Abstract
Background and purpose:
Studies with oestrogen receptorα (ERα)- and ERβ-selective compounds have already shown that the effects of 17β-estradiol (E2) on body weight, movement drive and bone-protection are mediated via ERα. This study was based on the hypothesis that activation of ERβ may antagonize ERα-mediated effects and designed to investigate potential effects of ERα/ERβ heterodimers.
Experimental approach:
Ovariectomized (OVX) female Wistar rats were treated with combinations of the ERα-specific agonist 16α-LE2 (ALPHA; 1 and 10 μg kg−1 d−1), the ERβ-specific agonist 8β-VE2 (BETA; 100 μg kg−1 d−1), the phytoestrogen, genistein (10 mg kg−1 d−1) and with the anti-oestrogen compound, ICI 182,780 (3 mg kg−1 d−1) for three weeks. The combined effects of the substances on body weight increase, tibial bone mineral density (BMD) and the influence on running wheel activity (RWA) were investigated.
Key results:
OVX-induced body weight increase was reduced by co-administration of genistein and BETA. Co-application of BETA or genistein with ALPHA had no effect on ALPHA-mediated bone-protection. The RWA of OVX animals was significantly reduced by treatment with genistein but stimulated by application of ALPHA. The stimulatory effect of ALPHA on RWA could be antagonized by co-treatment with the pure antioestrogen ICI 182,780 but also by co-administration of genistein or BETA.
Conclusions and implications:
Our results indicate that activation of ERβ may modulate ERα-mediated physiological effects in vivo. The observation that substances with selective affinity for ERβ are able to antagonize distinct physiological functions, like RWA, may be of great relevance to the pharmaceutical use of such drugs.
Keywords: oestrogens, phytoestrogens, oestrogen receptor, running wheel activity
Introduction
As the cloning of a second oestrogen receptor (ER) in the rat prostate in 1996 led to the description of ERβ (Kuiper et al., 1996), ER signalling has become much more multifaceted and complex (Gustafsson, 2000, 2003; Koehler et al., 2005). Even though oestrogens bind with similar affinity to ERα and ERβ (Kuiper et al., 1998), the ligand-binding domains of these two transcription factors differ significantly from each other (Kuiper et al., 1997).
Unlike 17β-estradiol (E2), several synthetic ER ligands, such as tamoxifen, raloxifene or other selective oestrogen receptor modulators, exhibit a distinct binding affinity to ERα and ERβ (Plouffe, 2000; Gluck and Maricic, 2003; Albertazzi and Sharma, 2005). It has been suggested that the molecular mechanisms involved in the tissue-selective action of these substances include ER subtype-specific effects (Diel, 2002; Ohmichi et al., 2005). Moreover, the effects of some phytoestrogens, compounds of plant origin with affinity to the ER, are also proposed to be mediated via ER subtype specific mechanisms (Fitzpatrick, 2003).
In this context, the phytoestrogen genistein is supposed to act as an ERβ-selective partial agonist that binds to the ligand-binding domain of both ER isoforms with moderate affinity (Kuiper et al., 1997; Barkhem et al., 1998), but preferentially to ERβ and in a manner similar to E2 (Pike et al., 1999). These differences in binding affinities suggest that phytoestrogens, such as genistein and coumestrol, may selectively trigger ERβ-mediated transcriptional pathways (Fitzpatrick, 2003). Both phytoestrogens influence many physiological processes in various oestrogen-sensitive tissues. Nevertheless, it has to be kept in mind that phytoestrogens, such as genistein, may also exert endocrine functions via multiple other mechanisms, besides its preferential ERβ-binding activity (Davis et al., 1999; Cassidy, 2003).
