Abstract
Background and purpose:
This study evaluated the signalling coupled to the α1-adrenoceptor-induced stimulation of the Cl−/HCO3− exchanger in hypertension.
Experimental approach:
The Na+-independent HCO3− transport system activity was assayed as the initial rate of pHi recovery after an alkaline load (CO2/HCO3 removal) in immortalized renal proximal tubular epithelial cells from spontaneously hypertensive rat (SHR) and their normotensive control (Wistar Kyoto rat; WKY).
Key results:
Noradrenaline increased Cl−/HCO3− exchanger activity with EC50 values of 0.6 and 5.3 μM in SHR and WKY cells, respectively. These effects were abolished by prazosin, but not by yohimbine. Phenylephrine increased Cl−/HCO3− exchanger activity in SHR and WKY cells (EC50 of 2.6 and 4.9 μM, respectively). Phenylephrine-mediated increase in Cl−/HCO3− exchanger activity in WKY and SHR cells was inhibited by protein kinase C (PKC), MAPK/ERK kinase (MEK) and p38 mitogen-activated protein kinase (p38 MAPK) inhibitors. The expression of α1A- and α1B-adrenoceptors was identical in WKY and SHR cells. SHR cells generated more H2O2 than WKY cells. In SHR cells, the NADPH oxidase inhibitor apocynin reduced their increased ability to generate H2O2 and abolished their hypersensitivity to phenylephrine, but failed to affect basal Cl−/HCO3− exchanger activity. H2O2-dependent stimulation of Cl−/HCO3− exchange activity was significantly higher in SHR than in WKY cells.
Conclusions and implications:
Differences between WKY and SHR cells on their sensitivity to α1-adrenoceptor stimulation did not correlate with the abundance of α1A- and α1B-adrenoceptors and may be related to the increased generation of H2O2, which may amplify the response downstream of α1-adrenoceptor activation.
Keywords: Cl−/HCO3− exchanger, α1-adrenoceptor, H2O2, hypertension, SHR, WKY
Introduction
A considerable part of filtered HCO3− is reabsorbed in the renal proximal tubules by the anion exchangers (Krapf and Alpern, 1993; Soleimani and Singh, 1995). The Na+/HCO3− cotransporter, the Na+-dependent Cl−/HCO3− exchanger and the Na+-independent Cl−/HCO3− exchanger have been described in the kidney (Alpern, 1990; Hara et al., 2000; Soleimani and Burnham, 2000; Petrovic et al., 2003; Mount and Romero, 2004). These anion exchangers facilitate the reversible electroneutral exchange of Cl− for HCO3− across the plasma membrane and regulate intracellular pH (pHi), intracellular chloride concentration, bicarbonate metabolism and cell volume. After an intracellular acid load, the cell responds with stimulation of the Na+/H+ exchanger (NHE) (Gomes et al., 2001; Pedrosa et al., 2004a, 2004b, 2004c; Gomes and Soares-da-Silva, 2006), the Na+/HCO3− cotransporter and the Na+-dependent Cl−/HCO3− exchanger to mediate the recovery of pHi (Lazdunski et al., 1985; Dart and Vaughan-Jones, 1992; Gomes et al., 2001; Gomes and Soares-da-Silva, 2006). In contrast, the Na+-independent Cl−/HCO3− exchanger usually mediates the recovery from an intracellular alkalinization (Xu and Spitzer, 1994). Our group recently demonstrated the presence of an apical Cl−/HCO3− exchanger in immortalized renal proximal tubular epithelial (PTE) cells from the spontaneously hypertensive rat (SHR) and Wistar Kyoto rat (WKY), which may correspond to the SLC26A6 protein (Pedrosa et al., 2004d).
One proposal for the initiation and maintenance of hypertension centres on a reduced capacity of the kidney to excrete salt and water in proper relation to intake (Guyton et al., 1972). Renal sympathetic nerves and circulating catecholamines are involved in the regulation of Na+ and water excretion in the kidney (Wilborn et al., 1998). The catecholamine noradrenaline is the major endogenous neurotransmitter in renal sympathetic nerves and mediates sympathetic regulation of blood pressure. Noradrenaline interacts with both the α- and β-adrenoceptors in the renal proximal tubules (DiBona, 1985; Wilborn et al., 1998; Kanagy, 2005). Studies have shown that renal nerves, acting through α-adrenoceptors, enhance proximal tubular sodium reabsorption in the kidney. These studies suggest that noradrenaline, acting via α- and/or β-adrenoceptors, may contribute to the development of hypertension (Baines and Ho, 1987; Gesek and Schoolwerth, 1990).
The present study evaluated the activity of the Cl−/HCO3− exchanger in immortalized renal PTE cells from SHR and WKY, and its sensitivity to noradrenaline. We found that SHR PTE cells express an enhanced sensitivity to α1-adrenoceptor-mediated stimulation of Cl−/HCO3− exchanger activity. This enhanced sensitivity of the Cl−/HCO3− exchanger to the α1-adrenoceptor stimulation appeared not to be linked to differences in the signal-transduction pathway coupled to α1-adrenoceptors, the activation of which involves PKC and p38 mitogen-activated protein kinase (MAPK) in both WKY and SHR cells; rather, this appeared to result from differences in hydrogen peroxide (H2O2) production.
Methods
Cell culture
Immortalized renal PTE cells from 4- to 8-week-old WKYs and SHRs (Woost et al., 1996) were maintained in a humidified atmosphere of 5% CO2/95% air at 37 °C. WKY and SHR PTE cells were grown in Dulbecco's modified Eagle's medium nutrient mixture F-12 Ham (Sigma Chemical Company, St Louis, MO, USA) supplemented with 100 U ml−1 penicillin G, 0.25 μg ml−1 amphotericin B, 100 μg ml−1 streptomycin (Sigma Chemical Company), 4 μg ml−1 dexamethasone (Sigma Chemical Company), 5 μg ml−1 transferrin (Sigma Chemical Company), 5 μg ml−1 insulin (Sigma Chemical Company), 5 ng ml−1 selenium (Sigma Chemical Company), 10 ng ml−1 epidermal growth factor (Sigma Chemical Company), 5% fetal bovine serum (Sigma Chemical Company) and 25 mM HEPES (Sigma Chemical Company).
