Abstract
Background and purpose:
Peroxisome proliferator-activated receptor (PPAR)-γ ligands have been shown to inhibit cardiac fibrosis. However, the underlying mechanisms are poorly understood. We investigated the regulation by PPAR-γ ligands of angiotensin (Ang) II-induced plasminogen activator inhibitor (PAI)-1, extracellular matrix (ECM) production and cell growth in cardiac fibroblasts.
Experimental approach:
The effects of PPAR-γ ligands on Ang II-induced PAI-1, ECM expression and cell growth were assessed in primary-cultured rat cardiac fibroblasts; cardiac PAI-1 and ECM production was examined in Ang II-infused rats.
Key results:
In growth-arrested cardiac fibroblasts, PPAR-γ ligands rosiglitazone and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) dose-dependently attenuated Ang II-induced cell proliferation and expression of PAI-1, collagen type-I, collagen type-III and fibronectin. An accompanying increase in PPAR-γ expression and activation was also observed. These suppressive effects were attenuated by the PPAR-γ antagonists GW9662 and bisphenol A diglycidyl ether (BADGE). Moreover, rosiglitazone and 15d-PGJ2 inhibited in part the expression and phosphorylation of Ang II-induced transforming growth factor (TGF)-β1, Smad2/3 and c-Jun NH(2)-terminal kinase (JNK). Ang II infusion in rats markedly increased left ventricular production of PAI-1, collagen and fibronectin, with a concurrent increase in the ratios of heart weight/body weight and left ventricle weight/body weight. Co-treatment with rosiglitazone significantly decreased these levels and upregulated PPAR-γ expression.
Conclusions and implications:
Rosiglitazone and 15d-PGJ2 suppress Ang II-induced production of PAI-1 and ECM probably via interactions between PPAR-γ and TGF-β1/Smad2/3 and JNK signalling pathways. It is suggested that PPAR-γ and its ligands may have potential applications in preventing cardiac fibrosis.
Keywords: PPAR-γ, angiotensin, cardiac fibroblast, plasminogen activator inhibitor-1, extracellular matrix, rosiglitazone, 15d-PGJ2, fibrosis
Introduction
Cardiac fibrosis promotes the progression of left ventricular hypertrophy associated with hypertension, post-myocardial infarction remodelling and heart failure, and thus is a key determinant of clinical outcome in heart diseases. The proximal effector cells in this process are cardiac fibroblasts (Brown et al., 2005). There is now convincing evidence that angiotensin (Ang) II, which has been shown to be aberrantly activated in hypertension, myocardial ischaemia and diabetes mellitus (Gibbons, 1998; Fiordaliso et al., 2000), has an important function in cardiac fibrosis by promoting cardiac fibroblast proliferation, transdifferentiation and the production of extracellular matrix (ECM) proteins such as collagen, fibronectin and laminin.
Plasminogen activator inhibitor (PAI)-1 is a member of the serine protease inhibitor family. It is strongly implicated in fibrosis of diverse tissues (Kaikita et al., 2001; Eddy, 2002) by preventing degradation of the ECM, regulating the activation of metalloproteinases and accelerating collagen deposition (Yamamoto and Saito, 1998). Ang II is a potent inducer of PAI-1 production in heart (Abrahamsen et al., 2002) and multiple cell types such as cardiac fibroblasts (Kawano et al., 2000) and cardiomyocytes (Takeshita et al., 2004). Moreover, the induction of PAI-1 has also been suggested as a possible mechanism through which Ang II promotes the development of cardiovascular remodelling (Weisberg et al., 2005).
Peroxisome proliferator-activated receptor (PPAR)-γ belongs to the nuclear hormone receptor superfamily. After being stimulated by PPAR-γ ligands, such as rosiglitazone, pioglitazone and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), it binds to specific PPAR-responsive elements (PPRE) in target genes to modulate gene transcription (Houseknecht et al., 2002). In the cardiovascular system, PPAR-γ is expressed in cardiac myocytes (Takano et al., 2000) and other cells, where it exerts pleiotropic effects in cardiovascular diseases (Hsueh and Bruemmer, 2004). Recently, considerable evidence points to a role of PPAR-γ and its ligands in inhibiting fibrotic remodelling of diverse organs and tissues (Galli et al., 2002; Masamune et al., 2002; Burgess et al., 2005; Zafiriou et al., 2005). In addition, our previous study also found antifibrotic activities of PPAR-γ ligands on vascular fibrosis (Gao et al., 2007). However, their role in cardiac fibrosis is less well investigated. In vivo studies have shown that PPAR-γ ligands attenuate myocardial fibrosis in several experimental models (Iglarz et al., 2003; Geng et al., 2006), and some in vitro data have demonstrated the inhibitory effects of these ligands on Ang II- or anoxia–reoxygenation-induced production of collagen I, matrix metalloproteinase-1 and brain natriuretic peptide in cardiac fibroblasts (Chen et al., 2004a, 2004b; Makino et al., 2006). Despite these findings, the underlying mechanisms for the regulatory effects of PPAR-γ ligands on cardiac fibrosis are largely unknown and the specific role of PPAR-γ in this process is less clear.
In the present study, we examined the effects of rosiglitazone (a high-affinity synthetic ligand) and 15d-PGJ2 (an endogenous ligand) on Ang II-induced production of PAI-1 and ECM components in cardiac fibroblasts as well as in cardiac fibrosis in vivo. Furthermore, we attempted to elucidate the molecular mechanisms and the probable implications of PPAR-γ underlying these actions. Our results revealed that rosiglitazone and 15d-PGJ2 attenuated Ang II-induced cell proliferation and expression levels of PAI-1 and ECM components in cardiac fibroblasts. More importantly, we showed that the beneficial effects of these ligands involved the interactions between PPAR-γ and transforming growth factor (TGF)-β1/Smad2/3, c-Jun NH(2)-terminal kinase (JNK) signalling pathways. Rosiglitazone administration in Ang II-infused rats for 7 days effectively lowered production of PAI-1 and ECM, which was accompanied by decreased collagen content of the left ventricle. These findings demonstrate the potential antifibrotic actions of PPAR-γ and its agonist ligands on Ang II-induced cardiac fibrosis.
Methods
Cell culture
All animal procedures and our study followed the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). Cardiac fibroblasts were obtained from ventricles of 1- to 2-day-old Sprague–Dawley rats by the collagenase and trypsin digestion methods as described (Kim et al., 1995a) and grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, penicillin (100 U ml−1) and streptomycin (100 U ml−1). A total of 95% of the cells displayed positive immunoreactivity towards vimentin. Cells within three passages were used for the studies. For subsequent experiments, cells at 80% confluence in culture dishes were growth-arrested by serum starvation for 48 h.
