Abstract
Background and purpose:
Ifosfamide nephrotoxicity is a serious adverse effect for children undergoing cancer chemotherapy. Our recent in vitro studies have shown that the antioxidant N-acetylcysteine (NAC), which is used extensively as an antidote for paracetamol (acetaminophen) poisoning in children, protects renal tubular cells from ifosfamide-induced toxicity at a clinically relevant concentration. To further validate this observation, an animal model of ifosfamide-induced nephrotoxicity was used to determine the protective effect of NAC.
Experimental approach:
Male Wistar albino rats were injected intraperitoneally with saline, ifosfamide (50 or 80 mg kg−1 daily for 5 days), NAC (1.2 g kg−1 daily for 6 days) or ifosfamide+NAC (for 6 days). Twenty-four hours after the last injection, rats were killed and serum and urine were collected for biochemical analysis. Kidney tissues were obtained for analysis of glutathione, glutathione S-transferase and lipid peroxide levels as well as histology analysis.
Key results:
NAC markedly reduces the severity of renal dysfunction induced by ifosfamide with a significant decrease in elevations of serum creatinine (57.8±2.3 vs 45.25±2.1 μmol l−1) as well as a reduced elevation of β2-microglobulin excretion (25.44±3.3 vs 8.83±1.3 nmol l−1) and magnesium excretion (19.5±1.5 vs 11.16±1.5 mmol l−1). Moreover, NAC significantly improved the ifosfamide-induced glutathione depletion and the decrease of glutathione S-transferase activity, lowered the elevation of lipid peroxides and prevented typical morphological damages in renal tubules and glomeruli.
Conclusions and implications:
Our results suggest a potential therapeutic role for NAC in paediatric patients in preventing ifosfamide nephrotoxicity.
Keywords: ifosfamide, N-acetylcysteine, nephrotoxicity, glutathione, lipid peroxidation, glutathione S-transferase, histological changes
Introduction
With the advance in treating paediatric cancers, almost 80% of children and adolescents become long-term survivors (Oeffinger et al., 2006). However, long-term organ damage caused by chemotherapy and radiation has become an alarming health concern. As a result, many of these paediatric cancer survivors experience life-long impairment in their quality of life. Ifosfamide is a highly effective chemotherapeutic agent for treating a variety of paediatric solid tumours, including rhabdomyosarcoma, Wilms' tumour, Ewing's sarcoma, bone sarcomas, osteosarcoma and neuroblastoma (Straka et al., 2003). Ifosfamide causes nephrotoxicity in 30% of paediatric cancer patients, which may present in more severe cases as Fanconi syndrome (Skinner et al., 1993; Loebstein and Koren, 1998). This renal disorder is characterized by urinary losses of amino acids, glucose, phosphate, bicarbonate and small-molecular-weight proteins such as β2-microglobulin as well as a decrease in the GFR (Loebstein et al., 1999; Rossi et al., 1999; Skinner, 2003). The clinical consequences can range from mild form presented as hypophosphataemic rickets, which can be corrected with phosphate or bicarbonate supplementations, to more severe conditions in which children may necessitate a kidney transplant (Skinner, 2003).
Ifosfamide is a prodrug that is hepatically metabolized by the cytochrome P450 enzymes 3A4, 3A5 and 2B6 to its active metabolite ifosfamide mustard (Springate et al., 1999). The reactive toxic metabolite chloroacetaldehyde, which is produced by the side-chain oxidation of ifosfamide in renal tubular cells (Woodland et al., 2000), is believed to be responsible for the nephrotoxic effect (Springate et al., 1999; Aleksa et al., 2005). In addition to the renally produced chloroacetaldehyde in tubular cells, studies have demonstrated that insufficient local levels of glutathione (GSH) may predispose tubular cells to damage by chloroacetaldehyde (Aleksa et al., 2005). A large number of in vivo studies of ifosfamide-induced nephrotoxicity have confirmed that GSH has an important function as a cellular protective mechanism against toxic metabolites such as chloroacetaldehyde (Badary, 1998, 1999a, 1999b; Sener et al., 2004; Sehirli et al., 2007). These studies have investigated various antioxidant compounds that may prevent the depletion of GSH. However, the potential use of these antioxidants has not yet been translated into clinical use, as their relative risks in children have not been determined. Although there are a large number of antioxidants, including methionine and amifostine, N-acetylcysteine (NAC) holds a greater potential for clinical use given that it is routinely used in children experiencing paracetamol (acetaminophen) poisoning (Marzullo, 2005).
N-acetylcysteine is a synthetic precursor of GSH, which stimulates the intracellular synthesis of GSH, acts as a nucleophile to conjugate with reactive metabolites and enhances glutathione S-transferase (GST) activity (Tylicki et al., 2003). A recent clinical trial demonstrated that NAC can reduce the incidence of contrast-induced nephropathy, including dialysis requirement and mortality, in patients undergoing angiographic procedures (Al-Ghonaim and Pannu, 2006). In animals, studies have confirmed nephroprotective properties of NAC in cyclosporin-induced nephrotoxicity, in cisplatin-induced nephrotoxicity (Mishima et al., 2006) as well as in ischaemia/reperfusion injury (Tariq et al., 1999).
We have recently shown, by using a porcine proximal tubular cell line (LLCPK-1), that NAC protects renal tubular cells from ifosfamide-induced nephrotoxicity at a clinically relevant concentration (Chen et al., 2007). The objective of our present study was to further investigate the protective effect of NAC on ifosfamide-induced nephrotoxicity in an in vivo model.
Materials and methods
Experimental design
All animal procedures and experimental protocols were approved by the University of Western Ontario Animal Care and Use Council. Male Wistar albino rats (weighing 200–225 g) were purchased from Charles River Canada (Montreal, QC, Canada). They were kept at a constant temperature (22±1 °C) with a regular 12-h light and dark cycles and fed a standard rat chow and water ad libitum. The rats were divided into four groups, each consisting of six animals. The experimental design included one control and three experimental groups as follows:
Saline-treated control group. Rats were injected intraperitoneally daily with 0.9% NaCl (∼0.5 ml) for 5 days.
