokyo, Japan) between 0900 and 1200 hours, biweekly. The average of five readings was used.
In another series of experiments, a fine polyethylene tubing (PE-10) was chronically implanted into the left femoral artery of control rats and FDR, under ether anaesthesia, as described by Kawasaki and Takasaki (1986). Three days after the surgery, SBP and the diastolic BP were directly measured by a pressure transducer (model DX-100; Nihon Kohden, Tokyo, Japan) and polygraph system (model RM-6000; Nihon Kohden) in unanaesthetized and unrestrained animals. The HR triggered by arterial pulses was measured using a cardiotachometer (model AT-600G; Nihon Kohden) and was recorded using a polygraph. After connecting the extension tubing to the arterial cannula, the animal was moved to an open-topped cylindrical Plexiglas cage (30 cm in diameter) that was placed in a shielded, sound-proof box. When the animal was completely relaxed in the cage, the arterial BP and HR were measured simultaneously, whereas behaviour was observed through a window in the sound-proof box.
Biochemical analysis
At 6, 14 and 16 weeks of age, blood samples were obtained from the heart after a 12-h fasting. The plasma levels of glucose and triglycerides and total cholesterol were measured using a glucose analyser (ADVANTAGE; Boehringer, Mannheim, Tokyo, Japan) and enzymatically using a commercially available kit (Wako Pure Chemical Industries Ltd, Osaka, Japan), respectively. Plasma insulin was measured by a double-antibody method with an ELISA insulin kit (Morinaga Biochemistry Co., Kanagawa, Japan).
Pithing and measurement
The animals were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p. ) at 16 weeks of age. Polyethylene catheters (PE-10) were positioned in the right and left jugular veins to administer drugs, and a bilateral vagotomy was performed at the midcervical level. A polyethylene catheter (PE-50) was inserted into the left carotid artery and connected to a pressure transducer (model DX-100; Nihon Kohden). The arterial BP and mean BP were recorded using a polygraph (model RM-6000; Nihon Kohden). HR triggered by arterial pulses was measured using a cardiotachometer (model AT-600G; Nihon Kohden) and was recorded using a polygraph.
After the trachea was cannulated, the animals were pithed by inserting a stainless steel rod (1.4 mm in diameter) through the right orbit and the foramen magnum and down into the spinal cord to the level of the sacral end. Then the tip of the rod was raised to the thoracolumbar vertebrae (Th 9–12) according to the method described previously by Taguchi et al. (1992) and Takatori et al. (2003). Artificial respiration (4.5 ml breath per kg, 70 breaths per min) with room air was immediately started using an artificial respirator (model 683; Harvard Apparatus, South Natic, MA, USA). The pithing rod served as the stimulating electrode, which was insulated except for 5 mm of the tip. The level of SCS was determined by varying the depth of rod insertion. The position of the rod within the vertebral canal was determined from the length of the rod. A stainless steel needle was inserted subcutaneously in the dorsum, parallel to the vertebral column, to serve as an indifferent electrode. After the animals were pithed, D-tubocurarine (1 mg kg−1, i.v.) was injected to prevent skeletal muscle contraction during electrical stimulation of the spinal cord. The rectal temperature was maintained at approximately 37 °C by means of a heater mat (model KN-475; Natsume, Tokyo, Japan).
Spinal cord stimulation
After allowing BP and HR to stabilize, electrical stimulation at 2, 4 and 8 Hz, which induced a sharp increase in BP without changing the HR, was applied to verify the position of the rod in the spinal column. Rectangular pulses (1 ms in duration and 20 V) were given for 30 s at 5–10 min intervals with an electronic stimulator (model SEN-3201; isolator 20865, Nihon Kohden).
After the experiment on pressor responsiveness had finished, the mean BP was increased and maintained at a level of approximately 120 mm Hg by continuous infusion of the α1-adrenoceptor agonist methoxamine (20 μg kg−1 min−1, i.v.). The autonomic ganglionic blocker hexamethonium (2 mg kg−1 min−1, i.v.) was also infused to block the autonomic outflow. The increased BP was allowed to stabilize, and then the spinal cord was electrically stimulated. Rectangular pulses (1 ms in duration and 20 V) at 2 and 4 Hz were given for 30 s with an electronic stimulator (model SEN-3201; isolator 20865, Nihon Kohden).
Experimental protocols
To assess the effect of pioglitazone on the altered vascular responsiveness in the hyperinsulinaemic state in vivo, the pressor and depressor responses to SCS and various vasoactive agents were evaluated in the pithed control rats and FDR treated with or without pioglitazone. After the animals were pithed and both BP and HR stabilized, electrical stimulation (2, 4 and 8 Hz), intravenous injections of noradrenaline (125, 250 and 500 ng kg−1, i.v.) and angiotensin II (Ang II; 40, 100 and 200 pmol kg−1, i.v.) were given. At the end of these experiments, the mean BP of the pithed rats was increased by continuous infusion of methoxamine (20 μg kg−1 min−1, i.v.) concomitant with an infusion of hexamethonium (2 mg kg−1 min−1, i.v.). After the elevated BP stabilized, SCS (2 and 4 Hz) and bolus injections of acetylcholine (0.05 and 0.5 nmol kg−1, i.v.), rat CGRP (0.05 and 0.1 nmol kg−1, i.v.) and sodium nitroprusside (SNP; 0.5 and 5 μg kg−1, i.v.) were applied.