For subculturing, the cells were dissociated with 0.10% trypsin–EDTA, split 1:8 and subcultured in Costar plates with 21-cm2 growth areas (Costar, Badhoevedorp, The Netherlands). For pHi measurement experiments, cells were grown in 96-well plates (Costar). For the measurement of α1-adrenoceptor expression, the cells were seeded in six-well plastic culture clusters (Costar). The cell medium was changed every 2 days, and the cells reached confluence after 3–5 days of incubation. The cells were maintained in fetal bovine serum-free medium for 24 h before each experiment. Experiments were generally performed 1–2 days after cells reached confluence and 4–5 days after the initial seeding; each cm2 contained about 50 μg of cell protein.
pHi measurements
In pHi measurement experiments, WKY and SHR PTE cells were grown in 96-well plates. pHi was measured as previously described (Pedrosa et al., 2004d). At days 4–5 after seeding SHR and WKY PTE cells cultured in 96-well plates, pHi measurements were performed after loading the cells with 10 μM acetoxymethyl ester of 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein at 37 °C for 30 min. Cells were placed in the sample compartment of a dual-scanning microplate spectrofluorometer (Spectramax Gemini XS; Molecular Devices, Sunnyvale, CA, USA), and fluorescence was measured every 17 s, alternating between 440 and 490 nm excitation at 535 nm emission, with a cutoff filter of 530 nm. The ratio of intracellular 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein fluorescence at 490 and 440 nm was converted to pHi values by comparison with values from an intracellular calibration curve using the nigericin (10 μM) and high-K+ method (Thomas et al., 1979).
Cl−/HCO3− exchanger activity
The Na+-independent HCO3− transport system activity was assayed as the initial rate of pHi recovery after an alkaline load (CO2/HCO3 removal), in the absence of Na+, as previously described (Pedrosa et al., 2004d; Fraga et al., 2006). In the Krebs HCO3−-free medium used, sodium was replaced by an equimolar concentration of choline. The Krebs HCO3−-free medium also contained pargyline (100 μM) and tolcapone (1 μM) to inhibit the enzymes monoamine oxidase and catechol-O-methyltransferase, respectively. The test compounds were added to the extracellular fluid 40 min before the start of the pHi recovery period after the alkaline load.
Immunoblotting
WKY and SHR PTE cells cultured to 90% of confluence were washed twice with phosphate-buffered saline and total cell protein was extracted for α1A- and α1B-adrenoceptor detection. Briefly, to obtain total cell extract, cells were lysed by brief sonication (15 s) in lysis buffer with protease inhibitors (150 mM NaCl, 50 mM Tris-HCl pH 7.4, 5 mM EDTA, 0.25% sodium deoxycholate, 1 mM phenylmethylsulphonyl fluoride, 1% Nonidet P-40 (Igepal), 1 mM Na3VO4, 1 mM NaF, aprotinin and leupeptin 1 μg ml−1 each) and incubated on ice for 30 min. After centrifugation (16 000 g, 30 min, 4 °C), the supernatant was collected and protein concentration determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) with BSA as standard. Sixty micrograms of protein was mixed in 2 × sample buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 2% 50 mM dithiothreitol, 0.1% w/v bromophenol blue) and boiled for 5 min. Proteins were subjected to SDS-polyacrylamide gel electrophoresis (10% SDS-polyacrylamide gel) and electrotransferred onto nitrocellulose membranes. The transblot sheets were blocked for 1 h with 5% of non-fat dry milk in 25 mM Tris-HCl pH 7.5 and 150 mM NaCl. The membranes were subsequently incubated overnight at 4 °C with appropriately diluted antibodies (goat polyclonal anti-α1A-adrenoceptor and anti-α1B-adrenoceptor, 1:400 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-β-actin primary antibody, 1:60 000, Santa Cruz Biotechnology). The membranes were subsequently washed and incubated with fluorescent-labelled donkey anti-goat (1:10 000; IRDye 800, Rockland, Gilbertsville, PA, USA) or a fluorescent-labelled goat anti-mouse secondary antibody (1:10 000; IRDye 680, LI-COR Biosciences, Lincoln, NE, USA), respectively, for 60 min at room temperature and protected from light. The membrane was washed and imaged by scanning at both 700 and 800 nm with an Odyssey Infrared Imaging System (LI-COR Biosciences).
Measurement of H2O2
Hydrogen peroxide was measured fluorometrically using the Amplex Red Hydrogen Peroxide Assay kit (Molecular Probes Inc., Eugene, OR, USA). Amplex Red is a fluorogenic substrate with very low background fluorescence that reacts with H2O2 with a 1:1 stoichiometry to produce a highly fluorescent reagent. Measurement of H2O2 was evaluated either directly by H2O2 released from the WKY and SHR monolayer cultured in 96-well plates or by H2O2 accumulated in the extracellular medium during 24 h after the cells achieved confluence. Fluorescence intensity was measured in multiplate reader (Spectromax Gemini; Molecular Devices) at an excitation wavelength of 530 nm and emission wavelength of 590 nm at room temperature. After subtracting background fluorescence, the concentration of H2O2 was calculated using a resorufin–H2O2 standard calibration curve generated from experiments using H2O2 and Amplex Red.
Data analysis
Arithmetic means are given with s.e.mean or geometric means with 95% confidence values. Statistical significance was determined using one-way ANOVA followed by Newman–Keuls test for multiple comparisons. A value of P<0.05 was assumed to denote a significant difference.
Drugs
Apocynin, chelerythrine chloride, L-(−)-noradrenaline (+)-bitartrate salt monohydrate, pargyline hydrochloride, (R)-(−)-phenylephrine hydrochloride, phorbol-12,13-dibutyrate (PDBu), prazosin hydrochloride, yohimbine hydrochloride, U 0126 and H2O2 were purchased from Sigma Chemical Company. PD 098059, SB 203580 and anisomycin were obtained from Research Biochemicals International (Natick, MA, USA). Acetoxymethyl ester of 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein nigericin and the Amplex Red Hydrogen Peroxide Assay kit were obtained from Molecular Probes Inc.. Tolcapone was kindly donated by the late Professor Mosé DaPrada (Hoffmann La Roche, Basel, Switzerland).
Results
The addition of noradrenaline before (40 min) and during (10 min) the HCO3−-dependent recovery of pHi increased the Cl−/HCO3− exchanger activity in a concentration-dependent manner in WKY and SHR PTE cells (Figure 1). However, as shown in Figure 1a, the sensitivity of noradrenaline-dependent stimulation of Cl−/HCO3− exchange activity was significantly higher in SHR than that in WKY PTE cells. This was also evidenced by the 10-fold difference in EC50 values for the noradrenaline-induced stimulation of Cl−/HCO3− activity between SHR (geometric means [95% confidence limits]: 0.6 [0.5, 0.8] μM) and WKY (5.3 [4.3, 6.4] μM) PTE cells. The effect of noradrenaline (30 μM) in WKY and SHR PTE cells on the activity of Cl−/HCO3− exchanger was completely blocked by the α1-adrenoceptor antagonist prazosin (3 μM) but not by the α2-adrenoceptor antagonist yohimbine (0.1 μM; Figures 1b and c).