NAC group. Rats were administered an intraperitoneal injection of 1.2 g kg−1 NAC daily for 6 days. The NAC dose was adopted from a study by Lauterburg et al. (1983).
Ifosfamide group. The recommended dose administered to cancer patients is 50 mg kg−1 daily for 5 days each cycle. In a rat model, 50 mg kg−1 for 5 days has been shown to induce damage to renal proximal tubules and the Fanconi syndrome (Badary, 1998, 1999a, 1999b; Sener et al., 2004). A higher dosage of ifosfamide, 80 mg kg−1, was administered to another group of rats to determine the effect of NAC at a more severe stage of renal toxicity.
NAC+ifosfamide group. Rats were administered ifosfamide just as the ifosfamide group, except that they were injected intraperitoneally with 1.2 g kg−1 NAC 1 day before and then NAC+ifosfamide daily for 5 days.
The time course of enzymuria and proteinuria reaches its peak between the third and sixth day of ifosfamide treatment (Nissim and Weinberg, 1996). Twenty-four hours after the last treatment, rats were killed in a CO2 chamber (65% mixed with 35% O2). Blood (mixture of arterial and venous blood) and urine samples were collected. Blood samples were taken by the intracardiac puncture. The left kidney was divided into three parts, and each third was immediately homogenized in the specified buffered solutions for different biochemical assays. Kidney homogenate was analysed for GSH levels, 4-hydroxyalkenals (HAE), malondialdehyde (MDA), an end product of lipid peroxidation, and GST activity.
Serum samples were analysed for sodium, potassium, urea, creatinine, phosphate, albumin, magnesium and glucose (Core Lab, University Hospital, London, ON, Canada). Urine samples were measured for potassium, urea, creatinine, phosphate, magnesium, protein and β2-microglobulin (Core Lab, University Hospital).
Glutathione determination
One-third of the left kidney including both the cortex and medulla was weighed and then homogenized in 4 volume of ice-cold 100 mM potassium phosphate buffer with 1.15% KCl (pH 7.4) using a Teflon–glass homogenizer (Quantum BD 1000-04, Black & Decker Inc., Brockville, ON, Canada). EDTA (2 mM) and acivicin (15 μM) were also added to prevent any oxidation of reduced GSH and to inhibit γ-glutamyl transpeptidase. Levels of reduced GSH in the kidney homogenate were determined by a GSH colorimetric assay kit, GT-30 (Oxford Biomedical Research Inc., Oxford, MI, USA), with modifications. Samples (50 μl) of diluted kidney homogenates were used for the GSH measurement to which 50 μl of 5% MPA were added. Samples were then vortexed for 20 s and then centrifuged at 12 000 g for 10 min at 4 °C. Supernatants (50 μl) were transferred into a 96-well plate, and 50 μl each of chromogen DTNB (5,5′-dithiobis-(2-nitrobenzoic acid)) and enzyme (glutathione reductase) was added. Samples were incubated in the dark for 5 min at room temperature, after which the samples were read at 412 nm using Tecan Safire2 microplate reader (MTX Lab Systems Inc., Vienna, VA, USA). GSH concentrations were quantified by comparing with a standard curve ranging in concentrations from 0 to 3 μM and expressed as per milligram protein.
Lipid peroxidation
Another one-third of the same kidney was weighed and then homogenized in 4 volume of ice-cold 20 mM potassium phosphate buffer (pH 7.4) using a Teflon–glass homogenizer. To prevent sample oxidation, 5 mM of butylated hydroxytoluene was added. The homogenate was centrifuged at 3000 g for 10 min at 4 °C, and the supernatant was transferred and used for the measurement of MDA and HAE using the lipid peroxidation colorimetric kit, FR 22 (Oxford Biomedical Research Inc.). Samples (100 μl) were mixed with 325 μl of diluted N-methyl-2-phenylindole and 75 μl of methanesulphonic acid. Samples were vortexed for 30 s and then incubated for 60 min at 45 °C. Thereafter, samples were centrifuged at 13 000 g for 15 min at room temperature. MDA and HAE levels were measured at 586 nm using a Tecan Safire2 microplate reader. Both MDA and HAE levels were plotted against a concentration range of 0–4 μM of MDA standard (1,1,3,3-tetramethoxypropane).
Glutathione S-transferase activity
The last one-third of the same kidney was weighed and then homogenized in 4 volume of ice-cold 100 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0) using a Teflon–glass homogenizer. GST activity was determined by GST assay kit (Cayman Chemical, Ann Arbor, MI, USA). Samples were centrifuged at 10 000 g for 15 min at 4 °C. Supernatant (20 μl) was transferred to a 96-well plate. GSH (20 μl) and assay buffer (150 μl) were added. The reaction was initiated by adding 10 μl of 1-chloro-2,4-dinitrobenzene. The reaction rate, which was read at 340 nm, was determined by (ΔA340 per min per 0.00503 μM−1)(0.2 ml/0.02 ml) × sample dilution. The background reading was subtracted from that of the samples.
Histological analysis
For light microscopic investigation, the right kidney and right thigh muscle were fixed in 10% buffered neutral formalin solution (VWR, Mississauga, ON, Canada) for 48 h. Then the samples were dehydrated in an ascending ethanol series and embedded in paraffin. Sections were cut at 4 μm on a rotary microtome, mounted on slides, stained with haematoxylin and eosin or periodic acid-Schiff for the muscle and kidney analysis, respectively. Periodic acid-Schiff staining detects changes in the basement membrane of epithelial cells. All tissue sections were examined microscopically for the appearance of histopathological changes by either an experienced renal or neuromuscular pathologist, without knowledge of the treatments.
Statistical analysis
All studies were performed using samples sizes of six or more. Differences in serum and urine biochemical values, intracellular GSH levels, lipid peroxides levels and GST activity after different treatments were determined by one-way ANOVA followed by the Student–Newman–Keuls post hoc test. Values are presented as mean±s.e.mean. Analysis was performed using GraphPad InStat 3.05 (San Diego, CA, USA).
Chemicals
N-acetylcysteine and butylated hydroxytoluene were purchased from Sigma-Aldrich Canada Ltd (Oakville, ON, Canada). Ifosfamide (IFEX) was purchased from Baxter Oncology GmbH and Baxter Corporation (Mississauga, ON, Canada).