Statistical analysis
The experimental results are presented as the mean±s.e. mean. Statistical analyses were performed using one-way ANOVA followed by Tukey's test or two-way ANOVA, as appropriate. A P-value less than 0.05 was considered statistically significant.
Drugs
The following drugs were used: acetylcholine chloride (Daiichi Pharmaceutical Co., Tokyo, Japan), Ang II (Peptide Institute, Osaka, Japan), D(−)-fructose (Wako Pure Chemical Industries Ltd), hexamethonium bromide (Sigma Chemical Co., St Louis, MO, USA), methoxamine hydrochloride (Nihon Shinyaku Co., Kyoto, Japan), noradrenaline hydrochloride (Sankyo Co., Tokyo, Japan), rat α-CGRP (Peptide Institute), pioglitazone (Takeda Co., Tokyo, Japan), D-tubocurarine (Sigma) and SNP (Sigma). All drugs except pioglitazone were dissolved in 0.9% saline and infused at a rate of 0.2 ml min−1 using an infusion pump (model 11; Harvard Apparatus) or given as bolus doses (0.2 ml kg−1).
Results
Effect of pioglitazone on body weight and food and liquid intake in FDR
As shown in Table 1, pioglitazone treatment did not affect the body weight of control rats or FDR. The food intakes in non- and pioglitazone-treated FDR were significantly lower than those in control rats (Table 1). The liquid intakes in untreated and pioglitazone-treated FDR were significantly higher than those in control rats (Table 1). However, there was no significant difference in food or liquid intakes between untreated and pioglitazone-treated FDR.
Table 1.
Changes in body weight and food and liquid intake in water-drinking rats (control) and pioglitazone-treated control or 15% fructose-drinking rats and PIO-treated FDR
| 6 weeks old | 14 weeks old | 16 weeks old | |
|---|---|---|---|
| Body weight (g) | |||
| Control | 195.1±3.0 | 360.7±6.2 | 370.1±6.6 |
| Control+PIO (10 mg kg−1 day−1) | 196.3±2.5 | 368.4±6.1 | 368.2±5.7 |
| FDR | 194.0±1.9 | 370.6±7.1 | 369.9±3.2 |
| FDR+PIO (2.5 mg kg−1 day−1) | 197.2±2.1 | 370.2±5.2 | 370.3±4.5 |
| FDR+PIO (10 mg kg−1 day−1) | 196.7±3.8 | 374.2±9.2 | 371.9±12.7 |
| Food intake (g per 100 g BW per day) | |||
| Control | 7.3±3.3 | 4.9±1.3 | 5.2±2.8 |
| Control+PIO (10 mg kg−1 day−1) | 7.1±2.0 | 5.2±2.8 | 5.8±1.3 |
| FDR | 6.5±1.6 | 3.8±0.7** | 2.3±0.5** |
| FDR+PIO (2.5 mg kg−1 day−1) | 6.8±1.5 | 4.0±0.4* | 2.2±1.7** |
| FDR+PIO (10 mg kg−1 day−1) | 7.0±0.5 | 4.2±0.1* | 2.4±0.3** |
| Liquid intake (g per 100 g BW per day) | |||
| Control | 21.5±0.4 | 18.0±0.4 | 19.5±3.3 |
| Control+PIO (10 mg kg−1 day−1) | 20.8±2.9 | 17.7±1.4 | 20.1±2.0 |
| FDR | 22.0±1.7 | 16.1±2.3** | 15.0±1.0* |
| FDR+PIO (2.5 mg kg−1 day−1) | 23.2±1.2 | 14.1±0.7** | 15.3±2.8* |
| FDR+PIO (10 mg kg−1 day−1) | 19.1±0.3 | 13.7±2.8** | 16.1±3.1* |
Abbreviations: BW, body weight; FDR, fructose-drinking rats; PIO, pioglitazone.
Normal water or 15% fructose was given from 6 to 16 weeks as drinking solution. Pioglitazone at a dose of 2.5 or 10 mg kg−1 day−1 was administered p.o. for 2 weeks from 14 to 16 weeks of age. Values are expressed as the mean±s.e.mean of n results. Control, n=6; PIO-treated, n=6; FDR, n=8 and PIO-treated FDR, n=12. *P<0.05, **P< 0.01 vs control.
Effect of pioglitazone on plasma levels of insulin, triglycerides, total cholesterol and glucose
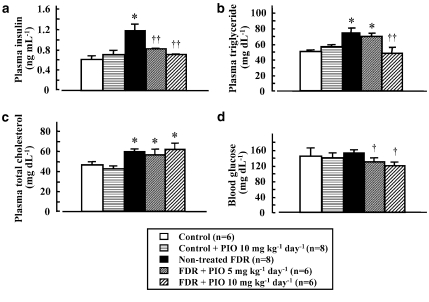
As shown in Figure 1, plasma levels of insulin, triglycerides and total cholesterol, but not plasma glucose, in untreated FDR at 16 weeks of age were significantly higher than those of control rats (except for total cholesterol level). In pioglitazone-treated FDR, plasma levels of insulin and triglycerides, but not total cholesterol, were significantly lower than those in untreated FDR and similar to those in control rats (Figures 1a and b). There was no significant difference in the plasma glucose level between control rats and FDR at 16 weeks of age. However, pioglitazone treatment significantly decreased plasma glucose levels compared with those in untreated FDR (Figure 1d). In pioglitazone-treated control rats, at 16 weeks of age, plasma levels of insulin, triglycerides, total cholesterol and glucose were similar to those in control rats (Figures 1a and b).
Figure 1.