Figure 1.
(a) Concentration-dependent effect of noradrenaline (40 min exposure) on Cl−/HCO3− exchanger activity in WKY and SHR PTE cells. The absolute control values for Cl−/HCO3− exchanger activity in pH units min−1 were 0.1532±0.01 (WKY cells) and 0.2413±0.01 (SHR cells). (b and c) Effect of noradrenaline (30 μM for 40 min exposure) on Cl−/HCO3− exchanger activity in the absence and presence of yohimbine (100 nM) and prazosin (3 μM) in (b) WKY and (c) SHR PTE cells. The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, in vehicle were 0.1492±0.01 (WKY cells) and 0.2388±0.01 (SHR cells). Symbols or columns represent the mean of 6–20 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and values for noradrenaline alone (#P<0.05). PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
As shown in Figure 2a, the sensitivity of SHR PTE cells to phenylephrine, a selective α1-adrenoceptor agonist, was also markedly different from that observed for WKY PTE cells. In fact, the phenylephrine-dependent stimulation of Cl−/HCO3− exchange activity was significantly higher in SHR PTE cells than that in WKY PTE cells. This was also evidenced by the significant twofold difference in EC50 values for the phenylephrine-induced stimulation of Cl−/HCO3− activity between SHR (2.6 [1.9, 3.4] μM) and WKY (4.9 [2.7, 8.8] μM) PTE cells. As already shown for noradrenaline, the effect of phenylephrine (30 μM) in WKY and SHR PTE cells on the activity of Cl−/HCO3− exchanger was completely blocked by the α1-adrenoceptor antagonist prazosin (3 μM) but not by the α2-adrenoceptor antagonist yohimbine (0.1 μM; Figures 2b and c). The expression of α1A- and α1B-adrenoceptors was evaluated in immortalized WKY and SHR PTE cells by immunoblot analysis. As shown in Figure 3, the level of expression of α1A- and α1B-adrenoceptors in WKY PTE cells was similar to that in SHR PTE cells.
Figure 2.
(a) Concentration-dependent effect of phenylephrine (40 min exposure) on Cl−/HCO3− exchanger activity in WKY and SHR PTE cells. The absolute control values for Cl−/HCO3− exchanger activity in pH units min−1 were 0.2238±0.02 (WKY cells) and 0.3419±0.01 (SHR cells). (b and c) Effect of phenylephrine (30 μM for 40 min exposure) on Cl−/HCO3− exchanger activity in the absence and presence of yohimbine (100 nM) and prazosin (3 μM) in (b) WKY and (c) SHR PTE cells. The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, in vehicle were 0.2054±0.01 (WKY cells) and 0.2988±0.01 (SHR cells). Symbols or columns represent the mean of 6–8 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and values for phenylephrine alone (#P<0.05). PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
Figure 3.
Expression of (a) α1A-adrenoceptors and (b) α1B-adrenoceptors in WKY and SHR PTE cells. Representative immunoblots are shown above the bar graphs. Columns represent mean of four independent immunoblots; vertical lines show s.e.mean. α1A-adrenoceptors ∼52 kDa; α1B-adrenoceptors ∼60 kDa; β-actin ∼40 kDa. PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
There is evidence suggesting that second messenger pathways involved in noradrenaline-induced stimulation of sodium and bicarbonate transport across proximal tubules involve stimulation of PKC (Gesek et al., 1989; Gesek and Strandhoy, 1990; Chan et al., 2000; Liu and Gesek, 2001; Hutchinson and Bengtsson, 2005). To evaluate whether this was the case in the phenylephrine-induced stimulation of the Cl−/HCO3− exchanger in WKY and SHR PTE cells, the effect of PDBu, an activator of classical and novel PKCs, was examined. Treatment of WKY and SHR PTE cells with PDBu (0.1 μM, 40 min) increased Cl−/HCO3− exchanger activity in both WKY and SHR PTE cells (Figure 4). Chelerythrine (1 μM) antagonized the effect of PDBu (100 nM) and that of phenylephrine in both WKY and SHR PTE cells (Figures 4a and b).
Figure 4.
Effect of PDBu (0.1 μM) and phenylephrine (30 μM) for 40 min on Cl−/HCO3− exchanger activity in (a) WKY and (b) SHR PTE cells in the absence and presence of chelerythrine (1 μM). The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, in vehicle were 0.1957±0.01 (WKY cells) and 0.2612±0.01 (SHR cells). Each column represents the mean of 7–15 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and values for PDBu or phenylephrine alone (#P<0.05). PDBu, phorbol-12,13-dibutyrate; PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
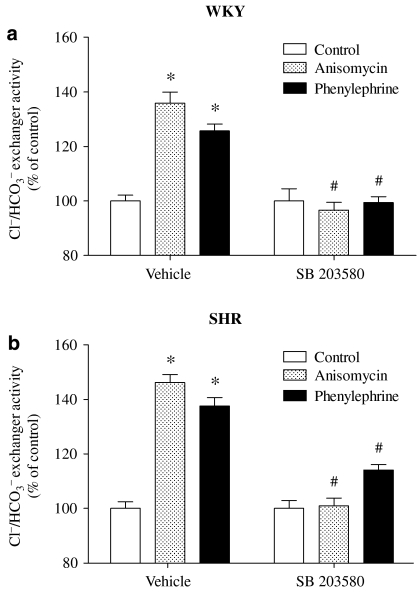
To evaluate the contribution of MAPK, which is normally related to signal transduction coupled to α1-adrenoceptors and also linked to noradrenaline-induced stimulation of sodium reabsorption in the kidney and vascular tissues (Liu and Gesek, 2001; Hutchinson and Bengtsson, 2005; Scarparo et al., 2006), we used specific inhibitors for MAPK/extracellular signal-regulated kinase kinase (MEK) and p38 MAPK. Pretreatment of cells for 15 min with the MEK 1 inhibitor PD 098059 abolished the phenylephrine-induced stimulation of Cl−/HCO3− exchanger activity in WKY PTE cells, but not in SHR PTE cells (Figure 5). As SHR PTE cells only express ERK 2, but not ERK 1 (Parenti et al., 2000), the effect of U 0126, a MEK 1/2 inhibitor, was also tested. A different effect from PD 098059 was obtained with U 0126 and the p38 MAPK inhibitor, SB 203580, which completely abolished the phenylephrine-induced stimulation of Cl−/HCO3− exchanger activity in both WKY and SHR PTE cells (Figures 5 and 6). Anisomycin, an activator of p38 MAPK, induced an increase in Cl−/HCO3− exchanger activity similar to that observed with phenylephrine in both WKY and SHR PTE cells. This effect was completely abolished by SB 203580 (Figure 6).