Oestrogens have been shown to influence running wheel activity (RWA) (Hertrampf et al., 2006) and anxiety behaviour (Walf and Frye, 2005) in ovariectomized (OVX) rats. Oestrogenic effects in the brain are multifaceted, including long-term ‘genomic' effects via intracellular ER-induced changes in gene expression and rapid ‘non-genomic' effects mediated by interactions of ERs with different signalling cascades in the cytoplasm (Belcher and Zsarnovszky, 2001; Deroo and Korach, 2006). Moreover, the widespread expression pattern of ERs in different compartments of the brain, the incidence of postmenopausal depression and hot flushes, and the fact that the RWA of rats can be significantly reduced by OVX and strongly enhanced by treatment of OVX animals with E2 (Hertrampf et al., 2006) are indicators of the diversity of oestrogenic actions in the CNS (Belcher and Zsarnovszky, 2001). Signalling via ERα and ERβ seems to be important in certain compartments of the brain, such as the medial preoptic area (mPOA) (Ogawa et al., 2003; Tanaka et al., 2003) and hippocampus (Walf and Frye, 2007). Studies with oestrogen receptor knockout mice and those performed with ER subtype-specific agonists consistently demonstrate that stimulation of RWA by E2 is the result of ERα-specific signalling (Ogawa et al., 2003; Hertrampf et al., 2007).
In the context of different menopausal symptoms and postmenopausal disorders, information on the tissue-specific action of oestrogens and phytoestrogens associated with ER subtype-specific signalling is very limited. Added to the data generated by the analysis of estrogen receptor knockout mice animals, the identification of isotype-selective ER ligands provides an alternative tool to study the biological role of ERα and ERβ.
The novel, highly selective steroidal ERβ-specific agonist 8β-VE2 (BETA) and the highly selective ERα-specific agonist 16α-LE2 (ALPHA) (Hegele-Hartung et al., 2004; Hillisch et al., 2004; Pelzer et al., 2005) (Figure 1) belong to the most potent and isotype selective oestrogens identified so far. Using these compounds, we recently demonstrated that the effects of E2 on body weight, movement drive and protection of bone mineral density (BMD) are mediated via ERα, whereas activation of ERβ has only a limited effect (Hertrampf et al., 2007).
Figure 1.
ER selective agonists. Chemical structures of the specific ER agonists: 16α-LE2 (ALPHA), 8β-VE2 (BETA).
Based upon the theory that ERβ may act as a modulator of the function of ERα (Weihua et al., 2003; Gustafsson, 2006) and in order to further investigate mechanisms of ER subtype-specific signaling, in this study, the effects of combinations of ALPHA, BETA and genistein have been studied. OVX rats were treated with E2, the phytoestrogen genistein, and ALPHA and BETA. To analyse agonistic and/or antagonistic effects of ER-specific signaling, an additional animal experiment was conducted, where effects of combined treatments of OVX rats with Alpha plus genistein, ALPHA plus BETA and BETA plus genistein were analysed. Vehicle-treated OVX rats and animals co-treated with ALPHA and the anti-oestrogen compound, ICI 182,780, served as controls. Besides voluntary RWA, other well-described oestrogen-responsive endocrine parameters, such as tibial BMD and the increase of body weight, were quantified. Uterine wet weights served as a reference for the oestrogenicity of the compounds.
Methods
Animals
All animal procedures were approved by the Committee on Animal Care and complied with accepted veterinary medical practice.
Adult female Wistar rats (200–220 g) were obtained from Janvier (Janvier, Le Genest St Isle, France) and were maintained under controlled conditions of temperature (20 °C±1, relative humidity 50–80%) and illumination (12 h light, 12 h dark). All rats had free access to a diet low in phytoestrogen content (ILD) (SSniff GmbH, Soest, Germany) and water.
Animal treatment and tissue preparation
Post-pubertal animals were OVX at the age of 3 months and weighed 200–220 g. After 14 days of endogenous hormonal decline, the animals were treated with the test compounds or vehicle for 3 weeks. The animals were randomly allocated to treatment and vehicle groups (n=6). E2 (4 μg kg−1 day−1), genistein (10 mg kg−1 day−1), ALPHA1 (1 μg kg−1 day−1), ALPHA10 (10 μg kg−1 day−1), BETA (100 μg kg−1 day−1) and ICI 182,780 (3 mg kg−1 day−1) were dissolved in dimethylsulphoxide (200 μl kg−1 day−1) and corn oil (800 μl kg−1 day−1) for s.c. administration. The treatment doses of the respective substances were chosen based on previous experiments. Genistein has been demonstrated to be effective at a dose of 10 mg kg−1 day−1 (Diel et al., 2004; Hertrampf et al., 2005). For isotype-specific ER activation, we used the selective ER-agonists ALPHA and BETA (Figure 1). Because these compounds activate both receptors at higher concentrations (Hegele-Hartung et al., 2004), doses of 10 μg kg−1 day−1 (ALPHA) and 100 μg kg−1 day−1 (BETA) were chosen. For these doses, action through either ERα or ERβ, respectively, can be anticipated (Hegele-Hartung et al., 2004; Hillisch et al., 2004). To analyse agonistic and/or antagonistic effects, the concentration of the highly selective and potent ALPHA was reduced to 1 μg kg−1 day−1 when combined with genistein or BETA, whereas the concentrations of genistein and BETA were the same for single or combined administration.