Results
Serum and urine biochemistry
Ifosfamide at 50 mg kg−1 day−1 significantly increased serum creatinine levels when compared with saline (P<0.001) (Table 1). Ifosfamide also significantly increased urinary magnesium (P<0.05) and β2-microglobulin (P<0.01) excretion (Table 2). In contrast, the NAC treatment significantly reversed the elevations of serum creatinine (P<0.001) (Table 1) as well as that of magnesium (P<0.01) and β2-microglobulin (P<0.001) excreted in urine (Table 2).
Table 1.
Effects of 50 mg kg−1 IF and/or NAC treatment on some serum biochemical parameters in rats
| Parameters | Saline | IF | NAC | NAC+IF |
|---|---|---|---|---|
| Na+ (mmol l−1) | 143.83±1.02 | 144.18±0.68 | 142.61±0.57 | 145.75±0.46 |
| K+ (mmol l−1) | 7.93±0.28 | 8.27±0.20 | 8.32±0.28 | 6.79±0.30 |
| Urea (mmol l−1) | 5.69±0.31 | 5.33±0.20 | 5.23±0.18 | 4.78±0.25 |
| Creatinine (mmol l−1) | 46.33±1.49 | 57.82±2.25*** | 47.94±2.01 | 45.25±2.05+++ |
| PO43− (mmol l−1) | 4.165±0.08 | 4.88±0.14** | 4.71±0.13* | 4.24±0.24+ |
| ALT (U l−1) | 90.56±21.93 | 88.12±7.95 | 56.50±2.71 | 66.33±7.17 |
| AST (U l−1) | 185.39±32.45 | 331±27.64*** | 135.61±12.52 | 240.82±30.66+ |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; IF, ifosfamide, NAC, N-acetylcysteine.
Data shown as mean±s.e.mean. n=12.
*P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, +++P<0.001 compared with IF group.
Table 2.
Effects of 50 mg kg−1 IF and/or NAC treatment on some urinary biochemical parameters in rats
| Parameters | Saline | IF | NAC | NAC+IF |
|---|---|---|---|---|
| Ucr (mmol l−1) | 3.95±0.34 | 5.55±0.51** | 3.82±0.37 | 3.83±0.51+ |
| Umg2+ (mmol l−1) | 15.02±1.77 | 19.50±1.48* | 13.51±1.24 | 11.17±1.49++ |
| Uurea (mmol l−1) | 403.5±38.56 | 433.35±31.78 | 396.76±38.85 | 346.17±46.41 |
| UK+ (mmol l−1) | 113.58±15.50 | 111.82±10.80 | 102.92±10.87 | 66.08±7.40 |
| UPO43− (mmol l−1) | 8.33±2.03 | 7.68±1.86 | 7.49±1.78 | 3.55±1.31 |
| Uprotein (g l−1) | 0.92±0.20 | 1.06±0.08 | 0.88±0.11 | 0.98±0.15 |
| Uβ2-micro (nmol l−1) | 13.92 ± 2.07 | 25.44±3.33** | 16.67±2.76 | 8.83±1.30+++ |
Abbreviations: IF, ifosfamide, NAC, N-acetylcysteine.
Data shown as mean±s.e.mean. n=12.
*P<0.05, **P<0.01, compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group.
The higher ifosfamide concentration (80 mg kg−1) caused more pronounced changes in serum parameters. Ifosfamide significantly decreased serum potassium, urea, creatinine, phosphate, albumin, magnesium and glucose (Table 3) when compared with saline. NAC treatment significantly prevented a decrease in serum potassium and magnesium (P<0.05). Urinary β2-microglobulin levels were significantly elevated at this ifosfamide dose level (P<0.01) and concurrent treatment with NAC prevented this elevation (Table 4). Changes in urinary excretion of magnesium and protein levels were not significant after treatment with the higher ifosfamide concentration.
Table 3.
Effects of 80 mg kg−1 IF and/or NAC treatment on some serum biochemical parameters in rats
| Parameters | Saline | IF | NAC | NAC+IF |
|---|---|---|---|---|
| Na+ (mmol l−1) | 143.83±1.02 | 145.78±0.55 | 142.61±0.57 | 144.44±0.65 |
| K+ (mmol l−1) | 7.93±0.28 | 6.42±0.31* | 8.32±0.28 | 7.74±0.77+ |
| Urea (mmol l−1) | 5.69±0.31 | 4.53±0.30** | 5.23±0.18 | 4.39±0.15** |
| Creatinine (mmol l−1) | 46.33±1.49 | 39.56±1.41* | 47.94±2.01 | 45.11±2.18 |
| PO43− (mmol l−1) | 4.165±0.08 | 3.50±0.12** | 4.71±0.13 | 3.96±0.28 |
| Albumin (g l−1) | 17.83±0.28 | 14.33±0.44*** | 16.05±0.65 | 13.33±0.73*** |
| Mg2+ (mmol l−1) | 1.845±0.03 | 1.40±0.033*** | 2.013±0.042 | 1.56±0.06+ |
| Glucose (mmol l−1) | 17.95±0.96 | 13.49±0.39** | 16.94±0.63 | 13.7±1.00** |
Abbreviations: IF, ifosfamide, NAC, N-acetylcysteine.
Data shown as mean±s.e.mean. n=12.
*P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05 compared with IF group.
Table 4.
Effects of 80 mg kg−1 IF and/or NAC treatment on some urinary biochemical parameters in rats
| Parameters | Saline | IF | NAC | NAC+IF |
|---|---|---|---|---|
| Ucr (mmol l−1) | 3.95±0.34 | 4.97±0.42 | 3.82±0.37 | 4.20±0.52 |
| Umg2+ (mmol l−1) | 15.02±1.77 | 19.51±2.59 | 13.51±1.24 | 14.2±1.41 |
| Uurea (mmol l−1) | 403.5±38.56 | 421.33±33.94 | 396.76±38.85 | 397.11±38.57 |
| UK+ (mmol l−1) | 113.58±15.50 | 107.5±10.39 | 102.92±10.87 | 79.78±8.67 |
| UPO43− (mmol l−1) | 8.33±2.03 | 2.82±1.37 | 7.49±1.78 | 2.09±0.83 |
| Uprotein (g l−1) | 0.92±0.20 | 1.22±0.19 | 0.88±0.11 | 1.10±0.11 |
| Uβ2-micro (nmol l−1) | 13.92±2.07 | 25.55±4.93** | 15.83±1.96 | 13.56±1.34+ |
Abbreviations: IF, ifosfamide, NAC, N-acetylcysteine.