Plasma insulin (a), plasma triglycerides (b), plasma total cholesterol (c) and blood glucose levels (d) in untreated (non-treated) or pioglitazone (PIO)-treated water-drinking rats (control) and fructose-drinking rats (FDR). PIO at a dose of 2.5 or 10 mg kg−1 day−1 was administered p.o. for 2 weeks from 14 to 16 weeks of age. *P<0.01 vs control rats. †P<0.01 vs untreated FDR.
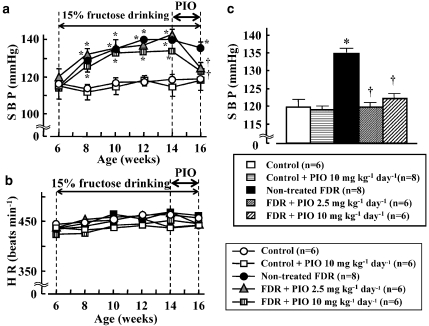
Effect of pioglitazone treatment on changes in SBP and HR
As shown in Figure 2a, systolic BP in FDR was markedly elevated 2 weeks after starting 15% fructose solution treatment and the elevation of systolic BP in FDR lasted until 16 weeks of age. Significant differences in systolic BP between FDR and control rats were found between 2 weeks (8 weeks of age) and 10 weeks (16 weeks of age) after fructose administration (Figure 2a). However, there was no significant difference in HR between FDR and control rats (Figure 2b).
Figure 2.
Changes in systolic BP (SBP) (a and c) and heart rate (HR) (b) in water (control)- and fructose-drinking rats (FDR) from 6 to 16 weeks of age and effect of pioglitazone (PIO). (c) SBP at 16 weeks. SBP and HR were measured by the tail-cuff method biweekly. Each value represents the mean and vertical bars show s.e.mean. PIO at a dose of 2.5 or 10 mg kg−1 day−1 was administered p.o. for 2 weeks from 14 to 16 weeks of age. *P<0.01 vs control rats. †P<0.01 vs untreated FDR.
In conscious, in unrestrained rats, the SBP, diastolic BP and HR of control rats and FDR, which were directly measured by a pressure transducer, were 115.0±1.5 mm Hg, 93.5±4.8 mm Hg and 352.4±10.6 beats min−1 (n=8) and 124.4±1.5 mm Hg, 107.8±3.6 mm Hg and 371.5±7.1 beats min−1 (n=5), respectively. There was significant difference (P<0.05) in SBP and diastolic BP but not in HR between the control and FDR groups. In addition, we analysed the relationship between the plasma level of insulin and SBP of control rats and FDR that were unanaesthetized and unrestrained and found that there was a significant positive correlation (r=0.686, P<0.05) between these two variables in both control rats and FDR.
Oral treatment with pioglitazone at 2.5 and 10 mg kg−1 day−1 for 2 weeks in FDR significantly decreased SBP, measured using a tail-cuff, compared with that in untreated FDR, a result similar to that found in control rats (Figures 2a and c). However, treatment with pioglitazone at 10 mg kg−1 day−1 in control rats did not affect SBP (Figures 2a and c). There was no significant difference in HR between all of the groups (Figure 2b).
Effect of pioglitazone treatment on changes in pressor responses to SCS and bolus injections of noradrenaline and Ang II
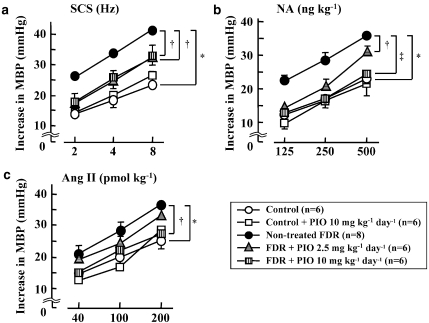
The basal mean BP and HR 30 min after pithing in control and untreated FDR were 33.1±2.4 mm Hg and 294.2±9.0 beats min−1 and 35.0±1.5 mm Hg and 296.3±7.9 beats min−1, respectively. There was no significant difference in mean BP and HR between the control and untreated FDR groups. The SCS at 2, 4 and 8 Hz in the pithed control rats and untreated FDR caused a sharp, frequency-dependent increase in BP (Figures 3A and D) without changing HR (data not shown). Also, bolus injections of noradrenaline (125, 250 and 500 ng kg−1) (Figures 3B and E) and Ang II (40, 100 and 200 pmol kg−1) (Figures 3C and F) induced dose-dependent increases in BP. Noradrenaline injection, but not Ang II injection, caused an increase in HR (data not shown). In the pithed untreated FDR, pressor responses to SCS (2, 4 and 8 Hz) (Figures 3D and 4a) and bolus injections of noradrenaline (125, 250 and 500 ng kg−1) (Figures 3E and 4b) and Ang II (200 pmol kg−1) (Figures 3F and 4c) were significantly greater than those in the pithed control rats. In addition, in the untreated FDR, the SCS-induced pressor responses were much greater than the noradrenaline-induced pressor responses (Figures 4a and b). Pressor responses to Ang II injection in the pithed untreated FDR were significantly greater than those in the pithed control rats (Figures 3F and 4c).