Animals were killed by decapitation after light anaesthesia with CO2 inhalation. Uteri were prepared free of fat and the wet weights were determined.
Determination of RWA
Animals had free access to running wheels that were kept in all cages during the 3-week period. Animals were placed in separate cages with individual admission to an appropriate running wheel. A computer connected to the wheel recorded the individual RWA of each animal for 21 days by counting the running wheel rotations and converting them into running distance. Because of the limited number of running wheels and based on previous studies (Hertrampf et al., 2007), where BETA and genistein had no stimulating influence on RWA, the BETA+GEN-treated animals had no access to running wheels. Thus, only combined effects of co-treatment of BETA and genistein on body weight increase and BMD were examined.
Determination of BMD
The right tibiae were snap frozen in liquid nitrogen. BMD was measured by peripheral quantitative computed tomography (XCT Research SA+, StraTec Medizintechnik, Pforzheim, Germany). Trabecular density (measured by density mode, ROI (region of interest) at 7.5% of bone length), cortical density (ROI at 50% of bone length) and total density (ROI at 7.5 and 50% of bone length) of the tibiae were measured at the end of the study after 3 weeks of treatment.
Statistical analysis
All data are expressed as arithmetic means±s.e.mean. Statistical significance of differences was calculated using one-way ANOVA with a following Tukey's HSD (honestly significant difference) test, where it was appropriate. Statistical tests were used for comparisons between every two groups and evaluated using P<0.05.
Materials
17β-Estradiol (Estra-1,3,5(10)-trien-3,16α,17 β-diol), and genistein (4′,5,7-trihydroxyisoflavone) were provided by Sigma-Aldrich (Deisenhofen, Germany). The specific ER agonists ALPHA (16α-LE2, 3,17-dihydroxy-19-nor-17α-pregna-1,3,5 (10)-triene-21,16α-lactone) and BETA (8β-VE2, 8-vinylestra-1,3,5 (10)-triene-3,17β-diol) (Figure 1) were provided by Bayer Schering Pharma AG (Berlin, Germany) and the pure anti-oestrogen compound, Faslodex (ICI 182,780), was provided by AstraZeneca (Wedel, Germany).
Results
The effects of of E2, genistein, ALPHA and BETA, each given alone, on uterine weight, body weight and trabecular BMD, were as expected (Hillisch et al., 2004; Hertrampf et al., 2007). Treatment of OVX rats with E2 led to a strong stimulation of uterine wet weights, whereas application of genistein resulted only in a small, although significant, increase (Figure 2). Application of ALPHA increased uterine wet weights in a dose-dependent manner, whereas BETA-treated animals showed no significant uterus stimulation. The stimulation of uterine wet weights by ALPHA could be antagonized by ICI 182,780 and was not influenced by co-treatment with genistein or BETA (Figure 2), which underlines previous findings showing that the induction of the uterine wet weight by E2 is mediated via ERα-specific signalling.
Figure 2.
Uterine wet weights after 3 weeks of treatment; data shown are means±s.e.mean. OVX rats were given daily s.c. injections of E2 (4 μg kg−1), genistein (10 mg kg−1), ALPHA1 (1 μg kg−1), ALPHA10 (10 μg kg−1), BETA (100 μg kg−1) or ICI 182,780 (3 mg kg−1), alone or in combinations as shown. Other experimental conditions and treatment procedures are given in Materials and methods. Mean values were significantly different for the following comparisons: * vs estradiol substituted group (E2), P<0.05; + vs OVX group; P<0.05, ANOVA, n=6. OVX, ovariectomized.