Data shown as mean±s.e.mean. n=12.
**P<0.01 compared with saline group; +P<0.05 compared with IF group.
Body weight changes
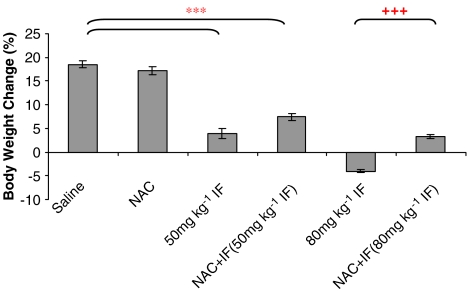
The lower dose of ifosfamide (50 mg kg−1) significantly reduced body weight gain (percentage of initial weight) as compared with the saline and NAC groups (P<0.001) (Figure 1). Concurrent administration of NAC, however, did not prevent the reduction in body weight gain. The higher dose of ifosfamide (80 mg kg−1) induced a more pronounced weight loss in the animals (P<0.001), and NAC treatment partially protected the animals against this weight loss (P<0.001). However, the body weight gain in this group of rats was still significantly lower than that of the saline group.
Figure 1.
The effect of ifosfamide (IF) and/or N-acetylcysteine (NAC) on body weight changes after treatment with IF 50 or 80 mg kg−1 for 5 days. n=6. *P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group.
Glutathione concentration
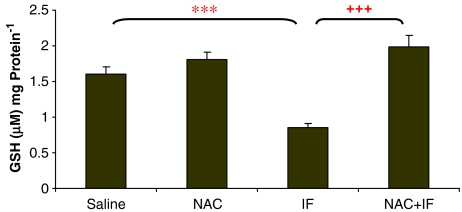
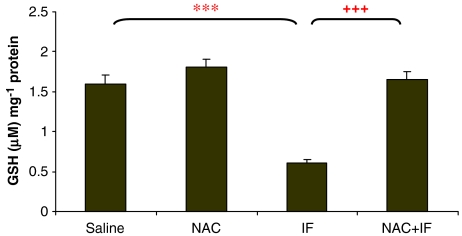
The 50 mg kg−1 ifosfamide dose significantly depleted GSH levels (P<0.01) to about 50% of the control levels (Figure 2), and NAC treatment was able to reverse this depletion. The higher ifosfamide dose significantly depleted GSH levels further (just below 40% of control) (P<0.001; Figure 3). Here also, NAC treatment reversed the depletion of GSH.
Figure 2.
The effect of ifosfamide (IF) and/or N-acetylcysteine (NAC) on the intracellular GSH levels of the kidney after treatment with IF 50 mg kg−1 for 5 days. n=6. *P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group.
Figure 3.
The effect of ifosfamide (IF) and/or N-acetylcysteine (NAC) on the intracellular GSH levels of the kidney after treatment with IF 80 mg kg−1 for 5 days. n=6. *P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group.
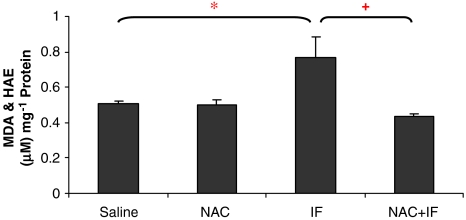
Lipid peroxidation
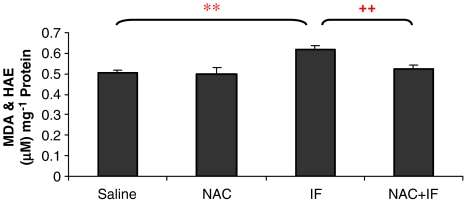
Malondialdehyde and HAE levels in the ifosfamide-treated animals were significantly higher than those of the control groups (P<0.01; Figure 4). The combined treatment of NAC and ifosfamide prevented these elevations. The higher ifosfamide concentration produced a further increase in lipid peroxide levels (Figure 5) and NAC prevented these elevations.
Figure 4.
The effect of ifosfamide (IF) and/or N-acetylcysteine (NAC) on lipid peroxidation in the kidney after treatment with IF 50 mg kg−1 for 5 days. n=6. *P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group.
Figure 5.
The effect of ifosfamide (IF) and/or N-acetylcysteine (NAC) on lipid peroxidation in the kidney after treatment with IF 80 mg kg−1 for 5 days. n=6. *P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group.
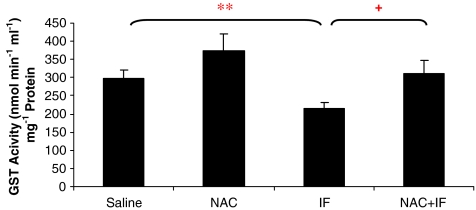
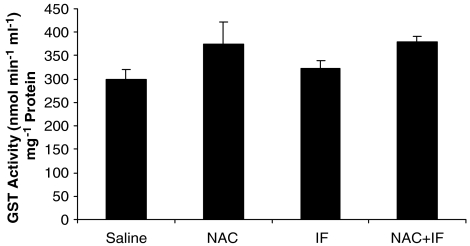
Glutathione S-transferase activity
In the rats treated with 50 mg kg−1 ifosfamide, GST activity was significantly lower than that in the group treated with saline (P<0.05; Figure 6). NAC treatment significantly prevented this decrease in GST activity (P<0.05). NAC alone tended to increase GST activity as compared to the control, but this trend was not significant. At the higher ifosfamide dose (80 mg kg−1), there was no change in GST activity as compared to the control (Figure 7), and there were no significant changes of GST activity in the NAC-treated animals.
Figure 6.
The effect of ifosfamide (IF) and/or N-acetylcysteine (NAC) on GST activity in the kidney after treatment with IF 50 mg kg−1 for 5 days. n=6. *P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group. GST, glutathione S-transferase.