Cell proliferation assay
Cell growth was determined by measuring MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide; Sigma, St Louis, MO, USA) dye absorbance of living cells. Cells were seeded in 96-well plates at 1 × 104 per well. After indicated treatments, 20 μl of MTT solution at 5 mg ml−1 was added to each well, and plates were incubated for an additional 4 h at 37 °C. The culture medium was removed; then 150 μl of dimethyl sulphoxide was added to each well. Absorbance was measured at 490 nm by using a microplate spectrophotometer (POLARstar OPTIMA, BMG LABTECH, Offenburg, Germany).
Immunocytochemistry
Immunocytochemical analysis was performed by using rabbit polyclonal antibodies against PPAR-γ (1:300), PAI-1 (1:200) as primary antibodies and biotinylated goat anti-rabbit IgG (Zymed, San Francisco, CA, USA) as the secondary antibody. A streptavidin enzyme conjugate was sequentially added for 20 min, and then cells were incubated with the substrate 3,3′-diaminobenzidine. In some experiments, tetramethylrhodamine isothiocyanate-labelled goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as secondary antibody, followed by the nuclear counterstaining with Hoechst 33342 (5 μg ml−1). Omission of primary antibodies was used as negative controls.
Real-time reverse transcription-PCR
Total RNA was extracted by using TRIzol reagent (Invitrogen Carlsbad, CA, USA), and DNA was removed by the use of the DNA-free kit (Ambion, Austin, TX, USA). Reverse transcription and PCR of each sample were performed in triplicates using the SYBR ExScript RT-PCR Kit (TaKaRa Bio, Shiga, Japan) and the ABI PRISM 7000 sequence detection PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. Sequence-specific PCR primers were designed using Beacon designer v4.0 (Premier Biosoft, Palo Alto, CA, USA) (see Table 1 for details). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control. Results were expressed as fold difference for each gene against GAPDH by the use of the 2−ΔΔCT method. Results were validated by demonstrating generation of a single PCR product of an expected size by gel electrophoresis and by the dissociation curve analysis. Serial 10-fold dilutions of the cDNA were used to confirm near-theoretical efficiencies of the assays.
Table 1.
Primer sets for PCR amplification
| Gene | Oligonucleotide primer sequences (5′–3′) |
|---|---|
| PPAR-γ | Forward: TGGAGCCTAAGTTTGAGTTTG |
| Reverse: ATCTTCTGGAGCACCTTGG | |
| PAI-1 | Forward: CCTCCTCATCCTGCCTAAGTTC |
| Reverse: GCCGCTCTCGTTCACCTC | |
| Collagen I | Forward: CTACCTCGTTCTTGTCTTTGTG |
| Reverse: TCTCTCCTCCCTTGTTAAATAGC | |
| Collagen III | Forward: AGATGCTGGTGCTGAGAAG |
| Reverse: TGGAAAGAAGTCTGAGGAAGG | |
| Fibronectin | Forward: GTGAAGAACGAGGAGGATGTG |
| Reverse: GTGATGGCGGATGATGTAGC | |
| GAPDH | Forward: GCCTTCTCCATGGTGGTGAA |
| Reverse: GGTCGGTGTGAACGGATTTG |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PAI-1, plasminogen activator inhibitor-1; PPAR-γ, peroxisome proliferator-activated receptor-γ.
Western blot analysis
Myocardial tissue or cells lysates were prepared with 200 μl ice-cold lysis buffer (pH 7.4) (50 mM HEPES, 5 mM EDTA, 100 mM NaCl, 1% Triton X-100, protease inhibitor cocktail; Roche, Mannheim, Germany) in the presence of phosphatase inhibitors (50 mM sodium fluoride, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 nM microcystin). The nuclear proteins were extracted additionally by the use of the Pierce NE-PER kit (Pierce, Rockford, IL, USA) according to the manufacturer's instruction. Protein concentrations were determined with the BCA protein assay kit (Pierce). In some studies, culture media were also collected for analysis. Samples were subjected to electrophoresis in 8% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gels and transferred onto a polyvinylidene difluoride membrane in a semi-dry system (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 10% fat-free milk in TBST buffer (20 mM Tris-HCl, 137 mM NaCl and 0.05% Tween 20), and subsequently incubated with primary antibodies in TBST buffer overnight. The membranes were then washed and incubated with secondary antibodies for 45 min. Antigen–antibody complexes were revealed by chemiluminescence and visualized by exposure to X-ray films. Optical densities of bands were scanned and quantified with the use of Gel Doc 2000 (Bio-Rad). β-Actin was used as loading control. Data are normalized against those of the corresponding β-actin. Results were expressed as fold increase over control.
DNA-binding assay
Peroxisome proliferator-activated receptor-γ DNA-binding activity was detected by an ELISA-based method with the PPAR-γ transcription assay kit (Cayman Chemical, Ann Arbor, MI, USA). Briefly, 10 μg of nuclear protein was added to the 96-well plate pre-coated with specific double-strand DNA sequence containing the PPRE and then incubated overnight at 4 °C. Bound PPAR-γ was detected by the use of specific PPAR-γ antibody. A horseradish peroxidase-conjugated secondary antibody was then added for colorimetric reading at 450 nm.
In vivo experiments
Male Sprague–Dawley rats (180 g) were divided into four groups (n=9 each) depending on the treatments: control rats, Ang II, Ang II+rosiglitazone and rosiglitazone. Rats were anaesthetized with methoxyflurane, and then osmotic minipumps (model 2001; Durect Corp, Cupertino, CA, USA) were inserted subcutaneously to deliver Ang II (150 ng kg−1 min−1) for 7 days. Rosiglitazone (5 mg kg−1 day−1) was administered in drinking water for 8 days, starting the day before Ang II infusion. Systolic blood pressure (SBP) was measured by the tail-cuff method. At the end of the experiment, animals were killed with an overdose of pentobarbital. Then hearts were excised and weighed. One portion of the ventricle was fixed in 4% formaldehyde solution and embedded in paraffin for collagen evaluation; the rest of the left ventricle was snap-frozen in liquid nitrogen and stored at −70 °C for subsequent biochemical assays.
Evaluation of collagen deposition
The median part of the left ventricle sections (5 μm) were hydrated and stained with Sirius Red F3BA (0.5% in saturated aqueous picric acid; Sigma) and viewed under polarized light. Ten fields in each region of the heart were selected randomly from three nonconsecutive serial sections, and collagen content was quantified as the Sirius Red-positive areas by the use of a Zeiss Axiovert S100 TV microscope (Zeiss, Jena, Germany) and ImageTool 2.0 (UTHSCSA, San Antonio, TX, USA).