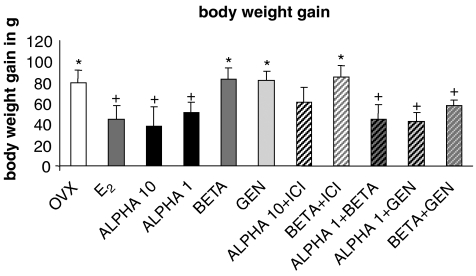
Apart from other disturbances, postmenopausal symptoms also include increase in fat mass that is thought to be correlated with a reduced output of ovarian steroids (Sorensen et al., 2001; Chen et al., 2005). In this context, OVX-induced body weight gain in rats can be antagonized by substitution of E2 (Hertrampf et al., 2006), an ERα-mediated effect (Hertrampf et al., 2007). In this study, the ERα-mediated reduction in body weight gain after OVX was not influenced by co-application of genistein or BETA (Figure 3). Interestingly and in contrast to the effects of separate treatment with genistein and BETA, co-treatment with these compounds led to a reduction of OVX-induced body weight gain (Figure 3).
Figure 3.
Increase in body weight (g) after 3 weeks of treatment; data shown are means±s.e.mean. OVX rats were given daily s.c. injections of E2 (4 μg kg−1), genistein (10 mg kg−1), ALPHA 1 (1 μg kg−1), ALPHA10 (10 μg kg−1), BETA (100 μg kg−1) or ICI 182,780 (3 mg kg−1), alone or in combinations as shown. Other experimental conditions and treatment procedures are given in Materials and methods. Mean values were significantly different for the following comparisons: + vs OVX group, P<0.05; * vs ALPHA 1 treated group; P<0.05; ANOVA, n=6. OVX, ovariectomized.
According to earlier studies from our laboratory (Hertrampf et al., 2007), E2, ALPHA and genistein have been shown to induce bone-protective effects in OVX rats, which is a suitable model for postmenopausal bone loss (Kalu, 1991). Co-administration of genistein and BETA had neither additive nor antagonistic effects on the protective effect of ALPHA on BMD (Figure 4).
Figure 4.
Trabecular BMD (region of interest was at 7.5% of tibia length); data shown are means±s.e.mean. OVX rats were given daily s.c. injections of E2 (4 μg kg−1), genistein (10 mg kg−1), ALPHA1 (1 μg kg−1), ALPHA10 (10 μg kg−1), BETA (100 μg kg−1) or ICI 182,780 (3 mg kg−1), alone or in combinations as shown. Other experimental conditions and treatment procedures are given in Materials and methods. + denotes values significantly different from OVX group; P<0.05; ANOVA, n=6. BMD, bone mineral density; OVX, ovariectomized.
Running wheel activity of OVX rats could be significantly elevated by application of E2 or ALPHA but not by treatment with BETA (Figure 5). Surprisingly and in contrast to ALPHA and E2, treatment of OVX animals with genistein led to a significant reduction of RWA (Figure 5). The stimulation of RWA by ALPHA could be antagonized by ICI 182,780, demonstrating that the oestrogenic influence on this parameter is also mediated by ERα (Figure 5). Interestingly, the elevation of RWA of OVX rats induced by treatment with ALPHA was prevented by co-treatment with either genistein or BETA (Figure 5).
Figure 5.
Running wheel activity. Average running distance in kilometers, completed by each treatment group after 3 weeks; data shown are means±s.e.mean. OVX rats were given daily s.c. injections of E2 (4 μg kg−1), genistein (10 mg kg−1), ALPHA 1 (1 μg kg−1), ALPHA10 (10 μg kg−1), BETA (100 μg kg−1) or ICI 182,780 (3 mg kg−1), alone or in combinations as shown. Other experimental conditions and treatment procedures are given in Materials and methods. Mean values were significantly different for the following comparisons: + vs OVX group P<0.05; * vs ALPHA 1 treated group, P<0.05; ANOVA, n=6. OVX, ovariectomized.
Discussion
Apart from those groups receiving combined treatments, the data collected with E2, genistein, ALPHA and BETA on uterine wet weight, body weight and trabecular BMD were as expected (Hillisch et al., 2004; Hertrampf et al., 2007).