Figure 7.
The effect of ifosfamide (IF) and/or N-acetylcysteine (NAC) on GST activity in the kidney after treatment with IF 80 mg kg−1 for 5 days. n=6. *P<0.05, **P<0.01, ***P<0.001 compared with saline group; +P<0.05, ++P<0.01, +++P<0.001 compared with IF group. GST, glutathione S-transferase.
Histological analysis
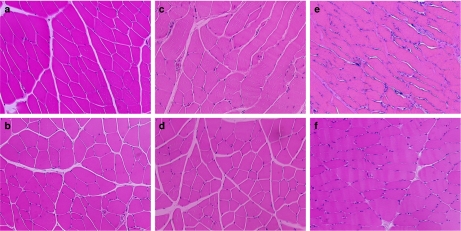
Haematoxylin and eosin staining of the thigh muscles demonstrated normal muscle architecture with closely packed polygonal muscle fibre profiles and abundant small peripherally located nuclei in samples taken from the groups treated with saline or NAC only (Figures 8a and b). Treatment with 50 mg kg−1 ifosfamide resulted in some distortion of the polygonal shape of muscle fibres with a few areas of nucleus clumps, which are signs of atrophy (Figure 8c). With 50 mg kg−1 ifosfamide and NAC treatment, the muscle fibres showed morphology similar to that in the saline and NAC-only groups (Figure 8d). At the higher dosage of ifosfamide, the shape and the size of the muscle fibre were more noticeably variable as compared with the 50 mg kg−1 dosage of ifosfamide. There was a greater extent of lymphatic infiltration (Figure 8e). These muscle abnormalities were improved when NAC was administered concurrently with 80 mg kg−1 ifosfamide, but the shape of the muscle fibre was still variable with signs of nucleus clumps (Figure 8f).
Figure 8.
Micrographs of rat thigh muscle tissues: (a) Saline group; (b) NAC group; (c) 50 mg kg−1 IF; (d) NAC+50 mg kg−1 IF; (e) 80 mg kg−1 IF; (f) NAC+80 mg kg−1 IF. H&E staining, × 200. H&E, haematoxylin and eosin.
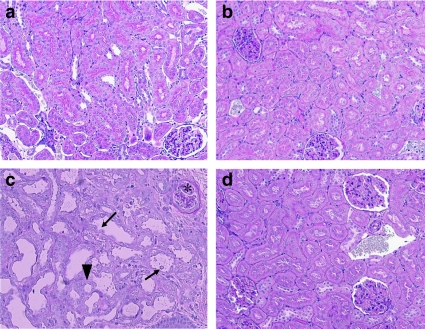
Periodic acid-Schiff staining of the kidney showed regular morphology of the tubules and glomeruli in both saline and NAC-only groups (Figures 9a and b). There were no signs of enlarged luminal space in the tubules and interstitial spaces. Moreover, the basal membrane of the tubules was well defined. The 50 mg kg−1 ifosfamide dose did not produce any significant changes compared to the control (data not shown). The 80 mg kg−1 ifosfamide caused severe renal damage (Figure 9c), as indicated by the distorted proximal tubules with sloughing cells, severe interstitial inflammation and oedema as well as a degenerated glomerulus with missing Bowman's space. There was also a significant amount of cell death. Concurrent treatment with NAC prevented the damage seen in the tubules and glomeruli (Figure 9d), with no signs of interstitial inflammation.
Figure 9.
Micrographs of rat kidney tissues: (a) Saline group, well-defined tubules and glomeruli; (b) NAC group, similar structure as saline group with normal tubules and glomeruli; (c) 80 mg kg−1 IF group, distorted proximal tubules with sloughing cells (arrows), severe interstitial inflammation and oedema (arrowhead) and degenerated glomerulus (*); (d) NAC+80 mg kg−1 IF, relatively normal morphology of tubules and glomeruli and interstitial inflammation is no longer present. PAS staining, × 200. PAS, periodic acid-Schiff.
Discussion
The present study is the first to describe that NAC is able to prevent ifosfamide-induced nephrotoxicity in an animal model. A clinically relevant dosage of ifosfamide (50 mg kg−1) resulted in altered renal functions as shown by elevations of serum creatinine and urinary excretion of magnesium and β2-microglobulin. At the same time, it produced remarkable oxidative damage as shown by the depletion in GSH levels, elevated lipid peroxide levels and decreased GST activity. However, as expected, it did not lead to detectable morphological changes in the renal tissue, as this model only captures the early stage of ifosfamide toxicity. At such an early stage of toxicity, NAC was able to prevent the elevated levels of serum creatinine, urinary magnesium and β2-microglobulin. To further evaluate the protective effect of NAC, a higher ifosfamide dose (80 mg kg−1) was used, resulting in decreases of more serum biochemical parameters, including potassium, urea, phosphate, albumin, magnesium and glucose, as well as the urinary excretion of β2-microglobulin. This was paralleled by a greater depletion of GSH levels and more elevated lipid peroxide levels as well as morphological damage in the tubules and glomeruli of the kidney. Critically, NAC reduced the severity of ifosfamide-induced renal toxicity, as evidenced by almost normal morphology of the tubules and glomeruli of animals that received combined treatment with NAC and ifosfamide.
The ifosfamide-induced animal model of Fanconi syndrome was described by Nissim and Weinberg (1996), and it has been subsequently used by several research groups to evaluate the effect of different antioxidants on renal functions in ifosfamide-treated rats (Nissim and Weinberg, 1996; Badary, 1998, 1999a, 1999b; Sener et al., 2004; Sehirli et al., 2007). In agreement with these studies, our results demonstrated a similar pattern of renal dysfunction in both serum and urine biochemistry. Furthermore, depletion of intracellular GSH levels was pronounced at the early stages of toxicity induced by ifosfamide. Our recent in vitro studies have suggested that depletion of GSH is a key determinant in ifosfamide-induced nephrotoxicity (Aleksa et al., 2005; Chen et al., 2007). As this crucial cellular defence mechanism weakens, renal cells are more susceptible to the oxidative stress caused by reactive toxic metabolites such as chloroacetaldehyde. In this case, NAC is an ideal candidate for replenishing tissue GSH levels, as its two major protective mechanisms are to act as a precursor for GSH synthesis as well as a nucleophile that scavenges reactive oxygen species and conjugates with reactive metabolites rendering them less toxic (De Vries and De Flora, 1993; Tylicki et al., 2003). Furthermore, NAC has advantages over direct GSH supplementation, as NAC can readily pass through cell membrane thereby resulting in rapid replenishment of intracellular GSH. In contrast, systemically administered GSH does not pass through the cell membrane and its action may be limited to the extracellular level only (Tariq et al., 1999).