Statistical analysis
Results are expressed as mean±s.e.mean. Statistical significance between groups was assessed by one-way ANOVA, followed by post hoc Duncan multiple comparisons, with the use of SPSS 11.5 (SPSS Inc., Chicago, IL, USA). A value of P<0.05 was considered significant.
Reagents
Dulbecco's modified Eagle's medium, fetal bovine serum, penicillin, streptomycin were purchased from Gibco BRL (Carlsbad, CA, USA). Ang II, 15d-PGJ2, GW9662, bisphenol A diglycidyl ether (BADGE) and anti-α-smooth muscle actin antibody were from Sigma. Rosiglitazone was from Alexis (Lausen, Switzerland). Rabbit anti-PPAR-γ antibody was from Upstate (Chicago, IL, USA). Rabbit polyclonal antibodies against PAI-1, collagen type-I, collagen type-III, fibronectin, Smad2/3 and phospho-Smad2/3 (Ser 433/435) were from Santa Cruz Biotechnology. Rabbit polyclonal antibodies against JNK and phospho-JNK (Thr183/Tyr185) were from Cell Signaling Technology (Beverly, MA, USA).
Results
Expression and activation of PPAR-γ in cardiac fibroblasts
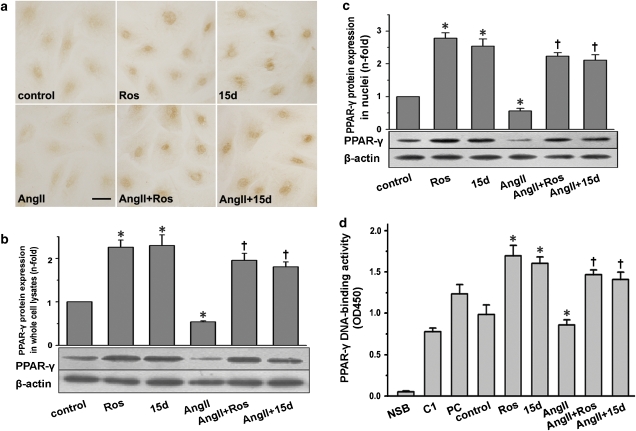
To examine PPAR-γ expression, primary cultured cardiac fibroblasts were growth-arrested for 48 h. Immunocytochemistry showed the presence of low level of PPAR-γ protein, particularly in and around nuclei without stimulation (Figure 1a). Treatment with Ang II led to more faint staining. Rosiglitazone or 15d-PGJ2 incubation resulted in substantial nuclear staining of PPAR-γ in Ang II-treated and untreated cells. No staining was detected in the absence of primary antibody (data not shown); then whole-cell lysates were analysed by western blot (Figure 1b). In non-stimulated cardiac fibroblasts, a single approximately 60 kDa band corresponding to PPAR-γ was detected. In cells incubated with Ang II, PPAR-γ level was significantly decreased. Treatment with PPAR-γ ligands substantially increased the cellular PPAR-γ level in Ang II-treated and untreated cells. Similar results were found for PPAR-γ mRNA (data not shown).
Figure 1.
Expression and activation of PPAR-γ in cardiac fibroblasts. Cells were pretreated with or without rosiglitazone (Ros; 5 μM) or 15d-PGJ2 (15d; 5 μM) for 1 h and subsequently stimulated with Ang II (0.1 μM) for 24 h. (a) A representative immunocytochemical staining of PPAR-γ in cardiac fibroblasts (n=5; bar=50 μm). (b) Detection of PPAR-γ protein in whole-cell lysates by western blot analysis. (c) Detection of PPAR-γ protein in nuclear extracts by western blot analysis. (d) PPAR-γ activation was analysed by DNA-binding assay using PPAR-γ transcription assay kit. NSB indicates nonspecific binding; C1 for competitor; PC for positive control. All the data shown here are the mean±s.e.mean of three independent experiments. *P<0.05 vs control; †P<0.05 vs Ang II. Ang II, angiotensin II, 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2; PPAR-γ, peroxisome proliferator-activated receptor-γ.
Then, nuclear protein was extracted to examine the activation of PPAR-γ by western blot analysis (Figure 1c) and DNA-binding assay (Figure 1d), respectively. Exposure to rosiglitazone and 15d-PGJ2 for 24 h resulted in a significant increase in the nuclear level of PPAR-γ and its DNA-binding activity to PPRE. Moreover, Ang II treatment led to a dramatic decrease in nuclear protein of PPAR-γ and its DNA-binding activity, whereas pretreatment with rosiglitazone or 15d-PGJ2 substantially increased the two parameters in comparison with Ang II treatment.
Effects of PPAR-γ agonist ligands on Ang II-induced proliferation and differentiation of cardiac fibroblasts
Angiotensin II induced a significant increase in the cell viability. Incubation with rosiglitazone and 15d-PGJ2 markedly prevented this increase in a dose-dependent manner. Pretreatment with BADGE and GW9662, two antagonists of PPAR-γ, blocked the effects of rosiglitazone and 15d-PGJ2 (Supplementary Figure 1).
We then investigated the effects of PPAR-γ ligands on transdifferentiation of cardiac myofibroblasts by examining α-smooth muscle actin expression with the use of western blot analysis. α-Smooth muscle actin expression was increased by 32% with 72-h Ang II treatment (P<0.05). But both rosiglitazone and 15d-PGJ2 failed to prevent this increase (data not shown).