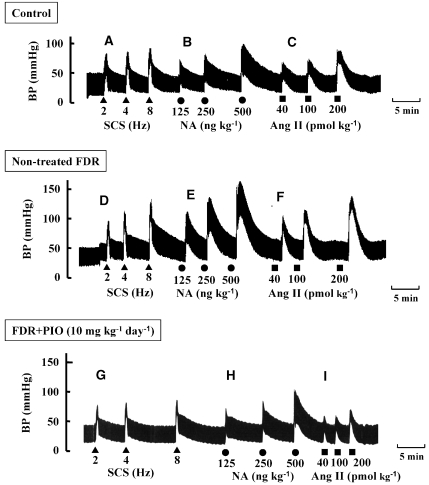
Figure 3.
Typical records showing effect of pioglitazone on vasoconstrictor responses to spinal cord stimulation (SCS) (A, D and G; 2–8 Hz; triangles) and bolus injections of noradrenaline (NA) (B, E and H; 125–500 ng kg−1; circles) and angiotensin II (Ang II) (C, F and I; 40–200 pmol kg−1; squares) in the pithed control rats (upper trace), untreated fructose-drinking rats (FDR; middle trace) and pioglitazone (PIO)-treated FDR (lower trace). PIO at a dose of 10 mg kg−1 day−1 was administered p.o. for 2 weeks from 14 to 16 weeks of age.
Figure 4.
Effect of pioglitazone (PIO) on pressor responses induced by spinal cord stimulation (SCS) (a; 2–8 Hz), noradrenaline (NA) (b; 125–500 ng kg−1) and angiotensin II (Ang II) (c; 40–200 pmol kg−1) in the pithed control rats, PIO-treated control rats, untreated fructose-drinking rats (FDR) and PIO-treated FDR. Each symbol represents the mean and vertical bars show s.e.mean. PIO at a dose of 2.5 or 10 mg kg−1 day−1 was administered p.o. for 2 weeks from 14 to 16 weeks of age. *P<0.01 vs control rats. †P<0.05, ‡P<0.01 vs untreated FDR. MBP, mean blood pressure.
The basal mean BP and HR 30 min after pithing in FDR treated with pioglitazone at 2.5 and 10 mg kg−1 were 37.4±2.9 mm Hg and 319.30±11.6 beats min−1 and 34.8±4.5 mm Hg and 303.0±9.7 beats min−1, respectively. There was no significant difference in mean BP or HR between untreated FDR and the pioglitazone-treated group. Treatment of FDR with pioglitazone at doses of 2.5 or 10 mg kg−1 day−1 for 2 weeks significantly suppressed SCS-induced vasoconstriction (Figures 3G and 4a). In addition, pioglitazone treatment at doses of 2.5 or 10 mg kg−1 day−1 significantly decreased the pressor responses to noradrenaline to the control levels (Figures 3H and 4b). As shown in Figures 3I and 4c, the Ang II-induced pressor responses in FDR were attenuated by treatment with pioglitazone (10 mg kg−1 day−1). A significant difference in the Ang II (200 pmol kg−1)-induced pressor response was found between the untreated FDR and pioglitazone (10 mg kg−1 day−1) group (Figure 4c).
The basal mean BP and HR 30 min after pithing in control rats treated with pioglitazone at 10 mg kg−1 were 37.2±2.1 mm Hg and 289.0±4.5 beats min−1. There was no significant difference in mean BP and HR between the untreated and pioglitazone-treated control groups. The pioglitazone (10 mg kg−1 day−1)-treated control group had SCS-, noradrenaline- and Ang II-induced pressor responses similar to those in the untreated control group (Figure 4).
Effect of pioglitazone on changes in depressor responses to SCS and bolus injections of CGRP, acetylcholine and SNP
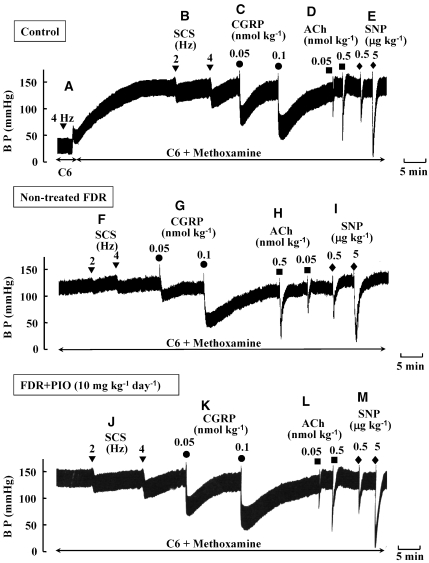
As shown in Figure 5A, the pressor response to SCS at 4 Hz in the control rats was abolished by hexamethonium, an autonomic ganglion blocker, indicating that the SCS-induced pressor response was mediated by sympathetic adrenergic nerves. When BP was increased by infusion of methoxamine in the presence of hexamethonium, SCS at 2 and 4 Hz caused a frequency-dependent depressor response (Figure 5B) without changing HR (data not shown). Bolus injections of CGRP at doses of 0.05 and 0.1 nmol kg−1 induced a dose-dependent and long-lasting fall in BP similar to the pattern of response to SCS (Figure 5C). Bolus injections of acetylcholine (0.05 and 0.5 nmol kg−1) (Figure 5D) and SNP (0.5 and 5 μg kg−1) (Figure 5E) caused a sharp fall in BP in a dose-dependent manner.
Figure 5.