Our animal model has demonstrated the effect of NAC on acute ifosfamide nephrotoxicity. To date, all recent papers addressing ifosfamide-induced nephrotoxicity also used an acute animal model (Sener et al., 2004). An attempt to establish an animal model for long-lasting ifosfamide-induced Fanconi syndrome was unsuccessful by Appenroth et al. (2007). However, the relationship between acute and long-term renal damage has been well established. Acute ifosfamide nephrotoxicity is often progressive, which may potentially lead to chronic damage (Heney et al., 1991). Hence, it is likely that early subclinical renal dysfunction may predict later nephrotoxicity. A prospective study has suggested that severe acute β2-microglobulinuria appeared to be a prognostic indicator for the development of chronic nephrotoxicity (Ho et al., 1995). In our study, NAC was able to prevent β2-microglobulinuria during an acute renal injury. On the basis of the relationship between acute and long-term renal damage, NAC would presumably have a protective effect on the chronic nephrotoxicity.
A close correlation between the depletion of tissue GSH and an elevation of lipid peroxidation has been well documented (Maddaiah, 1990). Lipid peroxidation becomes more likely in cell membranes as a result of impaired antioxidant defence mechanisms. Subsequently, the functional integrity of the cellular structures is compromised and in more severe cases, cell death may occur. The measurement of MDA and HAE has been used as an indicator of lipid peroxidation (Esterbauer et al., 1991). As shown in our findings, the levels of MDA and HAE in the ifosfamide-treated group were significantly higher than those of the control groups. In parallel, the GSH levels were significantly depleted. In agreement with our previous in vitro findings, NAC prevented renal tubular cell death as shown by almost normal morphology of the tubules. In addition to GSH depletion and lipid peroxidation, other toxicity mechanisms such as a drastic decrease in cellular Co-A, acetyl-CoA contents and cellular ATP levels, a key factor required for GSH synthesis (Jez and Cahoon, 2004), as well as an increase in lactate dehydrogenase release were suggested as contributing factors to ifosfamide-induced nephrotoxicity (Dubourg et al., 2001). For future mechanistic studies, it is important to investigate the effect of NAC on these factors in terms of preventing ifosfamide-induced renal injury.
Glutathione S-transferase is a phase II enzyme that facilitates the conjugation of GSH with reactive metabolites leading to the formation of a thioether bond that makes the conjugate less reactive than the parental compound. There is some evidence that NAC can enhance GST activity (Tylicki et al., 2003), and we observed, in the present study, an nonsignificant trend towards increased GST activity when NAC was administered alone. More importantly, NAC prevented the decrease of GST activity when it was administered together with the 50 mg kg−1 concentration of ifosfamide. This concurred with the findings from several studies conducted by Badary (1998, 1999a, 1999b). This suggested that NAC has an important function in preventing ifosfamide-induced nephrotoxicity by enhancing GST activity. Furthermore, the detoxification mechanisms were augmented by increasing both GSH synthesis and the conjugation between GSH and the toxic metabolites. However, the higher concentration of ifosfamide did not cause any decrease of GST activity in the present study. The induction of GST is not in itself unusual. GSTs have been shown to develop resistance towards chemotherapeutic agents (Townsend and Tew, 2003). Exposure to these agents may lead to the induction and expression of GSTs that protect the cell, and sometimes this becomes apparent after either a long-term exposure or at a higher dose. Mulders et al. (1995) reported that the effect of ifosfamide treatment has no effect on GST activity in patients with advanced cancers, whereas Dirven et al. (1995) reported an elevation in isoform GSTπ in the presence of ifosfamide mustard, the pharmacologically active metabolite. It appears that treatment with the higher dose of ifosfamide (80 mg kg−1) may have induced GST activity to detoxify the toxic metabolites.
One of the early signs of glomerular dysfunction is the elevation in serum creatinine (Levey et al., 1988). Although serum creatinine concentration is the most widely used marker in estimating GFR (Levey et al., 1988), it has limitations. Particularly in our study, an acute decrease in muscle mass by ifosfamide decreased the amount of creatinine available for filtration. As shown in the higher ifosfamide dose experiment, a decrease in serum creatinine was encountered as compared with the 50 mg kg−1 ifosfamide. This was best explained by the animals losing body weight significantly due to wastage in muscle mass, as shown in the histology. This is a well-described side effect in patients treated with anticancer drugs (Appenroth et al., 2007) and was also reported in ifosfamide-treated rats (Springate and Van Liew, 1995; Sener et al., 2004). As a result, the serum creatinine concentration could not be used adequately to reflect changes in renal function at the higher dose of ifosfamide (80 mg kg−1).
Along with the elevated serum creatinine, changes in urinary excretion of specific markers reflect the typical tubular injury of Fanconi syndrome (Han and Bonventre, 2004; Trof et al., 2006). β2-Microglobulin, a low-molecular-weight protein, is an early biological marker specifically for proximal tubular injury (Wibell, 1978; Tolkoff-Rubin et al., 1988), and this marker enabled us to detect tubular damage at both 50 and 80 mg kg−1 ifosfamide.
In our experiments, structural and functional changes were not always correlated in the high ifosfamide dose experiments. NAC protected the renal tubule cells from depletion of GSH, elevation of lipid peroxidation and morphological damages. However, unlike the effects of NAC at the 50 mg kg−1 ifosfamide dose level, some of the serum parameters, such as urea, phosphate, albumin and glucose, still remained significantly lower than those of control groups. This is consistent with a dose-dependent incomplete recovery from ifosfamide-induced nephrotoxicity. In some diseases characterized by acute renal failure, structural recovery precedes functional recovery (Solez and Whelton, 1984; Racusen, 1997).