PPAR-γ agonist ligands inhibited Ang II-induced expression of PAI-1, collagen I, collagen III and fibronectin in cardiac fibroblasts
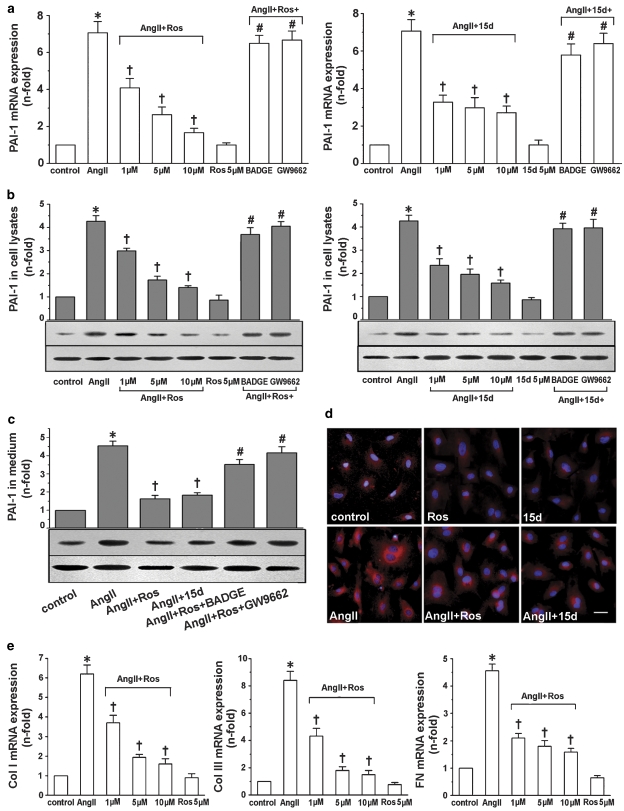
Results from real-time reverse transcription-PCR analysis showed that the increased levels of PAI-1 mRNA in response to Ang II were substantially reduced by both rosiglitazone and 15d-PGJ2 in a dose-dependent manner (Figure 2a). In cells pretreated with the PPAR-γ antagonists BADGE and GW9662, the suppression of PAI-1 by rosiglitazone and 15d-PGJ2 was no longer observed. Similar results were found by using western blot analysis for detecting PAI-1 protein both in cell lysates (Figure 2b) and in conditioned medium (Figure 2c). Immunofluorescence showed an apparent increase in cytoplasmic staining of PAI-1 when cardiac fibroblasts were stimulated with Ang II for 24 h (Figure 2d). This effect was also inhibited by pretreatment with the PPAR-γ agonists. Neither rosiglitazone nor 15d-PGJ2 administered alone modified the basal levels of PAI-1 mRNA and protein. In addition, rosiglitazone also concentration-dependently prevented the increase in Ang II-induced mRNA levels of collagen I, collagen III and fibronectin, as assessed by real-time reverse transcription-PCR (Figure 2e). Rosiglitazone alone had no significant effect on the basal levels of these parameters. Similar results were observed with 15d-PGJ2 treatment (data not shown).
Figure 2.
Rosiglitazone and 15d-PGJ2 inhibited PAI-1, collagen I (Col I), collagen III (Col III) and fibronectin (FN) expression in Ang II-stimulated cardiac fibroblasts. Cells were pretreated with or without GW9662 (3 μM) or BADGE (1 μM) for 30 min prior to the addition of rosiglitazone (Ros; 1, 5 and 10 μM) or 15d-PGJ2 (15d; 1, 5 and 10 μM). Ang II (0.1 μM) was then added for 24 h. (a) Real-time RT-PCR revealed that rosiglitazone (left panel) and 15d-PGJ2 (right panel) inhibited Ang II-induced PAI-1 mRNA expression in a PPAR-γ-dependent manner. Western blots showed that rosiglitazone and 15d-PGJ2 inhibited Ang II-induced PAI-1 protein synthesis (b) and secretion (c) in a PPAR-γ-dependent manner. β-Actin in cell fragment was served as an internal control. (d) A representative immunofluorescent staining showed that rosiglitazone and 15d-PGJ2 markedly inhibited PAI-1 cytoplasmic staining in cardiac fibroblasts (n=5; bar: 50 μm). (e) Rosiglitazone dose-dependently inhibited Ang II-induced collagen I, collagen III and fibronectin mRNA expression in cardiac fibroblasts. Results are expressed as fold increase over control and mean±s.e.mean data of three independent experiments are shown. *P<0.05 vs control; †P<0.05 vs Ang II; #P<0.05 vs Ang II+Ros or Ang II+15d-PGJ2. Ang II, angiotensin; BADGE, bisphenol A diglycidyl ether; 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2; PAI-1, plasminogen activator inhibitor-1; PPAR-γ, peroxisome proliferator-activated receptor-γ; real-time RT-PCR, real-time reverse transcription-PCR.
TGF-β1/Smad2/3 signalling pathway in the effects of PPAR-γ ligands on Ang II-induced PAI-1 and ECM expression
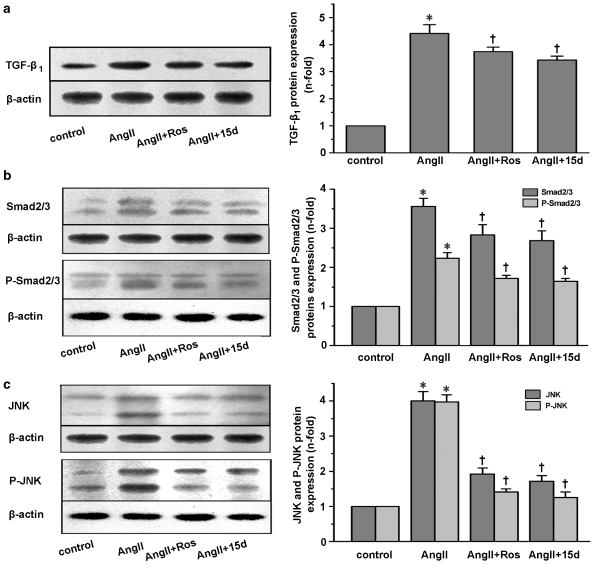
We sought to examine whether transforming growth factor-β1 (TGF-β1) served as a mediator of the effects of PPAR-γ ligands on Ang II-induced PAI-1 and ECM synthesis in cardiac fibroblasts. As shown in Figure 3a, the level of TGF-β1 protein was significantly increased with Ang II treatment for 24 h. Rosiglitazone and 15d-PGJ2 inhibited the increase by 17.1 and 22.4%, respectively (both P<0.05 vs Ang II).
Figure 3.
Effects of rosiglitazone (Ros) and 15d-PGJ2 (15d) on Ang II-induced TGF-β1/Smad2/3 and JNK signalling pathways in cardiac fibroblasts. Cells were pretreated with 5 μM rosiglitazone or 15d-PGJ2 for 1 h, and then stimulated with 0.1 μM Ang II for 24 h. Western blot analysis was subsequently used for detecting the expression of TGF-β1, Smad2/3 and JNK protein; cells were pretreated with 5 μM rosiglitazone or 15d-PGJ2 for 30 min, followed by 0.1 μM Ang II incubation for 20 min. Phosphorylated proteins were then detected. Rosiglitazone and 15d-PGJ2 inhibited Ang II-induced TGF-β1 expression (a), Smad2/3 (b) and JNK (c) expression and phosphorylation. All values are showed as mean±s.e.mean of three experiments. *P<0.05 vs control; †P<0.05 vs Ang II. Ang II, angiotensin II; 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2; JNK, c-Jun NH(2)-terminal kinase; TGF-β1, transforming growth factor-β1.
We then explored the regulation of rosiglitazone and 15d-PGJ2 on activation levels of Smad2/3, which were the major downstream effectors of TGF-β1. In parallel with their effect on TGF-β1, both PPAR-γ ligands had modest inhibitory effects on Ang II-induced expression of Smad2/3 and phospho-Smad2/3 (all P<0.05 vs Ang II; Figure 3b).
JNK signalling pathway in the effects of PPAR-γ agonists on Ang II-mediated PAI-1 and ECM expression
As shown in Figure 3c, the levels of both JNK and phopho-JNK proteins were significantly upregulated when cells were treated with Ang II. Rosiglitazone and 15d-PGJ2 pretreatment markedly diminished the increases.