The novel finding of the present study is that co-treatment with genistein or BETA can antagonize the ERα-mediated stimulation of RWA in a manner similar to the anti-oestrogen ICI 182,780 (Figure 5). In contrast, the ERα-mediated influence on uterine wet weights, BMD and body weight gain in OVX rats could only be antagonized by ICI 182,780 but not by co-treatment with genistein or BETA.
Based on the fact that both ER subtypes play essential roles in many different areas of the mammalian brain such as mPOA, suprachiasmatic nucleus, hippocampus and hypothalamus (Yokosuka et al., 1997; Scott et al., 2000; Perez et al., 2003; Tanaka et al., 2003; Fatehi and Fatehi-Hassanabad, 2007; Walf and Frye, 2007) and taking into consideration that oestrogens are known to increase RWA of rodents primarily by acting on the mPOA (Ogawa et al., 2003), the effects on RWA seen in this study may possibly be also the result of oestrogenic signalling in this compartment of the brain. This assumption is supported by the fact that both ER subtypes are expressed in the mPOA (Ogawa et al., 2003; Tanaka et al., 2003) and by the observation that the effect of E2 on RWA in OVX mice can be antagonized by co-treatment with coumestrol (Garey et al., 2001). This phytoestrogen has a two- to sevenfold preferential binding affinity for ERβ over ERα (Kuiper et al., 1997, 1998) and displays a preference for transactivation of ERβ over ERα (Mueller et al., 2004). However, it has to be taken into consideration that coumestrol is the most potent phytoestrogen on ERα as well (Mueller et al., 2004), which may be of great importance in tissues that express high quantities of ERα, such as ovary, mammary gland and uterus (Kuiper et al., 1997).
Moreover, studies with ERβ-KO mice (Rocha et al., 2005) and those performed with OVX rats treated centrally with selective oestrogen receptor modulators, with higher affinity to ERβ (Walf and Frye, 2007), suggest that E2-induced anti-anxiety and anti-depression effects are mediated through central activation of ERβ and are not influenced by ERα-specific signalling. Thus, the antagonistic influence of BETA and genistein is also potentially the result of antagonistic ERβ-specific signalling in the brain. In addition, this result could be taken as an indication for genistein, ALPHA and BETA to be able to pass the blood brain barrier. In this context, Patisaul et al. (2002) were able to show that genistein exerts anti-oestrogenic properties in the brain by affecting ERβ- but not ERα-dependent gene expression in the hypothalamus.
On the other hand, it remains questionable why the induction of RWA by ERα was also antagonized by ICI 182,780, a substance thought to be unable to cross the blood–brain barrier (Wade et al., 1993; Clark et al., 2003). This fact therefore challenges the supposition that the observed antagonistic effects of BETA and genistein occur in the CNS, because ICI 182,780 is only effective for assessing the peripheral actions of ER agonists (Wade et al., 1993; Howell et al., 2000). Peripheral administration of low dosages of ICI 182,780 has shown to be ineffective in the mPOA (Wade et al., 1993). Thus, it remains questionable whether the observed effects of ALPHA, BETA and genistein on RWA are central or peripheral oestrogenic actions. In consideration of the relatively high dosage of 3 mg kg−1 day−1 used in this study, future studies should evaluate if ICI 182,780 is able to cross the blood–brain barrier after administration of higher dosages.
Taken together, our data agree with other studies in which ERβ-selective agonists and ERβ subtype-selective phytoestrogens such as genistein mediate anti-oestrogenic as well as oestrogenic effects via ERβ (Patisaul et al., 2002; Walf et al., 2004). In hypothalamic neurons and in those of the mPOA, the activation of ERβ results in anti-oestrogenic effects (Patisaul et al., 2002), whereas ERβ-specific signalling in the hippocampus leads to reduced depression and anxiety levels, classed as oestrogenic actions (Walf et al., 2004; Walf and Frye, 2007). Our observation that ERβ-selective agonists can antagonize the ERα-mediated stimulation of RWA demonstrate that activation of ERβ functionally antagonizes ERα-specific signalling in vivo. Because RWA has been described to be an indication for oestrogenic signalling in the mPOA of the brain (Ogawa et al., 2003), our observation can also be taken as an indication that genistein and ERβ-selective agonists may be suitable substances for the prevention and treatment of postmenopausal depression through agonistic activity in the hippocampus via ERβ. Initially, future investigations have to prove whether the observed effects in this study are central or peripheral actions of ALPHA, BETA, genistein and ICI 182,780.