Figure 5.
Effect of phenylephrine (30 μM) for 40 min on Cl−/HCO3− exchanger activity in (a) WKY and (b) SHR PTE cells in the absence and presence of PD 098059 (10 μM) and U 0126 (10 μM). The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, in vehicle were 0.2157±0.02 (WKY cells) and 0.2912±0.02 (SHR cells). Each column represents the mean of 7–15 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and values for phenylephrine alone (#P<0.05). PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
Figure 6.
Effect of anisomycin (0.1 μM) and phenylephrine (30 μM) for 40 min on Cl−/HCO3− exchanger activity in the presence or absence of SB 203580 (10 μM) in (a) WKY and (b) SHR PTE cells. The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, in vehicle were 0.2067±0.02 (WKY cells) and 0.3012±0.02 (SHR cells). Each column represents the mean of 4–13 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and values for anisomycin or phenylephrine alone (#P<0.05). PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
These results suggest that stimulation of α1-adrenoceptors may lead to simultaneous activation of PKC, ERK 1/2 kinases and p38 MAPK transduction pathways in WKY PTE cells and PKC, ERK 2 and p38 MAPK transduction pathways in SHR PTE cells, with a common point in the cascade of events, as either chelerythrine or SB 203580 completely prevented the effects of phenylephrine. To confirm the involvement of both PKC and p38 MAPK in the stimulation of Cl−/HCO3− exchanger activity evoked by α1-adrenoceptor stimulation and to clarify the sequence of events in more detail, we performed complementary studies involving stimulation of PKC and p38 MAPK. To promote PKC activation, cells were incubated in the presence of PDBu (0.1 μM) and its effects on Cl−/HCO3− exchanger activity were evaluated in the absence and presence of PKC, MEK and p38 MAPK inhibitors. Under these experimental conditions, the effects of PDBu in both WKY and SHR PTE cells were abolished by the PKC inhibitor chelerythrine and the p38 MAPK inhibitor SB 203580, but not by the MEK inhibitor PD 098059 (Figure 7). To promote p38 MAPK activation, cells were incubated in the presence of anisomycin (0.1 μM) and its effects on Cl−/HCO3− exchanger activity were evaluated in the absence and presence of PKC, MEK and p38 MAPK inhibitors. Under these experimental conditions, the effect of anisomycin in both WKY and SHR PTE cells was abolished by the p38 MAPK inhibitor SB 203580, but not by the PKC inhibitor chelerythrine and the MEK 1 inhibitor PD 098059 (Figure 8). The Cl−/HCO3− exchanger in SHR PTE cells responded to PKC and p38 MAPK stimulation similarly to that observed in WKY PTE cells (Figures 7 and 8).
Figure 7.
Effect of PDBu (0.1 μM) for 40 min on Cl−/HCO3− exchanger activity in (a) WKY and (b) SHR PTE cells in the absence and presence of chelerythrine (1 μM), SB 203580 (10 μM) or PD 098059 (10 μM). The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, in vehicle were 0.1625±0.01 (WKY cells) and 0.2720±0.01 (SHR cells). Each column represents the mean of 7–15 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and values for PDBu alone (#P<0.05). PDBu, phorbol-12,13-dibutyrate; PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
Figure 8.
Effect of anisomycin (0.1 μM) for 40 min on Cl−/HCO3− exchanger activity in (a) WKY and (b) SHR PTE cells in the absence and presence of chelerythrine (1 μM), SB 203580 (10 μM) or PD 098059 (10 μM). The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, in vehicle were 0.1846±0.01 (WKY cells) and 0.2624±0.01 (SHR cells). Each column represents the mean of 7–15 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and values for anisomycin alone (#P<0.05). PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
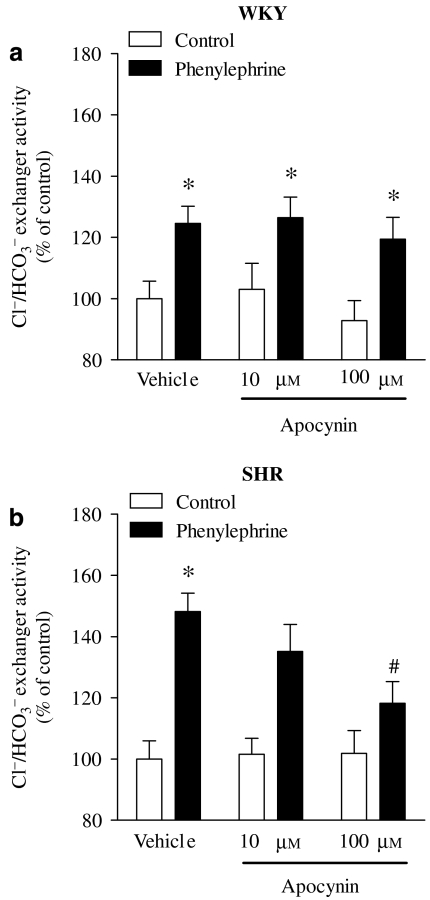
Because recent studies have demonstrated a role for renal H2O2 in the development of hypertension and renal dysfunction (Schnackenberg et al., 1998; Vaziri et al., 2000; Minuz et al., 2002; Makino et al., 2003), particularly in the SHR model (Adler and Huang, 2004; de Cavanagh et al., 2006; Sullivan et al., 2006), it seemed reasonable to measure H2O2 generation and evaluate the involvement of H2O2 in the regulation of Cl−/HCO3− exchanger activity by phenylephrine in WKY and SHR PTE cells. The SHR PTE cells had an increased rate of H2O2 production (50.7±0.4 versus 11.3±0.1 nmol min−1) when compared with WKY PTE cells (Figure 9a). Treatment of cells with apocynin (100 μM), an inhibitor of the nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase complex, for 4 days after seeding reduced the extracellular levels of H2O2 in SHR PTE cells, but not in WKY PTE cells (Figure 9b). The treatment with apocynin (100 μM) for 4 days after seeding did not change the basal Cl−/HCO3− exchanger activity in both WKY (0.084±0.004 versus 0.085±0.006 pH units min−1) and SHR (0.239±0.008 versus 0.240±0.020 pH units min−1) PTE cells. However, treatment of SHR PTE cells with apocynin (100 μM) almost completely blocked the ability of phenylephrine to stimulate Cl−/HCO3− exchanger activity with no effects in WKY PTE cells (Figure 10).
Figure 9.