SBP, body weight, heart weight and left ventricle weight in Ang II-infused rats
After the 7-day treatment, body weights were similar in all groups (Table 2). Ang II infusion induced a substantial increase in SBP, heart weight, the ratios of heart weight/body weight and left ventricle weight/body weight, which were markedly attenuated by rosiglitazone. Treatment with rosiglitazone alone had no significant effects on these parameters.
Table 2.
SBP, BW, HW and LVW in Ang II-infused rats
| Parameter | Control | Ang II | Ang II+Ros | Ros |
|---|---|---|---|---|
| BW (g) | 223±4 | 225±6 | 226±7 | 225±4 |
| SBP (mm Hg) | 110±4 | 165±9* | 127±5† | 114±3 |
| HW/BW (mg g−1) | 3.19±0.08 | 3.35±0.09* | 3.24±0.06† | 3.20±0.06 |
| LVW/BW (mg g−1) | 2.17±0.05 | 2.28±0.07* | 2.20±0.02† | 2.16±0.04 |
Abbreviations: Ang II, angiotensin II; BW, body weight; HW, heart weight; LVW, left ventricle weight; Ros, rosiglitazone; SBP, systolic blood pressure.
Data are mean±s.e.mean of nine rats.
*P<0.05 vs control, †P<0.05 vs Ang II.
Collagen deposition
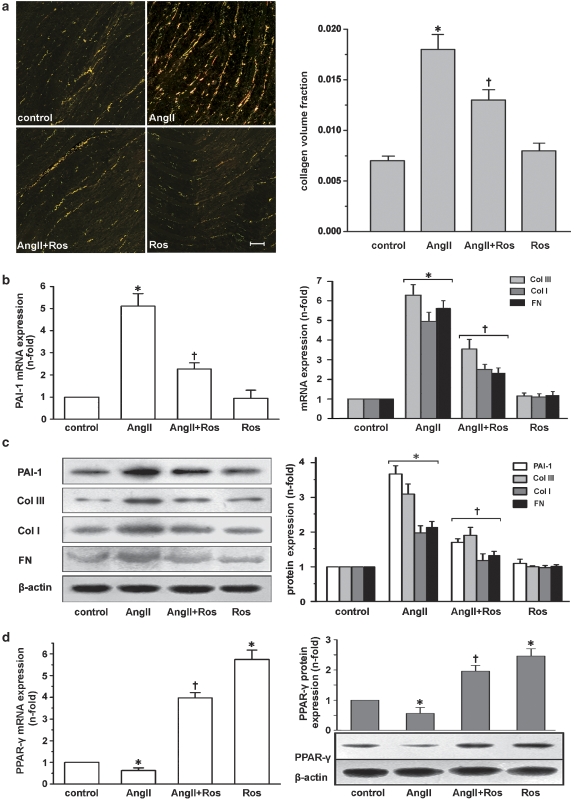
Sirius Red staining revealed that collagen content of the left ventricle was higher in the Ang II group compared with the control group (Figure 4a). Treatment with rosiglitazone could partially prevent this increase. Rosiglitazone treatment alone had no significant effect on collagen deposition.
Figure 4.
In vivo data from Ang II-infused rats. (a) Sirius Red staining showed a significant decrease in left ventricular collagen deposition of Ang II-infused rats by rosiglitazone (Ros). Intensity of staining was quantified and expressed as mean±s.e.mean of data from six animals in each group (left panel). Scale bar: 100 μm. (b) Real-time RT-PCR results of rosiglitazone inhibiting left ventricular levels of PAI-1, collagen I (Col I), collagen III (Col III) and fibronectin (FN) mRNA in Ang II-infused rats. (c) Western blots documented that rosiglitazone significantly inhibited left ventricular PAI-1, collagen I, collagen III and fibronectin protein expression. (d) Rosiglitazone upregulated left ventricular PPAR-γ mRNA and protein levels. Results are expressed as fold increase compared with control and mean±s.e.mean of data from six animals in each group is shown. *P<0.05 vs control; †P<0.05 vs Ang II. Ang II, angiotensin II; PAI-1, plasminogen activator inhibitor-1; PPAR-γ, peroxisome proliferator-activated receptor-γ; real-time RT-PCR, real-time reverse transcription-PCR.
Expression of PAI-1 and ECM in left ventricles of Ang II-infused rats
Both the mRNA (Figure 4b) and protein (Figure 4c) levels of PAI-1 were significantly elevated in the left ventricles of rats treated with Ang II, but only a slight increase was observed in the Ang II+rosiglitazone group (P<0.05 vs Ang II). The mRNA and protein levels of collagen I, collagen III and fibronectin were similar to that of PAI-1 as assessed by real-time reverse transcription-PCR (Figure 4b, right panel) and western blot (Figure 4c), respectively. Treatment with rosiglitazone alone had no significant effects on these variables.
PPAR-γ expression in Ang II-infused rats
Angiotensin II infusion caused a significant decrease in the PPAR-γ mRNA level. Pretreatment with rosiglitazone dramatically increased PPAR-γ levels in Ang II-treated and untreated rats (Figure 4d, left panel). A similar trend was found in the protein levels of PPAR-γ in these groups (Figure 4b, right panel).
Discussion
Peroxisome proliferator-activated receptor-γ was originally identified in adipocytes, and more recently, has been shown to be expressed in numerous tissues, where it exerts multiple effects, including regulation of tissue fibrosis. Although evidence has shown the inhibitory effects of PPAR-γ ligands on cardiac fibrosis, the involvement of PPAR-γ in this process has not been investigated. Our study demonstrated that quiescent normal cardiac fibroblasts expressed PPAR-γ, which could be decreased by Ang II stimulation. These results are consistent with recent studies performed on cardiac myofibroblasts (Chintalgattu and Katwa, 2004) and myocytes (Ye et al., 2006). Moreover, both rosiglitazone and 15d-PGJ2 upregulated the activation of PPAR-γ in response to Ang II, as indicated by enhanced nuclear accumulation of PPAR-γ protein and increased DNA-binding activity of PPAR-γ to PPRE. These results raise the possibility that PPAR-γ ligands may be involved in the effects of Ang II in cardiac fibroblasts through a functional PPAR-γ pathway. In addition, our data, which are in line with previous reports (Park et al., 1998; Su et al., 1999), showed that in vitro treatment with PPAR-γ ligands upregulated PPAR-γ expression, suggesting an additional mechanism by which PPAR-γ ligands regulate gene expression.