In conclusion, these observations provide evidence that combinations of ERβ- and ERα-specific agonists and genistein differ in their activity compared to E2. Further, these results show that their combined effects vary depending on the biological system investigated.
Abbreviations
- ALPHA
oestrogen receptor alpha-specific agonist 16α-LE2
- BETA
oestrogen receptor beta-specific agonist 8β-VE2
- BMD
bone mineral density
- E2
17β-estradiol
- ER
oestrogen receptor
- HRT
hormone replacement therapy
- ICI 182,780
Faslodex
- RWA
running wheel activity
- mPOA
medial preoptic area
- OVX
ovariectomized
Conflict of interest
The authors state no conflict of interest.
References
- Albertazzi P, Sharma S. Urogenital effects of selective estrogen receptor modulators: a systematic review. Climacteric. 2005;8:214–220. doi: 10.1080/13697130500117946. [DOI] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Zsarnovszky A.Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms J Pharmacol Exp Ther 2001299408–414.review [PubMed] [Google Scholar]
- Cassidy A.Potential risks and benefits of phytoestrogen-rich diets Int J Vitam Nutr Res 200373120–126.review [DOI] [PubMed] [Google Scholar]
- Chen Z, Bassford T, Green SB, Cauley JA, Jackson RD, LaCroix AZ, et al. Postmenopausal hormone therapy and body composition—a substudy of the estrogen plus progestin trial of the Women's Health Initiative. Am J Clin Nutr. 2005;82:651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- Clark AS, Guarraci FA, Megroz AB, Porter DM, Henderson LP.The display of sexual behaviors by female rats administered ICI 182,780 Horm Behav 200343454–464.Erratum in: Horm Behav. 2004 46(4): 506 [DOI] [PubMed] [Google Scholar]
- Davis SR, Dalais FS, Simpson ER, Murkies AL.Phytoestrogens in health and disease Recent Prog Horm Res 199954185–210.discussion 210–211. Review [PubMed] [Google Scholar]
- Deroo BJ, Korach KS.Estrogen receptors and human disease J Clin Invest 2006116561–570.review [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel P.Tissue-specific estrogenic response and molecular mechanisms Toxicol Lett 2002127217–224.review [DOI] [PubMed] [Google Scholar]
- Diel P, Geis RB, Caldarelli A, Schmidt S, Leschowsky UL, Voss A, et al. The differential ability of the phytoestrogen genistein and of estradiol to induce uterine weight and proliferation in the rat is associated with a substance specific modulation of uterine gene expression. Mol Cell Endocrinol. 2004;221:21–32. doi: 10.1016/j.mce.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Fatehi M, Fatehi-Hassanabad Z. Effects of 17beta-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat. Neuropsychopharmacology. 2007;30:664–672. doi: 10.1038/sj.npp.1301523. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LA.Phytoestrogens-mechanism of action and effect on bone markers and bone minAlphal density Endocrinol Metab Clin North Am 200332233–252.review [DOI] [PubMed] [Google Scholar]
- Garey J, Morgan MA, Frohlich J, McEwen BS, Pfaff DW. Effects of the phytoestrogen coumestrol on locomotor and fear-related behaviors in female mice. Horm Behav. 2001;40:65–76. doi: 10.1006/hbeh.2001.1660. [DOI] [PubMed] [Google Scholar]
- Gluck O, Maricic M.Skeletal and nonskeletal effects of raloxifene Curr Osteoporos Rep 20031123–128.review [DOI] [PubMed] [Google Scholar]
- Gustafsson JA.An update on estrogen receptors Semin Perinatol 20002466–69.review [DOI] [PubMed] [Google Scholar]
- Gustafsson JA.What pharmacologists can learn from recent advances in estrogen signalling Trends Pharmacol Sci 200324479–485.review [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. ERbeta scientific visions translate to clinical uses. Climacteric. 2006;9:156–160. doi: 10.1080/14689360600734328. [DOI] [PubMed] [Google Scholar]
- Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Muller G, Hillisch A, et al. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci USA. 2004;101:5129–5134. doi: 10.1073/pnas.0306720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrampf T, Degen GH, Kaid AA, Laudenbach-Leschowsky U, Seibel J, Di Virgilio AL, et al. Combined effects of physical activity, dietary isoflavones and 17beta-estradiol on movement drive, body weight and bone mineral density in ovariectomized female rats. Planta Med. 2006;72:484–487. doi: 10.1055/s-2006-931579. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Gruca MJ, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. The bone-protective effect of the phytoestrogen Genistein is mediated via ERα-dependent mechanisms and strongly enhanced by physical activity. Bone. 2007;40:1529–1535. doi: 10.1016/j.bone.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Schmidt S, Laudenbach-Leschowsky U, Seibel J, Diel P. Tissue-specific modulation of cyclooxygenase-2 (Cox-2) expression in the uterus and the v. cava by estrogens and phytoestrogens. Mol Cell Endocrinol. 2005;243:51–57. doi: 10.1016/j.mce.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hillisch A, Peters O, Kosemund D, Muller G, Walter A, Schneider B, et al. Dissecting physiological roles of estrogen receptor alpha and beta with potent selective ligands from structure-based design. Mol Endocrinol. 2004;18:1599–1609. doi: 10.1210/me.2004-0050. [DOI] [PubMed] [Google Scholar]
- Howell A, Osborne CK, Morris C, Wakeling AE.ICI 182,780 (Faslodex): development of a novel, ‘pure' antiestrogen Cancer 200089817–825.Erratum in: Cancer 2001 91(2): 455 [DOI] [PubMed] [Google Scholar]
- Kalu DN.The ovariectomized rat model of postmenopausal bone loss Bone Miner 199115175–191.review [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA.Reflections on the discovery and significance of estrogen receptor beta Endocr Rev 200526465–478.review [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS.Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells Toxicol Sci 20048014–25.Erratum in: Toxicol Sci. 2004 Oct; 81(2): 530–531 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases running wheel activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Tasaka K, Kurachi H, Murata Y.Molecular mechanism of action of selective estrogen receptor modulator in target tissues Endocr J 200552161–167.review [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Melby M, Whitten PL, Young LJ. Genistein affects ER beta- but not ER alpha-dependent gene expression in the hypothalamus. Endocrinology. 2002;143:2189–2197. doi: 10.1210/endo.143.6.8843. [DOI] [PubMed] [Google Scholar]
- Pelzer T, Jazbutyte V, Hu K, Segerer S, Nahrendorf M, Nordbeck P, et al. The estrogen receptor-alpha agonist 16alpha-LE2 inhibits cardiac hypertrophy and improves hemodynamic function in estrogen-deficient spontaneously hypertensive rats. Cardiovasc Res. 2005;67:604–612. doi: 10.1016/j.cardiores.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, et al. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe L., JrSelective estrogen receptor modulators (SERMs) in clinical practice J Soc Gynecol Investig 200071 SupplS38–S46.review [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology (Berlin) 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Scott CJ, Tilbrook AJ, Simmons DM, Rawson JA, Chu S, Fuller PJ, et al. The distribution of cells containing estrogen receptor-alpha (ERalpha) and ERbeta messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: comparison of males and females. Endocrinology. 2000;141:2951–2962. doi: 10.1210/endo.141.8.7622. [DOI] [PubMed] [Google Scholar]
- Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9:622–626. doi: 10.1038/oby.2001.81. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Shiina T, Hayashi S, Okamura H, Kamomae H, Kaneda Y. Estrogen receptor alpha expression in the medial preoptic area and the medial basal hypothalamus under different physiological conditions in cattle. J Reprod Dev. 2003;49:55–60. doi: 10.1262/jrd.49.55. [DOI] [PubMed] [Google Scholar]
- Wade GN, Blaustein JD, Gray JM, Meredith JM. ICI 182,780: a pure antiestrogen that affects behaviors and energy balance in rats without acting in the brain. Am J Physiol. 1993;265 6 Part 2:R1392–R1398. doi: 10.1152/ajpregu.1993.265.6.R1392. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–414. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Andersson S, Cheng G, Simpson ER, Warner M, Gustafsson JA. Update on estrogen signaling. FEBS Lett. 2003;546:17–24. doi: 10.1016/s0014-5793(03)00436-8. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]