(a) Rate of H2O2 (nmol min−1) released from WKY and SHR PTE cells in confluent monolayers (4 days after seeding). (b) Levels of extracellular H2O2 (nM) in WKY and SHR PTE cells in control cell culture conditions and in the presence of apocynin (100 μM; 4 days after seeding). Each column represents the mean of 6–16 experiments per group and vertical lines show s.e.mean. Significantly different from values for WKY (*P<0.05) or for control (#P<0.05). PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
Figure 10.
Effect of apocynin (10 and 100 μM; during 4 days after seeding) on phenylephrine (30 μM for 40 min) on Cl−/HCO3− exchanger activity in (a) WKY and (b) SHR PTE cells. The absolute control values for Cl−/HCO3− exchanger activity, in pH units min−1, for vehicle were 0.2238±0.02 (WKY cells) and 0.3419±0.01 (SHR cells). Each column represents the mean of 4–8 experiments per group; vertical lines indicate s.e.mean. Significantly different from values for control (*P<0.05) or for vehicle (#P<0.05). PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
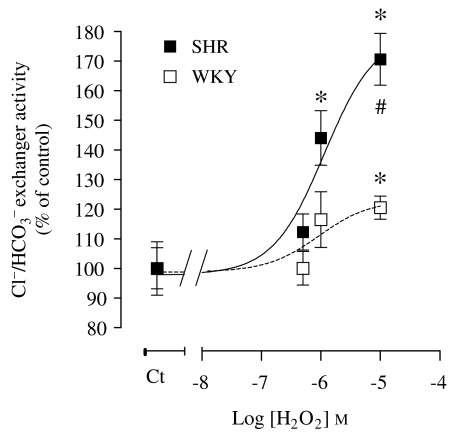
The effect of exogenous H2O2 on the Cl−/HCO3− exchanger activity in WKY and SHR PTE cells was also evaluated (Figure 11). As can be observed, H2O2 stimulated the Cl−/HCO3− exchanger activity in both WKY and SHR PTE cells, SHR PTE cells being much more sensitive to H2O2 (Figure 11).
Figure 11.
Concentration-dependent effect of H2O2 (40 min exposure) on Cl−/HCO3− exchanger activity in WKY and SHR PTE cells. The absolute control values for Cl−/HCO3− exchanger activity in pH units min−1 were 0.1725±0.02 (WKY cells) and 0.2432±0.01 (SHR cells). Symbols or columns represent the mean of 4–13 experiments per group; vertical lines indicate s.e.mean. Significantly different from corresponding control values (*P<0.05) and corresponding values in SHR PTE cells (#P<0.05). H2O2, hydrogen peroxide; PTE, proximal tubular epithelial; SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
Discussion
The present study was designed to evaluate the effects of noradrenaline and the signal-transduction pathway coupled to noradrenaline-induced stimulation of the Cl−/HCO3− exchanger in immortalized renal PTE cells from WKY and SHR. The data reported here show that SHR PTE cells were 4 and 10 times more sensitive to phenylephrine and noradrenaline, respectively, than WKY PTE cells in stimulating Cl−/HCO3− exchanger activity. Despite differences in sensitivity to phenylephrine and noradrenaline between SHR and WKY PTE cells, the results obtained indicate that noradrenaline- and phenylephrine-induced stimulation of Cl−/HCO3− exchanger activity was mediated through the activation of prazosin-sensitive α1-adrenoceptors coupled to PKC, ERK 2 and p38 MAPK pathways in both WKY and SHR PTE cells. However, the Cl−/HCO3− exchanger in SHR PTE cells responded to PKC and p38 MAPK stimulation similarly to that observed in WKY PTE cells. The enhanced sensitivity to phenylephrine-induced stimulation of the Cl−/HCO3− exchanger activity through the α1-adrenoceptors in SHR PTE cells was associated with the higher H2O2 generation.
The results presented here show that noradrenaline stimulated Cl−/HCO3− exchanger activity, in a concentration-dependent manner, in both WKY and SHR PTE cells. SHR PTE cells showed an enhanced sensitivity to noradrenaline-induced stimulation of the Cl−/HCO3− exchanger activity compared with that in WKY PTE cells. This type of response is consistent with previous experiments with another anti-natriuretic substance, angiotensin II, upon the Cl−/HCO3− exchanger (Pedrosa and Soares-da-Silva, 2006). The effect of noradrenaline on Cl−/HCO3− exchanger activity in WKY and SHR PTE cells was abolished by prazosin (α1-adrenoceptor antagonist), but not by yohimbine (α2-adrenoceptor antagonist). In addition, phenylephrine (α1-adrenoceptor agonist) stimulated the exchanger activity in a concentration-dependent manner in WKY and SHR PTE cells. SHR PTE cells have an enhanced sensitivity to phenylephrine comparatively to WKY PTE cells. The effect of phenylephrine upon Cl−/HCO3− exchanger activity was prevented by prazosin but not by yohimbine in WKY and SHR PTE cells. Taken together, these results indicate that noradrenaline-induced stimulation of the Cl−/HCO3− exchanger activity occurs through the α1-adrenoceptor. Major mechanisms intervening in renal proximal tubular NaCl absorption, pHi and cell volume regulation have been suggested to occur through the concerted action of the Cl−/HCO3− and NHEs. This fits well with previous studies that demonstrated that activation of the α1-adrenoceptor also increases NHE activity in the proximal tubule (Gesek et al., 1989; Gesek and Schoolwerth, 1990; Liu et al., 1997). The observation that α1A- and α1B-adrenoceptor expression was identical in WKY and SHR PTE cells excludes the possibility that the enhanced sensitivity to phenylephrine and noradrenaline in stimulating Cl−/HCO3− exchanger activity in SHR PTE cells might be related to differences in the density of α1-adrenoceptors. This result is in agreement with previous reports that detected no significant changes in the expression of α1-adrenoceptors in renal tissues from SHR and WKY (Yamada et al., 1986; Jeffries et al., 1988).
The α1-adrenoceptors initiate their physiological effects by activating PL (PLC) in the cell membrane, resulting in production of inositol 1,4,5-trisphosphate, which mobilizes intracellular Ca2+ and diacylglycerol, which in turn activates PKC (Theroux et al., 1996). More recently, α1-adrenoceptors were found to activate a variety of other effectors, such as the MAPK pathways, in various cell types (Michelotti et al., 2000; Piascik and Perez, 2001). Downstream to PKC activation, other mechanisms involved in signal transduction coupled to α1-adrenoceptor include activation of both the ERK and p38 MAPK pathways (Alexandrov et al., 1998; Snabaitis et al., 2000; Markou and Lazou, 2002). We examined some of these signalling pathways activated by α1-adrenoceptors in WKY and SHR PTE cells. Previously, studies with mouse PTE cells demonstrated that α1-adrenoceptor activation of NHE1 (NHE isoform 1) is also regulated by PKC, whereas NHE3 (NHE isoform 3) is controlled by MAPK (Liu and Gesek, 2001). In renal proximal tubules, the relationship between α1-adrenoceptor and MAPK is also a well-established process (Liu and Gesek, 2001). However, the link between α1-adrenoceptor-induced stimulation of sodium-reabsorptive mechanisms and MAPK is not so well established, although there is evidence suggesting that α1-adrenoceptors activate distinct signalling pathways to regulate specific NHE isoforms localized on opposite membranes in polarized renal epithelial cells (Liu and Gesek, 2001). α1-Adrenoceptor activation of NHE1 is regulated by PKC, whereas NHE3 is controlled by MAPK and serves to separately regulate pHi, Na+ absorption and Na+ excretion in PTE cells (Liu and Gesek, 2001).