Typical records showing effect of pioglitazone (PIO) on vasodilator responses to spinal cord stimulation (SCS) (B, F and J; 2 and 4 Hz; inversed triangles) and bolus injections of calcitonin gene-related peptide (CGRP) (C, G and K; 0.05 and 0.1 nmol kg−1; circles), acetylcholine (ACh) (D, H and L; 0.05 and 0.5 nmol kg−1; squares) and sodium nitroprusside (SNP) (E, I and M; 0.5 and 5 μg kg−1; diamonds) in the pithed control rats (upper trace), untreated fructose-drinking rats (FDR; middle trace) and pioglitazone (PIO)-treated FDR (lower trace). PIO at a dose of 10 mg kg−1 day−1 was administered p.o. for 2 weeks from 14 to 16 weeks of age. BP was artificially increased by continuous infusion of methoxamine (20 μg kg−1 min−1, i.v.) and in the presence of hexamethonium (C6) (2 mg kg−1 min−1, i.v., which abolished the SCS (4 Hz)-induced pressor response (A).
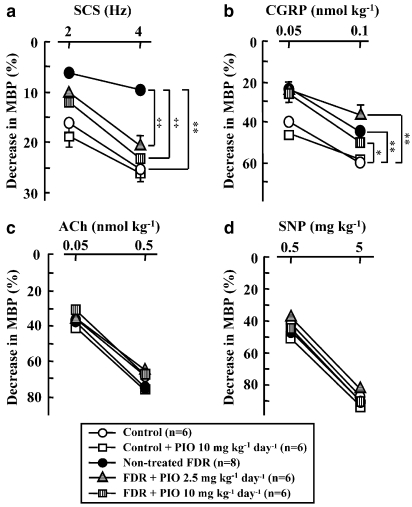
As shown in Figures 5 and 6, depressor responses to SCS (2 and 4 Hz) (Figures 5F and 6a) and to bolus injections of CGRP (0.05 and 0.1 nmol kg−1) (Figures 5G and 6b) in the pithed FDR were significantly smaller than those in the pithed control rats. However, depressor responses to bolus injections of acetylcholine (Figures 5H and 6c) and SNP (Figures 5I and 6d) in the pithed FDR were similar to those in the pithed control rats.
Figure 6.
Effect of pioglitazone (PIO) on vasodilator responses to spinal cord stimulation (SCS) (a; 2 and 4 Hz) and bolus injections of calcitonin gene-related peptide (CGRP) (b; 0.05 and 0.1 nmol kg−1), acetylcholine (ACh) (c; 0.05 and 0.5 nmol kg−1) and sodium nitroprusside (SNP) (d; 0.5 and 5 μg kg−1) in the pithed control rats, PIO-treated control rats, untreated fructose-drinking rats (FDR) and PIO-treated FDR. Each symbol represents the mean and vertical bars show s.e.mean. PIO at a dose of 2.5 or 10 mg kg−1 day−1 was administered p.o. for 2 weeks from 14 to 16 weeks of age. BP was artificially increased by continuous infusion of methoxamine (20 μg kg−1 min−1, i.v.) and in the presence of C6 (2 mg kg−1 min−1, i.v.). *P<0.05, **P<0.01 vs control rats. ‡P<0.01 vs non-treated FDR. MBP, mean blood pressure.
Treatment of FDR with pioglitazone at a dose of 2.5 or 10 mg kg−1 day−1 for 2 weeks augmented depressor responses to SCS (Figures 5J and 6a). Significant differences in the SCS-induced depressor responses were found between the untreated FDR and pioglitazone (2.5 and 10 mg kg−1 day−1)-treated FDR (Figure 6a). However, the SCS-induced depressor responses in the pioglitazone (10 mg kg−1 day−1)-treated control group were similar to those in the untreated control group (Figure 6a).
Depressor responses to bolus injections of CGRP at 0.1 and 0.05 nmol kg−1 were not significantly increased by treatment with pioglitazone compared with those in control rats (Figures 5K and 6b). Treatment with pioglitazone had no effect on the depressor responses to bolus injections of either acetylcholine (Figures 5L and 6c) or SNP (Figures 5M and 6d).
Discussion and conclusion
In the present study, FDR, which were given 15% fructose solution to drink instead of water, showed a marked increase in plasma levels of insulin and triglycerides without a significant increase in blood glucose. Treatment of FDR with pioglitazone, a PPARγ ligand, for 2 weeks, markedly increased insulin sensitivity, abolished hyperinsulinaemia and hypertriglyceridaemia and decreased the plasma glucose level, strongly suggesting that the hyperinsulinaemia of these animals was due to insulin resistance. In addition to these findings, FDR with hyperinsulinaemia developed hypertension. Furthermore, a significant positive correlation was found between the plasma levels of insulin and SBP (measured in conscious, unrestrained animals) in control rats and FDR. The untreated FDR showed maintained hypertension, whereas the SPB in the pioglitazone-treated FDR was markedly decreased to a level similar to that of the control rats. These results are in agreement with previous findings indicating that there is a positive correlation between SBP and plasma insulin or triglyceride levels in FDR (Suzuki et al., 1997). Thus, it is very likely that hypertension in FDR is closely associated with chronic hyperinsulinaemia and insulin resistance. Pioglitazone has been reported to decrease BP in patients with type 2 diabetic mellitus (Anan et al., 2007; De Rivas et al., 2007). Therefore, the present findings imply that pioglitazone is effective at treating hypertension resulting from insulin resistance and/or hyperinsulinaemia. Insulin has been shown to increase sympathetic activity (Lembo et al., 1992) and renal sodium reuptake (Defronzo, 1981), and promote the proliferation of vascular smooth muscle cells (Hsueh and Law, 1999), which could bring about an increase in BP. In the present study, the basal BP of the pithed FDR, which had no sympathetic outflow or vasoreflex, was similar to that in the pithed control rats. Therefore, it is unlikely that the increased BP in FDR is due to insulin-induced renal sodium uptake and proliferation of vascular smooth cells. It is more likely that increased sympathetic activity or dysfunction of the neuronal vascular control system induced by the hyperinsulinaemic state is responsible for the development of hypertension in FDR.