In light of the presence of PAI-1 in both cardiac fibroblasts and cardiomyocytes and its facilitative role in cardiac fibrosis (Kawano et al., 2000; Kaikita et al., 2001; Takeshita et al., 2004), we investigated the regulation of PPAR-γ ligands on PAI-1 production in cardiac fibroblasts. Consistent with the previous report (Kawano et al., 2000), a significant increase of PAI-1 mRNA and protein levels induced by Ang II was observed in our study. Furthermore, we found that both rosiglitazone and 15d-PGJ2 dose-dependently suppressed the increase of PAI-1 in response to Ang II. Similarly, PPAR-γ ligands have been found to inhibit the Ang II-enhanced PAI-1 level in cultured mesangial cells (Nicholas et al., 2001) as well as PAI-1 expression induced by tumour necrosis factor-α or TGF-β1 in many cell types, including hepatocyte cells, endothelial cells (Kato et al., 1999) and adipocytes (Zirlik et al., 2004). More importantly, results with the PPAR-γ inhibitors GW9662 and BADGE demonstrated that downregulation of PAI-1 by rosiglitazone and 15d-PGJ2 was mediated by PPAR-γ activation. In addition, PPAR-γ ligands dose-dependently decreased Ang II-induced mRNA levels of collagen I, collagen III and fibronectin, which are the major components of ECM in cardiac fibrosis. Pioglitazone, another PPAR-γ ligand, has also been shown to inhibit Ang II-induced expression of collagen I in cardiac fibroblasts (Chen et al., 2004a). Our data also revealed that both rosiglitazone and 15d-PGJ2 significantly attenuated Ang II-induced proliferation of cardiac fibroblasts through a PPAR-γ-dependent way, suggesting an additional means, whereby PPAR-γ ligands regulate fibrotic process.
Thus, our findings clearly indicate that PPAR-γ agonist ligands regulate the cellular effects induced by Ang II, which may mediate cardiac fibrosis. Moreover, our data imply a potential role of PPAR-γ in these processes. Thus, Ang II-induced production of ECM and PAI-1 was accompanied by a downregulation of PPAR-γ expression and activation, and when the expression and activation of PPAR-γ were upregulated by PPAR-γ ligands, PAI-1 and ECM production was also suppressed; the synthetic PPAR-γ ligand rosiglitazone and the endogenous ligand 15d-PGJ2 had parallel effects on PAI-1 and ECM synthesis; the PPAR-γ antagonists GW9662 and BADGE inhibited the effects of both rosiglitazone and 15d-PGJ2.
There is substantial evidence that Ang II is a potent inducer of TGF-β1 synthesis in a variety of cells and that this mechanism exerts important biological effects, including ECM accumulation, cell proliferation and hypertrophy (Verrecchia and Mauviel, 2002). In addition, a critical mediator controlling PAI-1 is TGF-β1 (Dennler et al., 1998). Previous studies have also found a downregulation of TGF-β1 by PPAR-γ ligands in renal tubular cells (Panchapakesan et al., 2005), peritoneal mesothelial cells (Peng et al., 2006) and hepatic stellate cells (Zhao et al., 2006). Our results demonstrated that the expression of TGF-β1 protein was substantially increased by Ang II treatment. However, rosiglitazone and 15d-PGJ2 modestly, but significantly, lowered this increase by 17 and 23%, respectively. In contrast, rosiglitazone and 15d-PGJ2 decreased Ang II-induced PAI-1 protein level by 59 and 71%. Meanwhile, results with Smad2/3, which are the main downstream effectors of TGF-β signalling and are central in most actions of the TGF-β family regarding ECM and PAI-1 gene expression (Ruiz-Ortega et al., 2007), also showed a modest decrease in their expression and phosphorylation by PPAR-γ ligands. The lack of correlation between the decreased levels of PAI-1 and TGF-β1/Smad2/3 indicates that the TGF-β1/Smad2/3 signalling pathway may contribute only, in part, to the inhibition of PPAR-γ ligands on Ang II-induced cell proliferation, PAI-1 and ECM production. Therefore, some other mechanisms may also be involved.
As JNK is implicated in cardiac diseases, and is preferentially activated by hypertrophic stimuli such as Ang II, leading to the activation of transcription factor activator protein (AP)-1 (Kudoh et al., 1997), which is present in the promoter of genes involved in remodelling such as collagen and skeletal α-actin (Karin, 1995), it may be possible that JNK, via AP-1, participates in Ang II-mediated gene expression in cardiac remodelling. Indeed, a recent study has suggested that Ang II-induced PAI-1 expression is mediated through JNK activation in neonatal cardiac fibroblasts and myocytes (Omura et al., 2005). Our study with JNK confirmed this possibility, and further demonstrated that rosiglitazone and 15d-PGJ2 markedly diminished Ang II-induced JNK expression and phosphorylation. Rosiglitazone and troglitazone, another PPAR-γ ligand, have been shown to inhibit tumour necrosis factor-α-induced JNK activation in 3T3-L1 adipocytes (Díaz-Delfín et al., 2007). In vivo, rosiglitazone also prevented post-ischaemic injury and significantly improved functional recovery by inhibiting JNK phosphorylation in both normal and diabetic rat hearts (Khandoudi et al., 2002). Thus, the decrease of PAI-1 and ECM by PPAR-γ ligands observed in our study is, at least in part, mediated through the JNK signalling pathway. Overall, our findings indicate that both TGF-β1/Smad2/3 and JNK signal pathways are implicated in the suppression of PAI-1 and ECM production in response to Ang II, mediated by PPAR-γ activation.
A possible concern arising from these in vitro studies is that the effects of PPAR-γ ligands on Ang II-induced PAI-1 and ECM production in cardiac fibroblasts may not reflect their role in cardiac PAI-1 and ECM production in vivo. To extend our in vitro studies, we used Ang II-infused rats as the in vivo model. Previous studies have shown the elevation of cardiac PAI-1 and ECM expression in this model (Kim et al., 1995b; Baltatu et al., 2000; Nakamura et al., 2000; Abrahamsen et al., 2002; Zirlik et al., 2004). Consistent with these reports, our findings demonstrated that Ang II infusion promotes cardiac fibrosis, as indicated by a substantial increase of PAI-1, collagen I, collagen III and fibronectin expression in the left ventricle of the rats. Furthermore, our results, together with the previous reports (Kim et al., 1995b; Baltatu et al., 2000), showed increased ratios of heart weight/body weight and left ventricle weight/body weight by Ang II infusion. More importantly, our data revealed that rosiglitazone prevented Ang II-induced collagen content, PAI-1 and ECM levels and inhibited the increase in the ratios of heart weight/body weight and left ventricular weight/body weight. These results are consistent with previous studies showing an inhibitory effect of PPAR-γ ligands on the stimulated collagen deposition and heart weight/body weight ratio in multiple animal models (Asakawa et al., 2002; Iglarz et al., 2003; Geng et al., 2006). And these in vivo effects observed in our study were accompanied with an increase in PPAR-γ expression and decrease in SBP. Clinical investigations and animal models have shown the downregulation of SBP by rosiglitazone (Diep et al., 2002). However, with respect to our in vitro studies on cardiac fibroblasts, which are not affected by pressure or haemodynamic-induced changes, and results of others demonstrating that Ang II-induced PAI-1 and ECM expression in rat heart is independent of blood pressure elevation (Kim et al., 1995b; Nakamura et al., 2000), the suppressive effects of rosiglitazone on PAI-1 and ECM expression in vivo is most likely to be due, in part, to the direct cellular effects of rosiglitazone on cardiac fibroblasts and may be independent of lowered blood pressure.