The data obtained in the present study provide evidence that activation of PKC, ERK 1/2 and p38 MAPK is required for α1-adrenoceptor-induced stimulation of Cl−/HCO3− exchanger in WKY PTE cells. On the other hand, in SHR cells only PKC, ERK 2 and p38 MAPK are required for α1-adrenoceptor-induced stimulation of Cl−/HCO3− exchanger. To evaluate the contribution of MAPK in the signal-transduction pathway coupled to phenylephrine-induced stimulation of Cl−/HCO3− exchanger activity, specific MEK and p38 MAPK inhibitors were used. The p38 MAPK inhibitor SB 203580 blocked the stimulation of Cl−/HCO3− exchanger activity by phenylephrine in both WKY and SHR PTE cells. The finding that anisomycin, an activator of p38 MAPK, stimulated Cl−/HCO3− exchanger activity in both WKY and SHR PTE cells, in a SB 203580-sensitive manner, supports the involvement of p38 MAPK in events downstream of α1-adrenoceptor activation in which the Cl−/HCO3− exchanger is the effector protein. The results presented here also clearly establish a connection between MEK and p38 MAPK in the signal-transduction pathways coupled to α1-adrenoceptor-induced stimulation of Cl−/HCO3− exchanger activity in both WKY and SHR PTE cells. In WKY PTE cells, the specific MEK 1 inhibitor PD 098059 blunted the stimulation of Cl−/HCO3− exchanger activity induced by phenylephrine, but it was devoid of effects in SHR PTE cells. By contrast, the MEK 1/2 inhibitor U 0126 blunted the stimulation of Cl−/HCO3− exchanger activity induced by phenylephrine in both WKY and SHR PTE cells. This fits well the evidence that SHR PTE cells only express ERK 2, whereas WKY PTE cells express both ERK 1 and ERK 2 (Parenti et al., 2000).
Transduction mechanisms set into motion during activation of α1-adrenoceptor in WKY and SHR PTE cells involve the activation of both PKC and p38 MAPK pathways in a single sequence of events with PKC activation occurring before p38 MAPK activation, which most likely includes phosphorylation of p38 MAPK by PKC. Both anisomycin and PDBu were able to stimulate Cl−/HCO3− exchanger activity to the same extent, with these effects being prevented by specific inhibitors of p38 MAPK (SB 203580) and PKC (chelerythrine). Similarly, p38 MAPK and PKC inhibition by SB 203580 and chelerythrine prevented the stimulation of Cl−/HCO3− exchanger activity by the α1-adrenoceptor agonist phenylephrine. This suggests the involvement of both kinases in the signal-transduction pathway following α1-adrenoceptor activation, but does not constitute evidence that stimulation of α1-adrenoceptor may lead to simultaneous activation of both p38 MAPK and PKC transduction pathways. In fact, the most likely possibility consists of a single sequence of events with PKC activation before p38 MAPK activation in the signalling cascade downstream to stimulation of α1-adrenoceptors. This view is compatible with the finding that p38 MAPK inhibition by SB 203580 abolished the inhibitory effects of anisomycin, phenylephrine and PDBu, whereas PKC inhibition by chelerythrine abolished the effects of PDBu and phenylephrine, but not those elicited by anisomycin. The observation that the Cl−/HCO3− exchanger in SHR PTE cells responded to PKC and p38 MAPK stimulation similarly to that observed in WKY PTE cells suggests that differences in sensitivity to phenylephrine and noradrenaline between SHR and WKY PTE cells may not be related to differences for the activation of these pathways in WKY and SHR PTE cells.
From the finding that the signal-transduction pathway associated with phenylephrine-induced stimulation of Cl−/HCO3− exchanger activity is similar in WKY and SHR PTE cells, with the exception of the involvement of MEK 1, and that differences in the level of expression of α1-adrenoceptors do not explain the differences in the sensitivity to phenylephrine, it was hypothesized that oxidative stress, which has been clearly implicated in hypertension (Makino et al., 2003; Adler and Huang, 2004; Asghar et al., 2006; de Cavanagh et al., 2006), could be involved in such differences in the response to phenylephrine in WKY and SHR PTE cells. To test this possibility, the generation of H2O2, a marker of oxidative stress, was evaluated in WKY and SHR PTE cells. SHR PTE cells were found to be endowed with an increased rate of H2O2 production, which was fivefold than that in WKY cells. As a result of this enhanced ability to generate H2O2, SHR PTE cells accumulated greater amounts of H2O2 in the extracellular medium. One of the mechanisms that may be involved in the enhanced generation of H2O2 in SHR PTE cells could be the overexpression of NADPH oxidase, as has been found in the SHR (Adler and Huang, 2004). The view that apocynin, an inhibitor of NADPH oxidase, clearly reduced the extracellular levels of H2O2 in SHR PTE cells, but not in WKY PTE cells, implicates this enzyme in the enhanced oxidative stress in SHR PTE cells. In SHR PTE cells, treatment with apocynin, apart from decreasing the levels of H2O2 in the extracellular medium, also abolished the enhanced sensitivity in phenylephrine-mediated stimulation of Cl−/HCO3− exchanger activity. It should be emphasized that, after treatment with 100 μM apocynin, WKY PTE cells exhibited the same sensitivity to phenylephrine in terms of the α1-adrenoceptor-mediated stimulation of Cl−/HCO3− exchanger activity. On the other hand, stimulation of Cl−/HCO3− exchanger activity by H2O2 was significantly higher in SHR PTE cells than that in WKY PTE cells. Altogether, this would agree with the view that the enhanced sensitivity to phenylephrine-mediated stimulation of Cl−/HCO3− exchanger activity in SHR cells is a consequence of the oxidative stress condition, resulting from increases in the generation of H2O2. The treatment with apocynin (100 μM) for 4 days after seeding did not change the basal Cl−/HCO3− exchanger activity in both WKY and SHR PTE cells, suggesting that apocynin only modifies responses after stimulus, but not the basal activities. In addition, we demonstrated that SHR PTE cells overexpressed the Cl−/HCO3− exchanger SLC26A6 (sevenfold more than WKY PTE cells) (Pedrosa and Soares-da-Silva, 2006), which may explain the fact that inhibition of NADPH oxidase had no effect on the enhanced basal exchanger activity in SHR PTE cells. One aspect that it is under evaluation in our laboratory is the elucidation of the H2O2-sensitive mechanism responsible for the enhanced phenylephrine-induced stimulation of Cl−/HCO3− exchanger activity in SHR PTE cells.