In conclusion, the present study has illustrated that the antioxidant NAC is able to protect the kidney from ifosfamide-induced nephrotoxicity in an in vivo model. Although it is a standard practise to administer 2-mercaptoethane sulphonate concurrently with ifosfamide to prevent urotoxicity, NAC has been shown to also have a protective effect in the bladder (Watson, 1984; Palma et al., 1986). This may benefit patients as long as NAC does not interact with 2-mercaptoethane sulphonate, in which case the protective effect of both compounds could be compromised. Future investigation addressing this issue is required. Several clinical studies have addressed the issue of whether NAC interferes the antitumour activity of chemotherapeutic agents and found that NAC did not affect the antitumour activity at least of cisplatin and ifosfamide for lung carcinomas (Morgan et al., 1982; Dickey et al., 2005). Furthermore, NAC has no effect on ifosfamide pharmacokinetics (Benvenuto et al., 1992). More studies on the effect of NAC on the antitumour activity of ifosfamide in relevant cell lines are needed and these studies are currently ongoing in our laboratory. Following the in vitro studies, the next logical and indispensable step is to demonstrate the potential action of NAC in a tumour-bearing animal model before continuing onto clinical studies. As NAC is approved and widely used in children with paracetamol (acetaminophen) toxicity, our results should lead to testing the ability of NAC to prevent ifosfamide nephrotoxicity clinically in paediatric patients.
Acknowledgments
We thank Dr Madeleine Moussa for her expertise in renal pathology and Dr Robert Hammond for his expertise in neuropathology. This study was supported by a grant of Canadian Institutes Health Research (CIHR) and GK holds the Ivey Chair in Molecular Toxicology, University of Western Ontario, London, Ontario, Canada.
Abbreviations
- GSH
glutathione
- GST
glutathione S-transferase
- HAE
4-hydroxyalkenals
- LLCPK-1
porcine proximal tubular cell line
- MDA
malondialdehyde
- NAC
N-acetylcysteine
Conflict of interest
The authors state no conflict of interest.
References
- Aleksa K, Halachmi N, Ito S, Koren G. A tubule cell model for ifosfamide nephrotoxicity. Can J Physiol Pharmacol. 2005;83:499–508. doi: 10.1139/y05-036. [DOI] [PubMed] [Google Scholar]
- Al-Ghonaim M, Pannu N. Prevention and treatment of contrast-induced nephropathy. Tech Vasc Interv Radiol. 2006;9:42–49. doi: 10.1053/j.tvir.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Appenroth D, Werner T, Lupp A, Patzer L, Misselwitz J, Fleck C. Efforts to establish an animal model of Fanconi syndrome after ifosfamide administration to rats. J Appl Toxicol. 2007;27:327–336. doi: 10.1002/jat.1197. [DOI] [PubMed] [Google Scholar]
- Badary OA. Taurine attenuates Fanconi syndrome induced by ifosfamide without compromising its antitumor activity. Oncol Res. 1998;10:355–360. [PubMed] [Google Scholar]
- Badary OA. L-Histidinol attenuates Fanconi syndrome induced by ifosfamide in rats. Exp Nephrol. 1999a;7:323–327. doi: 10.1159/000020620. [DOI] [PubMed] [Google Scholar]
- Badary OA. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J Ethnopharmacol. 1999b;67:135–142. doi: 10.1016/s0378-8741(98)00242-6. [DOI] [PubMed] [Google Scholar]
- Benvenuto JA, Ayele W, Legha SS, Raber MN, Nicaise C, Newman RA. Clinical pharmacokinetics of ifosfamide in combination with N-acetylcysteine. Anticancer Drugs. 1992;3:19–23. doi: 10.1097/00001813-199202000-00004. [DOI] [PubMed] [Google Scholar]
- Chen N, Aleksa K, Woodland C, Rieder M, Koren G. The effect of N-acetylcysteine on ifosfamide-induced nephrotoxicity: in vitro studies in renal tubular cells. Transl Res. 2007;150:51–57. doi: 10.1016/j.trsl.2007.02.001. [DOI] [PubMed] [Google Scholar]
- De Vries N, De Flora S. N-Acetyl-l-cysteine. J Cell Biochem Suppl. 1993;17F:270–277. doi: 10.1002/jcb.240531040. [DOI] [PubMed] [Google Scholar]
- Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- Dirven HA, Megens L, Oudshoorn MJ, Dingemanse MA, van Ommen B, van Bladeren PJ. Glutathione conjugation of the cytostatic drug ifosfamide and the role of human glutathione S-transferases. Chem Res Toxicol. 1995;8:979–986. doi: 10.1021/tx00049a012. [DOI] [PubMed] [Google Scholar]
- Dubourg L, Michoudet C, Cochat P, Baverel G. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J Am Soc Nephrol. 2001;12:1615–1623. doi: 10.1681/ASN.V1281615. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10:476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- Heney D, Wheeldon J, Rushworth P, Chapman C, Lewis IJ, Bailey CC. Progressive renal toxicity due to ifosfamide. Arch Dis Child. 1991;66:966–970. doi: 10.1136/adc.66.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PT, Zimmerman K, Wexler LH, Blaney S, Jarosinski P, Weaver-McClure L, et al. A prospective evaluation of ifosfamide-related nephrotoxicity in children and young adults. Cancer. 1995;76:2557–2564. doi: 10.1002/1097-0142(19951215)76:12<2557::aid-cncr2820761223>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Jez JM, Cahoon RE. Kinetic mechanism of glutathione synthetase from Arabidopsis thaliana. J Biol Chem. 2004;279:42726–42731. doi: 10.1074/jbc.M407961200. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71:980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- Loebstein R, Atanackovic G, Bishai R, Wolpin J, Khattak S, Hashemi G, et al. Risk factors for long-term outcome of ifosfamide-induced nephrotoxicity in children. J Clin Pharmacol. 1999;39:454–461. [PubMed] [Google Scholar]