Recently, several clinical trials have showed that rosiglitazone appears to be associated with an increase in the risk of myocardial infarction and congestive heart failure (Kermani and Garg, 2003; Nissen and Wolski, 2007). The underlying mechanisms remain uncertain and whether there is a role of local cardiac PPAR-γ in these observed adverse effects associated with rosiglitazone is still unclear. Importantly, pioglitazone appears to have cardiac protective effects (Dormandy et al., 2005). This discrepancy reminds us that the observed risks of rosiglitazone may not represent the effects of other PPAR-γ ligands, such as 15d-PGJ2. Indeed, pioglitazone and 15d-PGJ2 have been shown to reduce acute myocardial infarction with an increased PPAR-γ expression and activation (Wayman et al., 2002; Cao et al., 2007). In addition, pioglitazone has also been shown to inhibit pressure overload-induced cardiac hypertrophy through PPAR-γ-dependent pathways (Asakawa et al., 2002). These findings are consistent with our observations that rosiglitazone may reverse cardiac fibrosis through PPAR-γ activation in vitro and in vivo. However, further studies are needed to elucidate the precise role of cardiac PPAR-γ in cardiovascular diseases.
In conclusion, PPAR-γ is functionally expressed in cardiac fibroblasts. The PPAR-γ ligands rosiglitazone and 15d-PGJ2 decrease Ang II-induced PAI-1 expression and ECM production in vitro and in vivo. The interactions between PPAR-γ and TGF-β1/Smad2/3, JNK signalling pathways may contribute to the suppressive effects of the PPAR-γ ligands. These findings provide further insight into the beneficial cardiac effects of PPAR-γ ligands and the potential molecular mechanisms of PPAR-γ and its ligands in preventing cardiac fibrosis.
External data objects
Acknowledgments
This study was supported by the Major Basic Research Development Program of China from the Ministry of Science and Technology (no. 2006CB503802 to XLN and 2006CB503906 to NPW).
Abbreviations
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- Ang II
angiotensin II
- BADGE
bisphenol A diglycidyl ether
- ECM
extracellular matrix
- JNK
c-Jun NH(2)-terminal kinase
- PAI-1
plasminogen activator inhibitor-1
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- PPRE
PPAR-responsive element
- TGF-β1
transforming growth factor-β1
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Abrahamsen CT, Pullen MA, Schnackenberg CG, Grygielko ET, Edwards RM, Laping NJ, et al. Effects of angiotensins II and IV on blood pressure, renal function, and PAI-1 expression in the heart and kidney of the rat. Pharmacology. 2002;66:26–30. doi: 10.1159/000063252. [DOI] [PubMed] [Google Scholar]
- Asakawa M, Takano H, Nagai T, Uozumi H, Hasegawa H, Kubota N, et al. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105:1240–1246. doi: 10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- Baltatu O, Silva JA, Jr, Ganten D, Bader M. The brain renin–angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension. 2000;35:409–412. doi: 10.1161/01.hyp.35.1.409. [DOI] [PubMed] [Google Scholar]
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, et al. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- Cao Z, Ye P, Long C, Chen K, Li X, Wang H. Effect of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on ischemia–reperfusion injury in rats. Pharmacology. 2007;79:184–192. doi: 10.1159/000100870. [DOI] [PubMed] [Google Scholar]
- Chen K, Chen J, Li D, Zhang X, Mehta JL. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone. Hypertension. 2004a;44:655–661. doi: 10.1161/01.HYP.0000144400.49062.6b. [DOI] [PubMed] [Google Scholar]
- Chen K, Li D, Zhang X, Hermonat PL, Mehta JL. Anoxia–reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J Cardiovasc Pharmacol. 2004b;44:682–687. doi: 10.1097/00005344-200412000-00010. [DOI] [PubMed] [Google Scholar]
- Chintalgattu V, Katwa LC.The modulation of PPAR-gamma expression by angiotensin-II in cardiac myofibroblasts FASEB J 200418abstract no. 677.8 [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Delfín J, Morales M, Caelles C. Hypoglycemic action of thiazolidinediones/peroxisome proliferator-activated receptor gamma by inhibition of the c-Jun NH2-terminal kinase pathway. Diabetes. 2007;56:1865–1871. doi: 10.2337/db06-1293. [DOI] [PubMed] [Google Scholar]
- Diep QN, EI Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, et al. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Eddy AA. Plasminogen activator inhibitor-1 and the kidney. Am J Physiol Renal Physiol. 2002;283:F209–F220. doi: 10.1152/ajprenal.00032.2002. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, et al. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II-dependent. Lab Invest. 2000;80:513–527. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- Gao DF, Niu XL, Hao GH, Peng N, Wei J, Ning N, et al. Rosiglitazone inhibits angiotensin II-induced CTGF expression in vascular smooth muscle cells—role of PPAR-gamma in vascular fibrosis. Biochem Pharmacol. 2007;73:185–197. doi: 10.1016/j.bcp.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Geng DF, Wu W, Jin DM, Wang JF, Wu YM. Effect of peroxisome proliferator-activated receptor gamma ligand. Rosiglitazone on left ventricular remodeling in rats with myocardial infarction. Int J Cardiol. 2006;113:86–91. doi: 10.1016/j.ijcard.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Gibbons GH. The pathophysiology of hypertension: the importance of angiotensin II in cardiovascular remodeling. Am J Hypertens. 1998;11:177S–181S. doi: 10.1016/s0895-7061(98)00198-8. [DOI] [PubMed] [Google Scholar]
- Houseknecht KL, Cole BM, Steele PJ. Peroxisome proliferator-activated receptor gamma (PPARgamma) and its ligands: a review. Domest Anim Endocrinol. 2002;22:1–23. doi: 10.1016/s0739-7240(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor gamma: implications for cardiovascular disease. Hypertension. 2004;43:297–305. doi: 10.1161/01.HYP.0000113626.76571.5b. [DOI] [PubMed] [Google Scholar]
- Iglarz M, Touyz RM, Viel EC, Paradis P, Amiri F, Diep QN, et al. Peroxisome proliferator-activated receptor-alpha and receptor-gamma activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension. 2003;42:737–743. doi: 10.1161/01.HYP.0000083511.91817.B1. [DOI] [PubMed] [Google Scholar]
- Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001;104:839–844. doi: 10.1161/hc3301.092803. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 binding activity by mitogen-activated protein kinase. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kato K, Satoh H, Endo Y, Yamada D, Midorikawa S, Sato W, et al. Thiazolidinediones down-regulate plasminogen activator inhibitor type 1 expression in human vascular endothelial cells: a possible role for PPARgamma in endothelial function. Biochem Biophys Res Commun. 1999;258:431–435. doi: 10.1006/bbrc.1999.0648. [DOI] [PubMed] [Google Scholar]
- Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, et al. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation. 2000;101:1130–1137. doi: 10.1161/01.cir.101.10.1130. [DOI] [PubMed] [Google Scholar]
- Kermani A, Garg A. Thiazolidinedione-associated congestive heart failure and pulmonary oedema. Mayo Clin Proc. 2003;78:1088–1091. doi: 10.4065/78.9.1088. [DOI] [PubMed] [Google Scholar]
- Khandoudi N, Delerive P, Berrebi-Bertrand I, Buckingham RE, Staels B, Bril A. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma, inhibits the Jun NH(2)-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51:1507–1514. doi: 10.2337/diabetes.51.5.1507. [DOI] [PubMed] [Google Scholar]
- Kim NN, Villarreal FJ, Printz MP, Lee AA, Dillmann WH. Trophic effects of angiotensin II on neonatal rat cardiac myocytes are mediated by cardiac fibroblasts. Am J Physiol. 1995a;269:E426–E437. doi: 10.1152/ajpendo.1995.269.3.E426. [DOI] [PubMed] [Google Scholar]
- Kim S, Ohta K, Hamaguchi A, Yukimura T, Miura K, Iwao H. Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension. 1995b;25:1252–1259. doi: 10.1161/01.hyp.25.6.1252. [DOI] [PubMed] [Google Scholar]
- Kudoh S, Komuro I, Mizuno T, Yamazaki T, Zou Y, Shiojima IX, et al. Angiotensin II stimulates c-Jun NH2-terminal kinase in cultured cardiac myocytes of neonatal rats. Circ Res. 1997;80:139–146. doi: 10.1161/01.res.80.1.139. [DOI] [PubMed] [Google Scholar]
- Makino N, Sugano M, Satoh S, Oyama J, Maeda T. Peroxisome proliferator-activated receptor-gamma ligands attenuate brain natriuretic peptide production and affect remodeling in cardiac fibroblasts in reoxygenation after hypoxia. Cell Biochem Biophys. 2006;44:65–71. doi: 10.1385/CBB:44:1:065. [DOI] [PubMed] [Google Scholar]
- Masamune A, Kikuta K, Satoh M, Sakai Y, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J Biol Chem. 2002;277:141–147. doi: 10.1074/jbc.M107582200. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Nakamura I, Ma L, Vaughan DE, Fogo AB. Plasminogen activator inhibitor-1 expression is regulated by the angiotensin type 1 receptor in vivo. Kidney Int. 2000;58:251–259. doi: 10.1046/j.1523-1755.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- Nicholas SB, Kawano Y, Wakino S, Collins AR, Hsueh WA. Expression and function of peroxisome proliferator-activated receptor-gamma in mesangial cells. Hypertension. 2001;37:722–727. doi: 10.1161/01.hyp.37.2.722. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Omura T, Yoshiyama M, Matsumoto R, Kusuyama T, Enomoto S, Nishiya D, et al. Role of c-Jun NH2-terminal kinase in G-protein-coupled receptor agonist-induced cardiac plasminogen activator inhibitor-1 expression. J Mol Cell Cardiol. 2005;38:583–592. doi: 10.1016/j.yjmcc.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Panchapakesan U, Sumual S, Pollock CA, Chen X. PPARgamma agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol. 2005;289:F1153–F1158. doi: 10.1152/ajprenal.00097.2005. [DOI] [PubMed] [Google Scholar]
- Park KS, Ciaraldi TP, Lindgren K, Abrams-Carter L, Mudaliar S, Nikoulina SE, et al. Troglitazone effects on gene expression in human skeletal muscle of type II diabetes involve up-regulation of peroxisome proliferator-activated receptor-gamma. J Clin Endocrinol Metab. 1998;83:2830–2835. doi: 10.1210/jcem.83.8.5034. [DOI] [PubMed] [Google Scholar]
- Peng Y, Liu H, Liu F, Liu Y, Li J, Chen X. Troglitazone inhibits synthesis of transforming growth factor-beta1 and reduces matrix production in human peritoneal mesothelial cells. Nephrology (Carlton) 2006;11:516–523. doi: 10.1111/j.1440-1797.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Su JL, Winegar DA, Wisely GB, Sigel CS, Hull-Ryde EA. Use of a PPAR gamma-specific monoclonal antibody to demonstrate thiazolidinediones induce PPAR gamma receptor expression in vitro. Hybridoma. 1999;18:273–280. doi: 10.1089/027245799315934. [DOI] [PubMed] [Google Scholar]
- Takano H, Nagai T, Asakawa M, Toyozaki T, Oka T, Komuro I, et al. Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes. Circ Res. 2000;87:596–602. doi: 10.1161/01.res.87.7.596. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Hayashi M, Iino S, Kondo T, Inden Y, Iwase M, et al. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164:449–456. doi: 10.1016/S0002-9440(10)63135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, et al. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002;16:1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, et al. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Saito H. A pathological role of increased expression of plasminogen activator inhibitor-1 in human or animal disorders. Int J Hematol. 1998;68:371–385. doi: 10.1016/s0925-5710(98)00094-2. [DOI] [PubMed] [Google Scholar]
- Ye P, Sheng L, Zhang C, Liu Y. Atorvastatin attenuating down-regulation of peroxisome proliferator-activated receptor gamma in preventing cardiac hypertrophy of rats in vitro and in vivo. J Pharm Pharm Sci. 2006;9:365–375. [PubMed] [Google Scholar]
- Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005;16:638–645. doi: 10.1681/ASN.2004040278. [DOI] [PubMed] [Google Scholar]
- Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARgamma agonists prevent TGFbeta1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun. 2006;350:385–391. doi: 10.1016/j.bbrc.2006.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirlik A, Leugers A, Lohrmann J, Ernst S, Sobel BE, Bode C, et al. Direct attenuation of plasminogen activator inhibitor type-1 expression in human adipose tissue by thiazolidinediones. Thromb Haemost. 2004;91:674–682. doi: 10.1160/TH03-06-0384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.