In conclusion, SHR PTE cells were 10 and 4 times more sensitive to noradrenaline and phenylephrine than WKY PTE cells in stimulating Cl−/HCO3− exchanger activity, respectively. In both cell lines, the phenylephrine-mediated stimulation of Cl−/HCO3− exchanger activity proceeds through a molecular pathway that involves PKC and p38 MAPK downstream α1-adrenoceptor activation. Differences between WKY and SHR PTE cells in their sensitivity to phenylephrine do not correlate with the expression of the α1A- and α1B-adrenoceptors and may be related to the increased generation of H2O2, which may amplify the phenylephrine response downstream of α1-adrenoceptor activation.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia, POCTI, POCI, FEDER and Programa Comunitário de Apoio (POCI/SAU-FCF/59207/2004).
Abbreviations
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- NHE
Na+/H+ exchanger
- PDBu
phorbol-12,13-dibutyrate
- PTE
proximal tubular epithelial
- SHR
spontaneously hypertensive rat
- WKY
Wistar Kyoto rat
Conflict of interest
The authors state no conflict of interest.
References
- Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol. 2004;287:F907–F913. doi: 10.1152/ajprenal.00060.2004. [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Keffel S, Goepel M, Michel MC. Stimulation of alpha1A-adrenoceptors in Rat-1 cells inhibits extracellular signal-regulated kinase by activating p38 mitogen-activated protein kinase. Mol Pharmacol. 1998;54:755–760. doi: 10.1124/mol.54.5.755. [DOI] [PubMed] [Google Scholar]
- Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol Rev. 1990;70:79–114. doi: 10.1152/physrev.1990.70.1.79. [DOI] [PubMed] [Google Scholar]
- Asghar M, Banday AA, Fardoun RZ, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free Radic Biol Med. 2006;40:13–20. doi: 10.1016/j.freeradbiomed.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Baines AD, Ho P. Specific alpha 1-, alpha 2-, and beta-responses to norepinephrine in pyruvate-perfused rat kidneys. Am J Physiol. 1987;252:F170–F176. doi: 10.1152/ajprenal.1987.252.1.F170. [DOI] [PubMed] [Google Scholar]
- Chan JS, Wang TT, Zhang SL, Chen X, Carriere S. Catecholamines and angiotensinogen gene expression in kidney proximal tubular cells. Mol Cell Biochem. 2000;212:73–79. [PubMed] [Google Scholar]
- Dart C, Vaughan-Jones RD. Na+-HCO3− symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1616–R1625. doi: 10.1152/ajpregu.00615.2005. [DOI] [PubMed] [Google Scholar]
- DiBona GF. Neural control of renal function: role of renal alpha adrenoceptors. J Cardiovasc Pharmacol. 1985;7 Suppl 8:S18–S23. [PubMed] [Google Scholar]
- Fraga S, Luo Y, Jose P, Zandi-Nejad K, Mount DB, Soares-da-Silva P. Dopamine D1-like receptor-mediated inhibition of Cl−/HCO3− exchanger activity in rat intestinal epithelial IEC-6 cells is regulated by G protein-coupled receptor kinase 6 (GRK 6) Cell Physiol Biochem. 2006;18:347–360. doi: 10.1159/000097612. [DOI] [PubMed] [Google Scholar]
- Gesek FA, Cragoe EJ, Jr, Strandhoy JW. Synergistic alpha-1 and alpha-2 adrenergic stimulation of rat proximal nephron Na+/H+ exchange. J Pharmacol Exp Ther. 1989;249:694–700. [PubMed] [Google Scholar]
- Gesek FA, Schoolwerth AC. Hormonal interactions with the proximal Na+–H+ exchanger. Am J Physiol. 1990;258:F514–F521. doi: 10.1152/ajprenal.1990.258.3.F514. [DOI] [PubMed] [Google Scholar]
- Gesek FA, Strandhoy JW. Dual interactions between alpha 2-adrenoceptor agonists and the proximal Na+–H+ exchanger. Am J Physiol. 1990;258:F636–F642. doi: 10.1152/ajprenal.1990.258.3.F636. [DOI] [PubMed] [Google Scholar]
- Gomes P, Soares-da-Silva P. Upregulation of apical NHE3 in renal OK cells overexpressing the rodent alpha1-subunit of the Na+ pump. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1142–R1150. doi: 10.1152/ajpregu.00102.2005. [DOI] [PubMed] [Google Scholar]
- Gomes P, Vieira-Coelho MA, Soares-Da-Silva P. Ouabain-insensitive acidification by dopamine in renal OK cells: primary control of the Na+/H+ exchanger. Am J Physiol Regul Integr Comp Physiol. 2001;281:R10–R18. doi: 10.1152/ajpregu.2001.281.1.R10. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- Hara C, Satoh H, Usui T, Kunimi M, Noiri E, Tsukamoto K, et al. Intracellular pH regulatory mechanism in a human renal proximal cell line (HKC-8): evidence for Na+/H+ exchanger, CI−/HCO3− exchanger and Na+–HCO3− cotransporter. Pflugers Arch. 2000;440:713–720. doi: 10.1007/s004240000356. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Bengtsson T. alpha1A-adrenoceptors activate glucose uptake in L6 muscle cells through a phospholipase C-, phosphatidylinositol-3 kinase-, and atypical protein kinase C-dependent pathway. Endocrinology. 2005;146:901–912. doi: 10.1210/en.2004-1083. [DOI] [PubMed] [Google Scholar]
- Jeffries WB, Yang E, Pettinger WA. Renal alpha 1-adrenergic receptor response coupling in spontaneously hypertensive rats. Hypertension. 1988;12:80–88. doi: 10.1161/01.hyp.12.1.80. [DOI] [PubMed] [Google Scholar]
- Kanagy NL. Alpha2-adrenergic receptor signalling in hypertension. Clin Sci (Lond) 2005;109:431–437. doi: 10.1042/CS20050101. [DOI] [PubMed] [Google Scholar]
- Krapf R, Alpern RJ. Cell pH and transepithelial H/HCO3 transport in the renal proximal tubule. J Membr Biol. 1993;131:1–10. doi: 10.1007/BF02258529. [DOI] [PubMed] [Google Scholar]
- Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985;17:1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- Liu F, Gesek FA. Alpha1-adrenergic receptors activate NHE1 and NHE3 through distinct signaling pathways in epithelial cells. Am J Physiol Renal Physiol. 2001;280:F415–F425. doi: 10.1152/ajprenal.2001.280.3.F415. [DOI] [PubMed] [Google Scholar]