In pithed rats, the pressor response to SCS has been shown to be mediated by activation of sympathetic adrenergic nerves, as an adrenergic neuron blocker (guanethidine) and an autonomic ganglionic blocker (hexamethonium) abolished the response (Taguchi et al., 1992; Takatori et al., 2003). In the present study, the SCS-induced pressor response in pithed FDR, which were in a chronic hyperinsulinaemic state, was significantly greater than that in pithed control rats. In addition, FDR showed significantly increased pressor responses to exogenously applied noradrenaline; these responses are mediated by postsynaptic α1-adrenoceptors (Taguchi et al., 1992; Takatori et al., 2003). However, the pressor responses to SCS were more markedly increased in FDR than those to exogenously applied noradrenaline. Therefore, it is very likely that the release of the neurotransmitter noradrenaline from sympathetic nerve terminals is augmented in a chronic hyperinsulinaemic state. We have found, previously, that acute hyperinsulinaemia produced by continuous infusion of insulin results in the potentiation of pressor responses to SCS and noradrenaline injection in the euglycaemic pithed rat (Takatori et al., 2003). Thus, it appears that the augmented pressor response in FDR mainly results from chronic hyperinsulinaemia, which increases the sympathetic nerve activity and vasoreactivity of the blood vessels. This conclusion is supported by the findings from the present study as the treatment of FDR with pioglitazone for 2 weeks abolished the hyperinsulinaemic state, resulting in a significant attenuation of the augmented vasoconstriction in response to SCS and exogenous noradrenaline and Ang II present in this hyperinsulinaemic state.
Another important finding of the present study is that the depressor response to SCS was strongly suppressed in the pithed FDR with artificially elevated BP and blockade of autonomic outflow. Previous studies have demonstrated that the depressor response to SCS in pithed rats with artificially elevated BP and blockade of autonomic outflow is mediated by endogenous CGRP released from CGRPergic vasodilator nerves, as the response was abolished by CGRP (8–37), a CGRP receptor antagonist, and capsaicin, which causes CGRP depletion in CGRPergic nerves (Taguchi et al., 1992; Nuki et al., 1993; Takatori et al., 2003). Also, it has been shown that acute hyperinsulinaemia induced by exogenously applied insulin in euglycaemic pithed rats results in a marked reduction of the vasodilatation mediated by stimulation of CGRPergic nerves and exogenous CGRP (Takatori et al., 2003). In the present study, the vasodilator responses induced by stimulating CGRPergic nerves or by the addition of CGRP were significantly smaller in FDR than in control rats. Furthermore, the decreased depressor response in FDR was more pronounced for SCS than for exogenously applied CGRP. These results suggest that CGRPergic nerve activity, probably the transmitter (CGRP) release, is decreased in FDR. Moreover, we demonstrated that treatment of FDR with pioglitazone restored the blunted CGRPergic nerve-mediated vasodilator response, but not the exogenous CGRP-induced response, to that of the control group. Therefore, it appears that the chronic hyperinsulinaemia in FDR suppresses the function of CGRPergic vasodilator nerves. The present and previous (Takatori et al., 2003) findings that acute or chronic hyperinsulinaemia induced the dysfunction of neurogenic vascular control and altered vascular responsiveness to several vasoconstrictors, leading to development of hypertension, suggest that insulin plays a very important role in the neurogenic vascular control system.
Pioglitazone has been shown to have many diverse vascular effects. It has been found to suppress inflammatory and proliferative processes that occur after vascular injury, improve endothelial dysfunction, activate endothelial regeneration and directly lower BP (Buchnan et al., 1995; Kato et al., 1999; Kim et al., 2004; Campbell, 2005; Forst et al., 2005; Tanaka et al., 2005). However, in the present study, pioglitazone had no effect on the SBP of control rats. Also, the vascular responses to SCS and the vasoactive agents in pithed control rats treated with pioglitazone were similar to those in pithed untreated control rats. Therefore, it is unlikely that direct vascular actions of pioglitazone contribute to the restoration of vascular responsiveness and hypertension in FDR with chronic hyperinsulinaemia.
In vitro studies have shown that the mesenteric resistance arteries of the rat are densely innervated by sympathetic adrenergic nerves and CGRPergic nerves, both of which reciprocally control the vascular tone (Kawasaki et al., 1988, 1990; Takenaga and Kawasaki, 1999). The SCS-induced pressor response in pithed rats without an autonomic ganglion blocker is probably due to activation of both sympathetic nerves and CGRPergic nerves, and can be attenuated by the simultaneous activation of CGRPergic nerves. Therefore, it is likely that the decrease in pressor response to SCS observed in the pioglitazone-treated FDR results in part from the increased vasodilatation mediated by CGRPergic nerves. However, it is unlikely that pioglitazone exerts direct effects on adrenergic and CGRPergic nerves, as the drug inhibited adrenergic nerve function and facilitated CGRPergic nerve function.