- Loebstein R, Koren G. Ifosfamide-induced nephrotoxicity in children: critical review of predictive risk factors. Pediatrics. 1998;101:E8–E12. doi: 10.1542/peds.101.6.e8. [DOI] [PubMed] [Google Scholar]
- Maddaiah VT. Glutathione correlates with lipid peroxidation in liver mitochondria of triiodothyronine-injected hypophysectomized rats. FASEB J. 1990;4:1513–1518. doi: 10.1096/fasebj.4.5.2307329. [DOI] [PubMed] [Google Scholar]
- Marzullo L. An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children. Curr Opin Pediatr. 2005;17:239–245. doi: 10.1097/01.mop.0000152622.05168.9e. [DOI] [PubMed] [Google Scholar]
- Mishima K, Baba A, Matsuo M, Itoh Y, Oishi R. Protective effect of cyclic AMP against cisplatin-induced nephrotoxicity. Free Radic Biol Med. 2006;40:1564–1577. doi: 10.1016/j.freeradbiomed.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Morgan LR, Donley PJ, Harrison EF, Hunter HL. The control of ifosfamide-induced hematuria with N-acetylcysteine in patients with advanced carcinoma of the lung. Semin Oncol. 1982;9:71–74. [PubMed] [Google Scholar]
- Mulders TM, Keizer HJ, Ouwerkerk J, van der Velde EA, Breimer DD, Mulder GJ. Effect of ifosfamide treatment on glutathione and glutathione conjugation activity in patients with advanced cancers. Clin Cancer Res. 1995;1:1525–1536. [PubMed] [Google Scholar]
- Nissim I, Weinberg JM. Glycine attenuates Fanconi syndrome induced by maleate or ifosfamide in rats. Kidney Int. 1996;49:684–695. doi: 10.1038/ki.1996.97. [DOI] [PubMed] [Google Scholar]
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- Palma PC, Villaca Junior CJ, Netto Junior NR. N-acetylcysteine in the prevention of cyclophosphamide induced haemorrhagic cystitis. Int Surg. 1986;71:36–37. [PubMed] [Google Scholar]
- Racusen LC. Pathology of acute renal failure: structure/function correlations. Adv Ren Replace Ther. 1997;4:3–16. [PubMed] [Google Scholar]
- Rossi R, Pleyer J, Schafers P, Kuhn N, Kleta R, Deufel T, et al. Development of ifosfamide-induced nephrotoxicity: prospective follow-up in 75 patients. Med Pediatr Oncol. 1999;32:177–182. doi: 10.1002/(sici)1096-911x(199903)32:3<177::aid-mpo3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sehirli O, Sakarcan A, Velioglu-Ogunc A, Cetinel S, Nursal G, Yegen BC, et al. Resveratrol improves ifosfamide-induced Fanconi syndrome in rats. Toxicol Appl Pharmacol. 2007;222:33–41. doi: 10.1016/j.taap.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Sener G, Sehirli O, Yegen BC, Cetinel S, Gedik N, Sakarcan A. Melatonin attenuates ifosfamide-induced Fanconi syndrome in rats. J Pineal Res. 2004;37:17–25. doi: 10.1111/j.1600-079X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Skinner R. Chronic ifosfamide nephrotoxicity in children. Med Pediatr Oncol. 2003;41:190–197. doi: 10.1002/mpo.10336. [DOI] [PubMed] [Google Scholar]
- Skinner R, Sharkey IM, Pearson AD, Craft AW. Ifosfamide, mesna, and nephrotoxicity in children. J Clin Oncol. 1993;11:173–190. doi: 10.1200/JCO.1993.11.1.173. [DOI] [PubMed] [Google Scholar]
- Solez K, Whelton A. Acute Renal Failure: Correlations between Morphology and Function. Dekker: New York; 1984. [Google Scholar]
- Springate J, Chan K, Lu H, Davies S, Taub M. Toxicity of ifosfamide and its metabolite chloroacetaldehyde in cultured renal tubule cells. In Vitro Cell Dev Biol Anim. 1999;35:314–317. doi: 10.1007/s11626-999-0080-y. [DOI] [PubMed] [Google Scholar]
- Springate JE, Van Liew JB. Nephrotoxicity of ifosfamide in rats. J Appl Toxicol. 1995;15:399–402. doi: 10.1002/jat.2550150510. [DOI] [PubMed] [Google Scholar]
- Straka C, Hebart H, Adler-Reichel S, Werding N, Emmerich B, Einsele H. Blood stem cell collections after mobilization with combination chemotherapy containing ifosfamide followed by G-CSF in multiple myeloma. Oncology. 2003;65 Suppl 2:94–98. doi: 10.1159/000073368. [DOI] [PubMed] [Google Scholar]
- Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant. 1999;14:923–929. doi: 10.1093/ndt/14.4.923. [DOI] [PubMed] [Google Scholar]
- Tolkoff-Rubin NE, Rubin RH, Bonventre JV. Noninvasive renal diagnostic studies. Clin Lab Med. 1988;8:507–526. [PubMed] [Google Scholar]
- Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trof RJ, Di Maggio F, Leemreis J, Groeneveld AB. Biomarkers of acute renal injury and renal failure. Shock. 2006;26:245–253. doi: 10.1097/01.shk.0000225415.5969694.ce. [DOI] [PubMed] [Google Scholar]
- Tylicki L, Rutkowski B, Horl WH. Antioxidants: a possible role in kidney protection. Kidney Blood Press Res. 2003;26:303–314. doi: 10.1159/000073936. [DOI] [PubMed] [Google Scholar]
- Watson RA. Ifosfamide: chemotherapy with new promise and new problems for the urologist. Urology. 1984;24:465–468. doi: 10.1016/0090-4295(84)90323-6. [DOI] [PubMed] [Google Scholar]
- Wibell L. The serum level and urinary excretion of beta2-microglobulin in health and renal disease. Pathol Biol. 1978;26:295–301. [PubMed] [Google Scholar]
- Woodland C, Ito S, Granvil CP, Wainer IW, Klein J, Koren G. Evidence of renal metabolism of ifosfamide to nephrotoxic metabolites. Life Sci. 2000;68:109–117. doi: 10.1016/s0024-3205(00)00915-2. [DOI] [PubMed] [Google Scholar]