- Liu F, Nesbitt T, Drezner MK, Friedman PA, Gesek FA. Proximal nephron Na+/H+ exchange is regulated by alpha 1A- and alpha 1B-adrenergic receptor subtypes. Mol Pharmacol. 1997;52:1010–1018. doi: 10.1124/mol.52.6.1010. [DOI] [PubMed] [Google Scholar]
- Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension. 2003;42:25–30. doi: 10.1161/01.HYP.0000074903.96928.91. [DOI] [PubMed] [Google Scholar]
- Markou T, Lazou A. Phosphorylation and activation of mitogen- and stress-activated protein kinase-1 in adult rat cardiac myocytes by G-protein-coupled receptor agonists requires both extracellular-signal-regulated kinase and p38 mitogen-activated protein kinase. Biochem J. 2002;365:757–763. doi: 10.1042/BJ20011828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelotti GA, Price DT, Schwinn DA. Alpha 1-adrenergic receptor regulation: basic science and clinical implications. Pharmacol Ther. 2000;88:281–309. doi: 10.1016/s0163-7258(00)00092-9. [DOI] [PubMed] [Google Scholar]
- Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R, et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation. 2002;106:2800–2805. doi: 10.1161/01.cir.0000039528.49161.e9. [DOI] [PubMed] [Google Scholar]
- Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- Parenti A, Cui XL, Hopfer U, Ziche M, Douglas JG. Activation of MAPKs in proximal tubule cells from spontaneously hypertensive and control Wistar-Kyoto rats. Hypertension. 2000;35:1160–1166. doi: 10.1161/01.hyp.35.5.1160. [DOI] [PubMed] [Google Scholar]
- Pedrosa R, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Gialpha3 protein-coupled dopamine D3 receptor-mediated inhibition of renal NHE3 activity in SHR proximal tubular cells is a PLC–PKC-mediated event. Am J Physiol Renal Physiol. 2004a;287:F1059–F1066. doi: 10.1152/ajprenal.00139.2004. [DOI] [PubMed] [Google Scholar]
- Pedrosa R, Gomes P, Soares-da-Silva P. Distinct signalling cascades downstream to Gsalpha coupled dopamine D1-like NHE3 inhibition in rat and opossum renal epithelial cells. Cell Physiol Biochem. 2004b;14:91–100. doi: 10.1159/000076930. [DOI] [PubMed] [Google Scholar]
- Pedrosa R, Gomes P, Zeng C, Hopfer U, Jose PA, Soares-da-Silva P. Dopamine D3 receptor-mediated inhibition of Na+/H+ exchanger activity in normotensive and spontaneously hypertensive rat proximal tubular epithelial cells. Br J Pharmacol. 2004c;142:1343–1353. doi: 10.1038/sj.bjp.0705893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa R, Jose PA, Soares-da-Silva P. Defective D1-like receptor-mediated inhibition of the Cl−/HCO3− exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2004d;286:F1120–F1126. doi: 10.1152/ajprenal.00433.2003. [DOI] [PubMed] [Google Scholar]
- Pedrosa R, Soares-da-Silva P. AT1 receptors mediate the enhanced sensitivity to angiotensin II and upon overexpressed Cl−/HCO3− exchanger (SLC26A6) in hypertension. Nephrol Dial Transplant. 2006;21 Suppl 4:iv24. [Google Scholar]
- Petrovic S, Ma L, Wang Z, Soleimani M. Identification of an apical Cl−/HCO−3 exchanger in rat kidney proximal tubule. Am J Physiol Cell Physiol. 2003;285:C608–C617. doi: 10.1152/ajpcell.00084.2003. [DOI] [PubMed] [Google Scholar]
- Piascik MT, Perez DM. Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther. 2001;298:403–410. [PubMed] [Google Scholar]
- Scarparo AC, Visconti MA, Castrucci AM. Signalling pathways evoked by alpha1-adrenoceptors in human melanoma cells. Cell Biochem Funct. 2006;24:119–129. doi: 10.1002/cbf.1309. [DOI] [PubMed] [Google Scholar]
- Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- Snabaitis AK, Yokoyama H, Avkiran M. Roles of mitogen-activated protein kinases and protein kinase C in alpha1A-adrenoceptor-mediated stimulation of the sarcolemmal Na+–H+ exchanger. Circ Res. 2000;86:214–220. doi: 10.1161/01.res.86.2.214. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Burnham CE. Physiologic and molecular aspects of the Na+:HCO3− cotransporter in health and disease processes. Kidney Int. 2000;57:371–384. doi: 10.1046/j.1523-1755.2000.00857.x. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Singh G. Physiologic and molecular aspects of the Na+/H+ exchangers in health and disease processes. J Investig Med. 1995;43:419–430. [PubMed] [Google Scholar]
- Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;292:R764–R768. doi: 10.1152/ajpregu.00322.2006. [DOI] [PubMed] [Google Scholar]
- Theroux TL, Esbenshade TA, Peavy RD, Minneman KP. Coupling efficiencies of human alpha1-adrenergic receptor subtypes: titration of receptor density and responsiveness with inducible and repressible expression vectors. Mol Pharmacol. 1996;50:1376–1387. [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Ni Z, Oveisi F, Trnavsky-Hobbs DL. Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension. 2000;36:957–964. doi: 10.1161/01.hyp.36.6.957. [DOI] [PubMed] [Google Scholar]
- Wilborn TW, Sun D, Schafer JA. Expression of multiple alpha-adrenoceptor isoforms in rat CCD. Am J Physiol. 1998;275:F111–F118. doi: 10.1152/ajprenal.1998.275.1.F111. [DOI] [PubMed] [Google Scholar]
- Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, et al. Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int. 1996;50:125–134. doi: 10.1038/ki.1996.295. [DOI] [PubMed] [Google Scholar]
- Xu P, Spitzer KW. Na-independent Cl−–HCO3− exchange mediates recovery of pHi from alkalosis in guinea pig ventricular myocytes. Am J Physiol. 1994;267:H85–H91. doi: 10.1152/ajpheart.1994.267.1.H85. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nakamoto M, Hayashi M, Tomita T, Hayashi E. Tubular and glomerular adrenoceptors in stroke-prone spontaneously hypertensive rats. J Hypertens. 1986;4:S209–S211. [Google Scholar]