In a previous in vitro study, insulin was shown to act on CGRP receptors and cause vasodilatation in rat mesenteric resistance arteries (Mimaki et al., 1998). In additiony, CGRPergic nerve terminals have presynaptic CGRP receptors that inhibit neurotransmitter CGRP release via a negative feedback mechanism (Nuki et al., 1994). Therefore, it is possible that acute or chronic increases in plasma insulin levels stimulate presynaptic CGRP receptors to inhibit neurotransmitter CGRP release from CGRPergic nerve terminals, which results in a decreased vasodilator response to SCS in pithed rats. This hypothesis may explain the present findings as pioglitazone, by abolishing hyperinsulinaemia, could thereby attenuate the insulin-induced presynaptic inhibition of CGRPergic nerve function and enhance CGRPergic nerve-mediated vasodilatation in FDR. However, further studies will be needed to clarify whether insulin activates presynaptic CGRP receptors in CGRPergic nerves.
Pioglitazone has been shown to lower BP and restore blunted endothelium-dependent vasodilatation of the aorta in fructose-fed rats (Kotchen et al., 1997). Therefore, it is possible that the BP lowering effect of pioglitazone observed in the present study may have resulted from enhanced endothelium function. However, we also showed that the vasodilatation in response to acetylcholine in vivo, which is mediated by endothelium-derived relaxing factors, was similar to that in control rats, and the treatment of FDR with pioglitazone did not affect the vasodilatation. The discrepancies between the present and previous findings are probably because the results from the present study were obtained in vivo in pithed rats whereas the previous study was in vitro using the aorta.
In conclusion, the results from the present study suggest that pioglitazone, a PPARγ ligand, has multiple effects that improve not only insulin resistance, but also dysfunctions of vascular control regulated by adrenergic and CGRPergic nerves in the hyperinsulinaemic state. In addition, the findings suggest that pharmacotherapy using pioglitazone would be very effective in preventing the development of hypertension in diabetes mellitus with insulin resistance.
Abbreviations
- Ang II
angiotensin II
- CGRP
calcitonin gene-related peptide
- FDR
fructose-drinking rat
- HR
heart rate
- PPARγ
peroxisome proliferator-activated receptor-γ
- SBP
systolic blood pressure
- SCS
spinal cord stimulation
- SNP
sodium nitroprusside
Conflict of interest
The authors state no conflict of interest.
References
- Anan F, Masaki T, Fukunaga N, Teshima Y, Iwao T, Kaneda K, et al. Pioglitazone shift circadian rhythm of blood pressure from non-dipper to dipper type in type 2 diabetes mellitus. Eur J Clin Invest. 2007;37:709–714. doi: 10.1111/j.1365-2362.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- Buchnan TA, Meehan WP, Jeng YY, Yang D, Chan TM, Nadler JL, et al. Blood pressure lowering by pioglitazone. Evidence for a direct vascular effect. J Clin Invest. 1995;96:354–360. doi: 10.1172/JCI118041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IW. The clinical significance of PPAR gamma agonism. Curr Mol Med. 2005;5:349–363. doi: 10.2174/1566524053766068. [DOI] [PubMed] [Google Scholar]
- Defronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21:165–171. doi: 10.1007/BF00252649. [DOI] [PubMed] [Google Scholar]
- De Rivas B, Luque M, Martell N, Fernandez C, Fernandez-Cruz A. Pioglitazone decreases ambulatory blood pressure in type 2 diabetes with difficult-to-control hypertension. J Clin Hypertens. 2007;9:530–537. doi: 10.1111/j.1524-6175.2007.06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Jarvinen H. Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR) Hypertension. 1997;30:1144–1149. doi: 10.1161/01.hyp.30.5.1144. [DOI] [PubMed] [Google Scholar]
- Forst T, Hohberg C, Fuellert SD, Lubben G, Konrad T, Lobig M, et al. Pharmacological PPARgamma stimulation in contrast to beta cell stimulation results in an improvement in adiponectin and proinsulin intact levels and reduces intima media thickness in patients with type 2 diabetes. Horm Metab Res. 2005;37:521–527. doi: 10.1055/s-2005-870322. [DOI] [PubMed] [Google Scholar]
- Grinsell JW, Lardinois CK, Swislocki A, Gonzalez R, Sare JS, Michaels JR, et al. Pioglitazone attenuates basal and postprandial insulin concentrations and blood pressure in the spontaneously hypertensive rat. Am J Hypertens. 2000;13:370–375. doi: 10.1016/s0895-7061(99)00216-2. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- Hsueh WA, Law RE. Insulin signaling in the arterial wall. Am J Cardiol. 1999;84:21–24. doi: 10.1016/s0002-9149(99)00353-7. [DOI] [PubMed] [Google Scholar]
- Kato K, Satoh H, Endo Y, Yamada D, Midorikawa S, Sato W, et al. Thiazolidinediones down-regulate plasminogen activator inhibitor type 1 expression in human vascular endothelial cells: a possible role for PPARgamma in endothelial function. Biochem Biophys Res Commun. 1999;258:431–435. doi: 10.1006/bbrc.1999.0648. [DOI] [PubMed] [Google Scholar]
- Kaufman LN, Peterson MM, Degrange LM. Pioglitazone attenuates diet-induced hypertension in rats. Metabolism. 1995;44:1105–1109. doi: 10.1016/0026-0495(95)90000-4. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Nuki C, Saito A, Takasaki K. Role of calcitonin gene-related peptide-containing nerves in the vascular adrenergic neurotransmission. J Pharmacol Exp Ther. 1990;252:403–409. [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K. Central alpha-2 adrenoceptor-mediated hypertensive response to clonidine in conscious, normotensive rats. J Pharmacol Exp Ther. 1986;236:810–818. [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kim H, Haluzik M, Gavrilova O, Yakar S, Portas J, Sun H, et al. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidaemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia. 2004;47:2215–2225. doi: 10.1007/s00125-004-1581-6. [DOI] [PubMed] [Google Scholar]
- Kotchen TA, Reddy S, Zhang HY. Increasing insuline sensitivity lowers blood pressure in the fructose fed rats. Am J Hypertens. 1997;10:1020–1026. doi: 10.1016/s0895-7061(97)00164-7. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Diet, obesity and hypertension: a hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, et al. Abnormal sympathetic overactivity evoked by insulin in skeletal muscle of patients with essential hypertension. J Clin Invest. 1992;90:24–29. doi: 10.1172/JCI115842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner L, Bengtsson C, Lapidus L, Kristjansson K, Wedel H. Fasting insulin in relation to subsequent blood pressure changes and hypertension in women. Hypertension. 1992;20:797–801. doi: 10.1161/01.hyp.20.6.797. [DOI] [PubMed] [Google Scholar]
- Marx N, Schonbeck U, Lazar MA, Libby P, Plutzky J. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–1103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimaki Y, Kawasaki H, Okazaki M, Nakatsuma A, Araki H, Gomita Y. Involvement of calcitonin gene-related peptide (CGRP) receptors in insulin-induced vasodilatation in mesenteric resistance blood vessels of rats. Br J Pharmacol. 1998;123:1684–1690. doi: 10.1038/sj.bjp.0701779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985;75:809–817. doi: 10.1172/JCI111776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuki C, Kawasaki H, Takasaki K, Wada A. Pharmacological characterization of presynaptic calcitonin gene-related peptide (CGRP) receptors on CGRP-containing vasodilator nerves in rat mesenteric resistance vessels. J Pharmacol Exp Ther. 1994;268:59–64. [PubMed] [Google Scholar]
- Nuki Y, Kawasaki H, Taguchi T, Takasaki K, Wada A. Effects of dorsal rhizotomy on depressor response to spinal cord stimulation mediated by endogenous calcitonin gene-related peptide in the pithed rat. J Neurosurg. 1993;79:899–904. doi: 10.3171/jns.1993.79.6.0899. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Wu HD, Willerson JT, Yen ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hoffman BB. A role for insulin in the aetiology and course of hypertension. Lancet. 1987;2:435–437. doi: 10.1016/s0140-6736(87)90968-8. [DOI] [PubMed] [Google Scholar]
- Skarfors ET, Lithell HO, Selinus I. Risk factors for the development of hypertension: a 10-year longitudinal study in middle-aged men. J Hypertens. 1991;9:217–223. doi: 10.1097/00004872-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nomura C, Odaka H, Ikeda H. Effect of an insulin sensitizer, pioglitazone, on hypertension in fructose-drinking rats. Jpn J Pharmacol. 1997;74:297–302. doi: 10.1254/jjp.74.297. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Kawasaki H, Imamura T, Takasaki K. Endogenous calcitonin gene-related peptide mediates nonadrenergic noncholinergic depressor response to spinal cord stimulation in the pithed rat. Circ Res. 1992;71:357–364. doi: 10.1161/01.res.71.2.357. [DOI] [PubMed] [Google Scholar]
- Takatori S, Mizote M, Zamami Y, Kurosaki Y, Kawasaki H. Effects of insulin on vascular responses to spinal cord stimulation and vasoactive agents in pithed rats. Br J Pharmacol. 2003;140:1137–1145. doi: 10.1038/sj.bjp.0705539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori S, Zamami Y, Mio M, Kurosaki Y, Kawasaki H. Chronic hyperinsulinemia enhances adrenergic vasoconstriction and decreases calcitonin gene-related peptide-containing nerve-mediated vasodilation in pithed rats. Hypertens Res. 2006;29:361–368. doi: 10.1291/hypres.29.361. [DOI] [PubMed] [Google Scholar]
- Takenaga M, Kawasaki H. Endogenous calcitonin gene-related peptide suppresses vasoconstriction mediated by adrenergic nerves in rat mesenteric resistance blood vessels. Eur J Pharmacol. 1999;367:239–245. doi: 10.1016/s0014-2999(98)00949-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Fukunaga Y, Itoh H, Doi K, Yamashita J, Chun TH, et al. Therapeutic potential of thiazolidinediones in activation of peroxisome proliferator-activated receptor gamma for monocyte recruitment and endothelial regeneration. Eur J Pharmacol. 2005;508:255–265. doi: 10.1016/j.ejphar.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Tobey TA, Mondon CE, Zavaroni I, Reaven GM. Mechanism of insulin resistance in fructose-fed rats. Metabolism. 1982;31:608–612. doi: 10.1016/0026-0495(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Uchida A, Nakata T, Hatta T, Kiyama M, Kawa T, Morimoto S, et al. Reduction of insulin resistance attenuates the development of hypertension in sucrose-fed SHR. Life Sci. 1997;61:455–464. doi: 10.1016/s0024-3205(97)00403-7. [DOI] [PubMed] [Google Scholar]
- Verma S, Bhanot S, Arikawa E, Yao L, Mcneill JH. Direct vasodepressor effects of pioglitazone in spontaneously hypertensive rats. Pharmacology. 1998;56:7–16. doi: 10.1159/000028177. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Reddy SR, Kotchen TA. Antihypertensive effect of pioglitazone is not invariably associated with increased insulin sensitivity. Hypertension. 1994;24:106–110. doi: 10.1161/01.hyp.24.1.106. [DOI] [PubMed] [Google Scholar]